An Andersson lesion (AL) is a rare complication of ankylosing spondylitis (AS). It was first described by Andersson in 1,937 (1). Clinical symptoms manifest as persistent pain in the lower back, with obvious tenderness at the spinous process upon physical examination. Few patients with an AL will have symptoms of compression of dura mater or nerves caused by fracture displacement. Often, an AL is misdiagnosed as purulent spondylitis or spinal tuberculosis, and the treatment for these diseases varies considerably. Therefore, an accurate diagnosis is of paramount importance. Many patients may experience persistent dull pain and can be treated conservatively. For those with severe pain or neurological symptoms, surgical treatment is recommended to rebuild spinal stability and relieve nerve compression. The surgical techniques employed are varied and encompass open fixation, open fixation with osteotomy, open fixation with lumbar interbody fusion (LIF), and minimally invasive surgery (MIS) fixation with and without LIF. Here, we report an atypical case of a 43-year-old man diagnosed with a neglected AL. We completed LIF with unilateral biportal endoscopy (UBE) and fixation using percutaneous pedicle screws (PPSs).
Case presentationA 43-year-old man complaining of lower back pain that had persisted for 8 years was admitted to our hospital. The progressively increasing pain and unresponsiveness to analgesics or physiotherapy based on traditional Chinese medicine encouraged him to seek additional treatment in our hospital. The patient appeared to be physically healthy. He was not using medication, had not undergone surgery, and did not have inherited diseases in his family. He had not experienced fever, weight loss, coughing, or night sweats. At the initial visit, the patient only had magnetic resonance imaging (MRI) data from a different hospital, which showed endplate erosion and damage to intervertebral disks (Figure 1). Physical examination showed no other signs of nerve damage except for obvious tenderness at the spinous process of the lower back with limited activity upon flexion and extension. Based on symptoms and imaging data, we preliminarily suspected spinal infection, and he was admitted to our hospital.
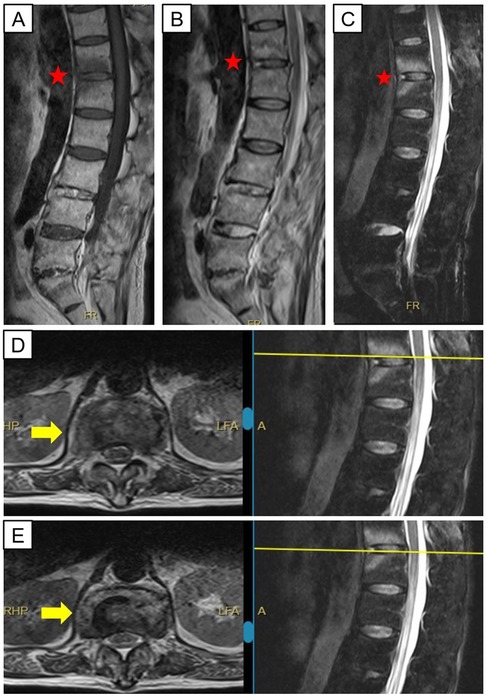
Figure 1. Magnetic resonance images of the patient before admission. The red pentagrams indicate the lesion mimicking ModicⅠchanges, which are hypointense on T1-weighted sequences (A), hyperintense on T2-weighted sequences (B) and STIR sequences (C) Yellow arrows show inhomogeneous signal in the disc space at T12/L1 in keeping with discitis (D,E).
Blood samples for hematologic indices and infection-related indices were collected as part of the routine examination after admission (Table 1). We found no abnormalities except for a slightly higher erythrocyte sedimentation rate (ESR). Therefore, we could exclude bacterial or viral infections. Radiography showed separation of T12-L1 vertebrae and sclerotic endplates in the spine. Computed tomography (CT) showed corresponding widening of the intervertebral space, the “vacuum sign” in the joint space, destruction of endplate bone, and peripheral-bone sclerosis. Based on imaging (CT and radiography) findings (Figure 2), medical history, and clinical examination, we performed an HLA-B27 blood test to confirm the diagnosis of AS with an AL.
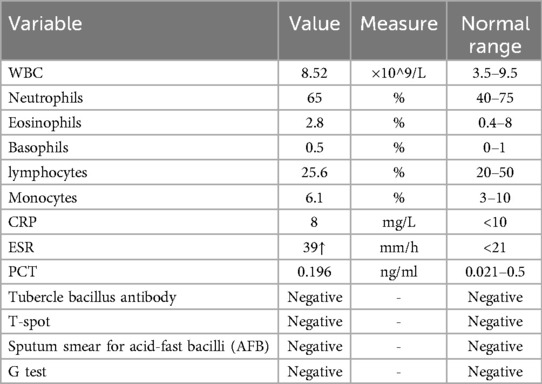
Table 1. Laboratory test indicators during the hospitalization of this patient.
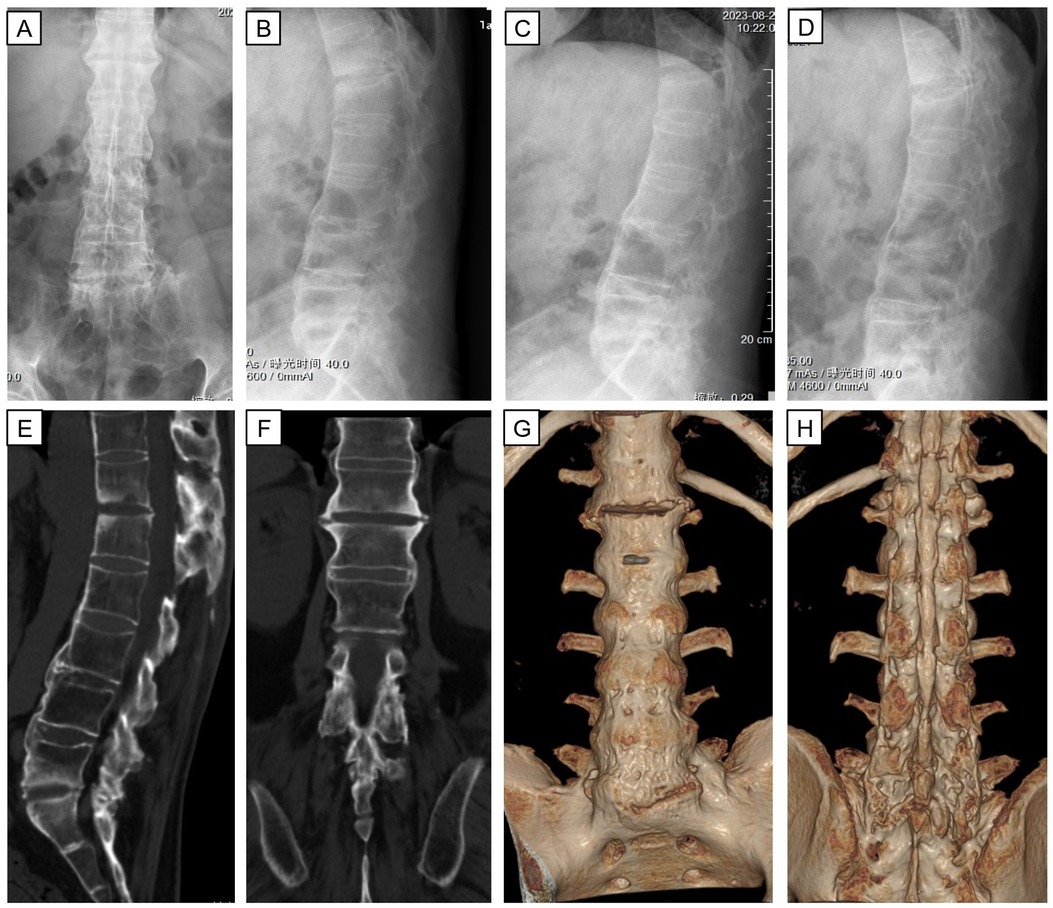
Figure 2. X-ray and CT three-dimensional reconstruction of thoraco-lumbar vertebrae after admission. Anterior and lateral radiographs (A,B) show abnormal paravertebral and anterior longitudinal ligament ossification. Flexion and extension lateral radiographs (C,D) represent a loss of lumbar mobility. Sagittal, coronal, and reconstructed coronal CT images (E–H) demonstrate instability of the lumbar spine result from no bone bridging between T12 and L1.
After communication between the treating physicians and patient, the latter refused the original plan of oral intake of non-steroidal anti-inflammatory drugs (NSAIDs) and immunosuppressive drugs. Sticking to medication and physiotherapy did not get rid of his back pain. On the contrary, the patient is still unable to carry out long-term physical activities every day. So he requested surgical treatment. Considering the minimally invasive requirements of the patient, we adopted the fully endoscopic LIF procedure with UBE. Under general anesthesia, the patient was placed prone on a radiolucent operating table. Precise preoperative planning can be aided by 3D printing technology. Considering the difficulty in inserting screws caused by many posterior osteophytes, we designed the insertion positions, angles, lengths of eight pedicle screws, and curvature and length of the titanium rods based on a 3D-printing model in advance. Simultaneously, stiffness and deformity in the back of the patient hampered threading of the long titanium rods. We could also determine the curvature and length of the titanium rods based on a 3D-printing model. This preoperative planning based on a 3D-printing model ensured the safety of instrumentation placement and decompression process, and it also shortened the duration of the surgical procedure significantly. Eight pedicle guidewires were inserted sequentially into the T11-L2 pedicle using G-arm fluoroscopy. Subsequently, decompression and interbody fusion were completed under endoscopic monitoring. The position of the intervertebral fusion cage was identified by G-arm fluoroscopy. Then, eight PPSs were inserted (Figure 3). Finally, we used two pre-bent titanium rods to connect the pedicle screws. After confirming the position of the internal fixation position again with G-arm fluoroscopy, we placed a drainage tube and sutured the wound.
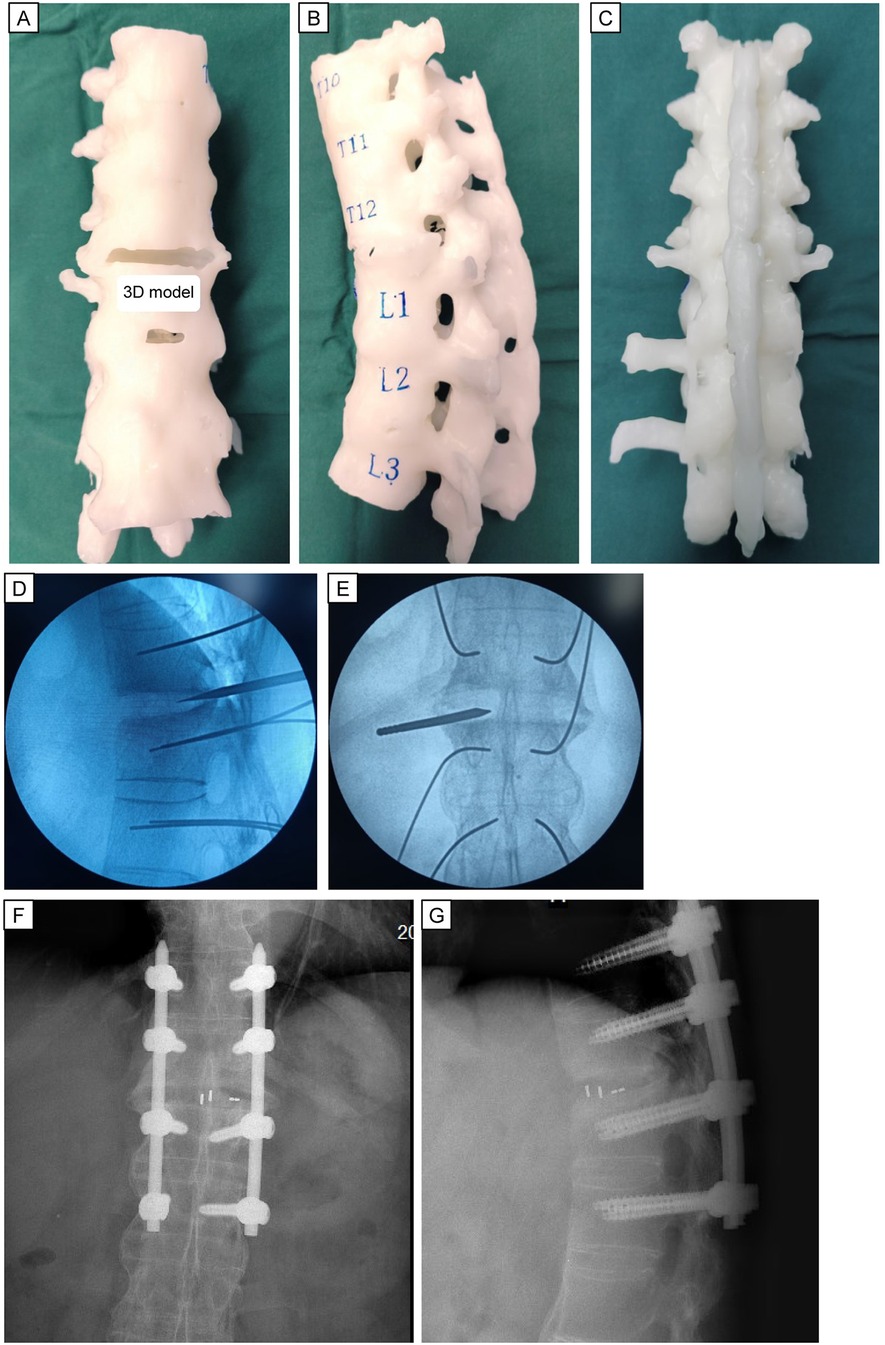
Figure 3. Preoperative 3D model printing and intraoperative fluoroscopic images. Anterior (A), lateral (B), and posterior (C) view of the 3D printed model. Intraoperative anterior and lateral radiographs (D,E) demonstrate the proper position for the diseased lesion using location-needle. Anterior and lateral radiographs (F,G) of excellent fusion cage and percutaneous pedicle screws placement is seen.
Through LIF and posterior fixation of PPSs, spinal stability was improved and postoperative lower back pain was significantly alleviated. Compared with traditional open surgery, this surgical method had less blood loss and smaller incision wounds, which resulted in a faster recovery (Figure 4). We did not attempt to correct kyphosis through osteotomy, but in situ fusion can give the AL a biomechanically stable environment to heal.
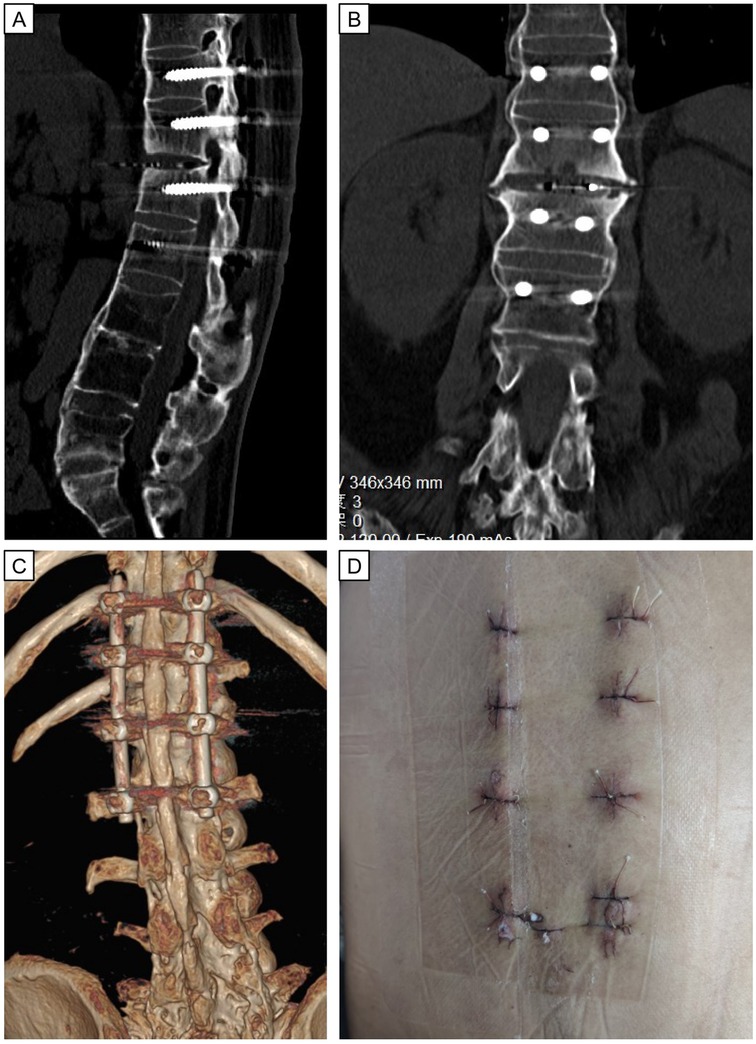
Figure 4. Postoperative immediate CT images and wound scars after minimally invasive surgery of LIF. Postoperative CT images (A–C) show good positioning of the internal fixation and normal sagittal sequence of the lumbar spine. Eight minimally invasive incisions (D) for internal fixation and fusion surgery.
DiscussionAn AL refers specifically to the destructive inflammatory lesion at the intervertebral disk-vertebral interface caused by AS. This scenario can affect the anterior, middle, and posterior columns, and it often occurs in the thoracolumbar region. In general, an AL is considered to be a rare complication in the late stage of AS. Due to the low incidence and lack of awareness of this lesion for most spinal surgeons, an AL is often misdiagnosed as infective spondylodiscitis, especially in geographic areas with a high incidence of tuberculosis. In this case, MRI data from a different hospital that was presented by the patient at the initial visit to our hospital influenced our initial diagnosis. Subsequent radiographic and CT findings revealed that the patient had AS with an AL.
Conservative treatment, including drugs (e.g., NSAIDs and anti-tumor necrosis factor-α agents), braces, rest, and physiotherapy, is feasible if symptoms are mild or absent (1–3). Etanercept, a dimeric fusion protein that inhibits the activity of TNF-α, has shown efficacy in the treatment of ankylosing spondylitis (4). Research indicates that certain single nucleotide polymorphisms (SNPs) in the TNF-α promoter can predict etanercept treatment response, aiding in personalized treatment based on genetic differences (4, 5). If the diagnosis is correct and the pain is unbearable, then decompression fusion and internal fixation surgery can be undertaken (as in our case) (6). The main purpose of the surgical procedure is to rebuild the stability of the spine and promote fusion of the spinal lesion, thereby alleviating lower back pain (6, 7). Various open surgical procedures through anterior, posterior, or combined approaches have been proposed (8–14). Fixation using PPSs can be considered only if axial back pain is present and only in situ fixation is required (15, 16). C. Zhang et al.'s recent study investigates the clinical and radiological outcomes of early MIS vs. open spinal fusion (OSF) for the treatment of AL, suggesting that MIS offers comparable efficacy to OSF with the advantages of reduced blood loss and shorter operation times (17). However, reports of minimally invasive cases that necessitate decompression, fusion, and percutaneous fixation are scarce. The most fundamental reason may arise from the characteristics of the disease: the rapidly growing and hard bone blurs the surgical anatomic structure, thereby increasing the risk of accidental injury to nerves and blood vessels. Moreover, outdated devices used for minimally invasive surgery have hampered endoscopic surgery. Recently, Zhou et al. showed that oblique lateral interbody fusion combined with posterior pedicle-screw implantation through the Wiltse paraspinal approach could be undertaken in a minimally invasive fusion for patients diagnosed with an AL (18). Compared to MIS tubular technology, the utilization of UBE provides a more direct and minimally invasive approach to spinal interventions, which is especially advantageous given the distinct anatomical considerations of our patient cohort. This technique facilitates enhanced visualization and manipulation within the spinal canal, a critical factor for the successful execution of the procedures undertaken. In brief, this is the first case report of a fully endoscopic LIF using UBE to treat an AL.
In this case, there are four main considerations when using UBE for LIF. First, due to the large number of osteophytes in pedicle attachments and poor imaging rendered by C-arm fluoroscopy, precise positioning and puncture of the pedicle are necessary before intraoperative insertion of pedicle wires. Second, a Kirschner wire must be employed to locate the lesion before endoscopy-assisted decompression and fusion. Third, considering that patients with AS have a faster rate of bone formation than other healthy counterparts, the amount of bone grafting can be reduced appropriately. Fourth, deep overextension should be avoided when inserting the interbody fusion cage because it can lead to damage of blood vessels/organs due to ossification and rupture of the anterior longitudinal ligament.
Precise preoperative planning can be aided by 3D printing technology. Due to challenges from posterior osteophytes, we pre-planned the positions, angles, and lengths of eight pedicle screws using a 3D-printed model. The model also helped us determine the curvature and length of titanium rods, overcoming stiffness and deformity in the patient's back. This preoperative planning ensured safe instrumentation and decompression, while also reducing surgery time.
This study is subject to certain limitations. Notably, the sample size was relatively small due to the rarity of AL as a complication of ankylosing spondylitis. Long-term follow-up is essential for evaluating the sustained efficacy of the treatment protocol. In future clinical studies, we intend to expand our case series and incorporate medium- to long-term follow-up assessments to enhance the robustness of our conclusions. Simultaneously, due to the limited number of surgical cases, we did not observe any potential surgical complications. As we all know, every surgery carries risks, and understanding these is crucial for the technology's development. From past experience, complications may include vascular or spinal cord injury, internal fixation loosening, fusion failure, and adjacent segment degeneration.
ConclusionsAccurate diagnosis of an AL and AS is the foundation of treatment. Once the diagnosis has been established, if the patient has unbearable pain or symptoms indicative of neurological damage, then posterior fusion can be considered. Compared with open surgical procedures, combining UBE and 3D-printing technologies for fully endoscopic LIF can offer the advantages of minimal trauma, minimal bleeding, the same effect as open surgery, and faster postoperative recovery. Accurate preoperative planning based on a 3D-printing model is strongly recommended for patients suffering from an AL with ambiguous anatomic landmarks.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsXY: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. RT: Supervision, Writing – original draft, Writing – review & editing. MZ: Supervision, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Youth Talent Development Plan of Changzhou Health Commission (CZQM2023006).
AcknowledgmentsWe thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Bron JL, de Vries MK, Snieders MN, van der Horst-Bruinsma IE, van Royen BJ. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol. (2009) 28(8):883–92. doi: 10.1007/s10067-009-1151-x
PubMed Abstract | Crossref Full Text | Google Scholar
4. Murdaca G, Spano F, Contatore M, Guastalla A, Magnani O, Puppo F. Pharmacogenetics of etanercept: role of TNF-alpha gene polymorphisms in improving its efficacy. Expert Opin Drug Metab Toxicol. (2014) 10(12):1703–10. doi: 10.1517/17425255.2014.970165
PubMed Abstract | Crossref Full Text | Google Scholar
5. Murdaca G, Gulli R, Spano F, Lantieri F, Burlando M, Parodi A, et al. TNF-alpha gene polymorphisms: association with disease susceptibility and response to anti-TNF-alpha treatment in psoriatic arthritis. J Invest Dermatol. (2014) 134(10):2503–9. doi: 10.1038/jid.2014.123
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wu M, Yan F, Ping A, Lei J. Effects of Andersson lesion treatment in ankylosing spondylitis: a medical record review study focused on medium- to long-term outcomes. Int J Rheum Dis. (2020) 23(6):753–62. doi: 10.1111/1756-185X.13826
PubMed Abstract | Crossref Full Text | Google Scholar
7. Wang Y, Xie J, Zhao Z, Zhang Y, Li T, Bi N, et al. Perioperative major non-neurological complications in 105 patients undergoing posterior vertebral column resection procedures for severe rigid deformities. Spine (Phila Pa 1976). (2015) 40(16):1289–96. doi: 10.1097/BRS.0000000000000995
PubMed Abstract | Crossref Full Text | Google Scholar
8. Debarge R, Demey G, Roussouly P. Sagittal balance analysis after pedicle subtraction osteotomy in ankylosing spondylitis. Eur Spine J. (2011) 20 Suppl 5(Suppl 5):619–25. doi: 10.1007/s00586-011-1929-9
PubMed Abstract | Crossref Full Text | Google Scholar
9. Ling T, Zhou B, Zhu C, Yang X, Song Y, Qiang Z, et al. One-stage posterior grade 4 osteotomy and bone graft fusion at pseudarthrosis for the treatment of kyphotic deformity with Andersson lesions in ankylosing spondylitis. Clin Neurol Neurosurg. (2017) 159:19–24. doi: 10.1016/j.clineuro.2017.05.017
PubMed Abstract | Crossref Full Text | Google Scholar
10. Chang KW, Tu MY, Huang HH, Chen HC, Chen YY, Lin CC. Posterior correction and fixation without anterior fusion for pseudoarthrosis with kyphotic deformity in ankylosing spondylitis. Spine (Phila Pa 1976). (2006) 31(13):E408–13. doi: 10.1097/01.brs.0000219870.31561.c2
PubMed Abstract | Crossref Full Text | Google Scholar
11. Liang Y, Tang X, Zhao Y, Wang Z. Posterior wedge osteotomy and debridement for andersson lesion with severe kyphosis in ankylosing spondylitis. J Orthop Surg Res. (2017) 12(1):54. doi: 10.1186/s13018-017-0556-5
PubMed Abstract | Crossref Full Text | Google Scholar
12. Chen LH, Kao FC, Niu CC, Lai PL, Fu TS, Chen WJ. Surgical treatment of spinal pseudoarthrosis in ankylosing spondylitis. Chang Gung Med J. (2005) 28(9):621–8.16323553
PubMed Abstract | Google Scholar
13. Guo C, Li T, Zhang H, Gao Q, Zhang G, Liu J, et al. Treatment of ankylosing spondylitis complicated with a thoracolumbar Andersson lesion by posterior closed osteotomy, debridement and fusion through the fracture line. BMC Musculoskelet Disord. (2022) 23(1):815. doi: 10.1186/s12891-022-05770-3
PubMed Abstract | Crossref Full Text | Google Scholar
14. Zhang H, Hu J, Zhang C, Yang Z, Gao M, Zhao H. Treating thoracic-lumbar andersson lesion in patients with ankylosing spondylitis: case series. Ann Med Surg (Lond). (2023) 85(5):1420–4. doi: 10.1097/MS9.0000000000000674
PubMed Abstract | Crossref Full Text | Google Scholar
15. Wang G, Sun J, Jiang Z, Cui X. The surgical treatment of andersson lesions associated with ankylosing spondylitis. Orthopedics. (2011) 34(7):e302–6. doi: 10.3928/01477447-20110526-09
PubMed Abstract | Crossref Full Text | Google Scholar
16. Okada E, Shiono Y, Nishida M, Mima Y, Funao H, Shimizu K, et al. Spinal fractures in diffuse idiopathic skeletal hyperostosis: advantages of percutaneous pedicle screw fixation. J Orthop Surg (Hong Kong). (2019) 27(2):2309499019843407. doi: 10.1177/2309499019843407
PubMed Abstract | Crossref Full Text | Google Scholar
17. Zhang C, Li Y, Wang G, Sun J. Treatment of andersson lesion-complicating ankylosing spondylitis via early minimally invasive surgery. Bone Jt Open. (2024) 5(10):886–93. doi: 10.1302/2633-1462.510.BJO-2024-0023.R1
PubMed Abstract | Crossref Full Text | Google Scholar
18. Zhou H, Li X, Liu Y, Wang H, Jiang W. Surgical treatment of andersson lesion of the lumbar spine with minimal invasion: a case report. Orthop Surg. (2022) 14(11):3129–33. doi: 10.1111/os.13426
留言 (0)