Lung cancer is the most prevalent cancer in China and the second most common in the United States, with approximately 85% being non-small cell lung cancer (NSCLC) (1). Accurate stratification of NSCLC patients based on survival outcomes is crucial for effective treatment. The American Joint Committee on Cancer Tumor Lymph Node Metastasis (TNM) Lung Cancer Staging System classifies lymph node staging based on the anatomical location of the lymph nodes involved, disregarding the absolute number of lymph nodes affected. Moreover, it does not specify the number of lymph nodes or stations examined but recommends sampling at least 6–10 lymph nodes or stations (2, 3). Ludwig et al. demonstrated that postoperative survival in NSCLC patients correlates with the number of lymph nodes resected during surgery, suggesting that the optimal number is 11 (3).
In an analysis of 38,806 NSCLC cases from the SEER registry and 5,706 NSCLC cases from a Chinese registry, Liang et al. found that a higher number of examined lymph nodes was associated with more accurate lymph node staging and improved long-term survival in resected NSCLC cases, recommending 16 examined lymph nodes as a threshold for postoperative prognostic stratification in patients deemed lymph node-negative (4).
Additionally, other methods of lymph node assessment have been shown to better predict prognosis in lymph node-positive NSCLC patients (5–10). Emerging evidence suggests that the number of histologically positive lymph nodes and the lymph node ratio have prognostic significance across various cancers, including esophageal, thyroid, breast, peripancreatic, gastric, colorectal, and cervical cancers, with similar findings in NSCLC studies (11–17). The nPLN and LNR have been identified as independent predictors of survival post-NSCLC resection. However, the superiority of one method over the other in NSCLC remains unclear. Another ratio-based approach, the LODDS, is currently employed as a prognostic indicator for various malignancies in several countries (10, 18–23). When a single lymph node is examined, the log odds of positive lymph nodes (LODDS) is calculated as the natural logarithm of the ratio between the probability of a positive lymph node and that of a negative lymph node. A preliminary report indicated that LODDS outperforms the number of positive lymph nodes (nPLNs) and pathological N-staging in predicting outcomes for stage I-IIIa NSCLC (24). However, this study did not address the relationship between LODDS and cancer-specific survival (CSS) nor analyze lymph nodes within stratified subgroups based on the number of nodes examined.
Thus far, no population-based study has elucidated the prognostic significance of LODDS for NSCLC. We posited that LODDS serves as an independent prognostic factor for these patients. To validate this hypothesis, we evaluated the comparative efficacy of LODDS, nPLNs, and the lymph node ratio (LNR) in predicting overall survival (OS) and CSS among lymph node-positive stage I-IIIA NSCLC patients who underwent lobectomy or total lung resection. We also explored the associations between these variables and survival outcomes.
2 Materials and methods 2.1 Data source and patient selectionWe extracted data from the Surveillance, Epidemiology, and End Results Program (SEER) 17 registry study database, encompassing the most recent data available from 2010 to 2019. The SEER 17 database includes registries across various urban and rural regions of the United States. Cancer data collection initiates with the identification of cancer patients diagnosed or treated in hospitals, outpatient clinics, radiology departments, physician offices, laboratories, surgery centers, or other healthcare providers such as pharmacists. All 50 states mandate that newly diagnosed cancers be reported to a central registry. Cancer registries then review these reported cases to ascertain if the information is legally reportable. If deemed reportable, the registry extracts cancer information from the medical records as stipulated by the North American Association of Central Cancer Registries (NAACCR) Data Standards External Site Policy.
Initially, we identified patients aged 18 or older using center codes C34.0-C34.9, which correspond to lung and bronchus diagnoses. Subsequently, we refined our inclusion criteria to encompass individuals diagnosed with NSCLC between January 1, 2010, and December 31, 2019. We focused on patients who underwent primary site-specific surgery and presented with N1 or N2 disease, with histological confirmation of at least one lymph node. The histological tumor types were restricted to those specified in the International Classification of Diseases for Oncology, Third Edition, including squamous cell carcinoma, adenocarcinoma, and other variants. Inclusion criteria also mandated a minimum survival period of one month post-surgery and active follow-up. We extracted clinicopathological data such as age at diagnosis, N stage, histology, gender, race, surgical approach, grade, T stage and primary site.
Exclusion criteria encompassed the presence of multiple primary tumors, unexamined lymph nodes, unknown nPLN, distant metastases, and IIIB or IV stage. The study protocol was reviewed and approved by the Ethics Committee of the Second Hospital of Nanchang University, ensuring adherence to ethical standards and regulations.
2.2 Statistical methodsOS and CSS were the primary endpoints of this study. Correlations among the LODDS, LNR, and the nPLN were examined through smoothed fitted curves. The Kaplan-Meier method was employed to estimate OS and CSS, with the log-rank test used for comparative analysis of the survival estimates. X-tile software facilitated the determination of optimal thresholds for LNR and nPLNs. The X-tile software analyzes different thresholds using Kaplan-Meier survival curves to determine the optimal cutoff values for LNR and nPLNs. The software selects thresholds that maximize the differentiation of patient prognosis based on survival rate comparisons, ensuring clinical relevance. Specifically, the output heatmap visualizes the impact of various thresholds on survival, aiding researchers in making informed decisions. For the analysis of the number of positive lymph nodes (nPLN), patients were stratified into two groups using x-tile software (Supplementary Figure S1): npLN ≤ 2 and nPLN > 2. Similarly, for the LNR analysis, patients were classified into LNR ≤ 0.5 and LNR > 0.5.
Cox proportional hazards models were utilized to evaluate the impact of demographic, pathological, and treatment variables in both univariate and multivariate contexts. Stratified analysis was used to evaluate the impact of LNR, nPLN, and LODDS on survival across different histologic types, T stages, and N stages. The goodness of fit for the models was assessed using the Akaike Information Criterion (AIC) and the Log Likelihood Ratio (LLR), with lower AIC or higher LLR values signifying superior model fit.
The LODDS were calculated as the logarithm of the ratio of the probability of a positive lymph node to that of a negative lymph node, according to the formula: LODDS=positivelymphnodes+0.5totallymphnodes−positivelymphnodes+0.5. The addition of 0.5 to both the numerator and the denominator was employed to avoid computational singularities. For LODDS analysis, patients were categorized into LODDS < 0.26 and LODDS ≥ 0.26. The values of LODDs were identified by recursive partitioning. Additionally, the number of lymph nodes examined was classified into <10 or ≥10, as the removal of 10 or more lymph nodes was associated with the longest median survival.
A predictive model for OS was constructed using the eXtreme Gradient Boosting (XGBoost) algorithm. The dataset was randomly divided into a training set (2,514 patients) and a validation set (618 patients). The primary endpoint was OS, with censoring applied to patients alive at the end of follow-up. Three lymph node-related features were selected based on their prognostic relevance in NSCLC: the LODDS, LNR, and nPLN. Model training was conducted on the training set, with hyperparameters optimized using five-fold cross-validation, and performance was evaluated on the independent validation set. SHAP (SHapley Additive exPlanations) values were calculated to quantify the contribution of each feature to the model's output, providing global rankings of feature importance as well as local interpretations for individual predictions. The predictive capacity of LODDS, LNR, and PLN was assessed by comparing their SHAP value distributions and mean SHAP importance scores. Statistical significance of differences in feature contributions was evaluated using non-parametric tests. All analyses were performed using Python (version 3.10), with key libraries including XGBoost, SHAP, and Scikit-learn. A p-value < 0.05 was considered statistically significant.
3 Results 3.1 Baseline characteristics of study participantsA total of 3,132 eligible patients meeting the criteria were identified from the SEER database. Patient demographics and tumor characteristics are detailed in Table 1. Of these patients, the majority were male (1,662, 53.07%), and the predominant histological types were adenocarcinoma (43.65%) and squamous cell carcinoma (29.76%). Regarding surgical procedures, approximately 87.71% underwent lobectomy, while 12.29% received total pneumonectomy. The mean number of lymph nodes resected was 13.89 (±SD: 9.75), with a median nPLN of 1. The median LNR was 0.14 (Q1-Q3: 0.08–0.27), and the median LODDS was −0.65 (Q1-Q3: −0.89 to −0.37) (Table 1).
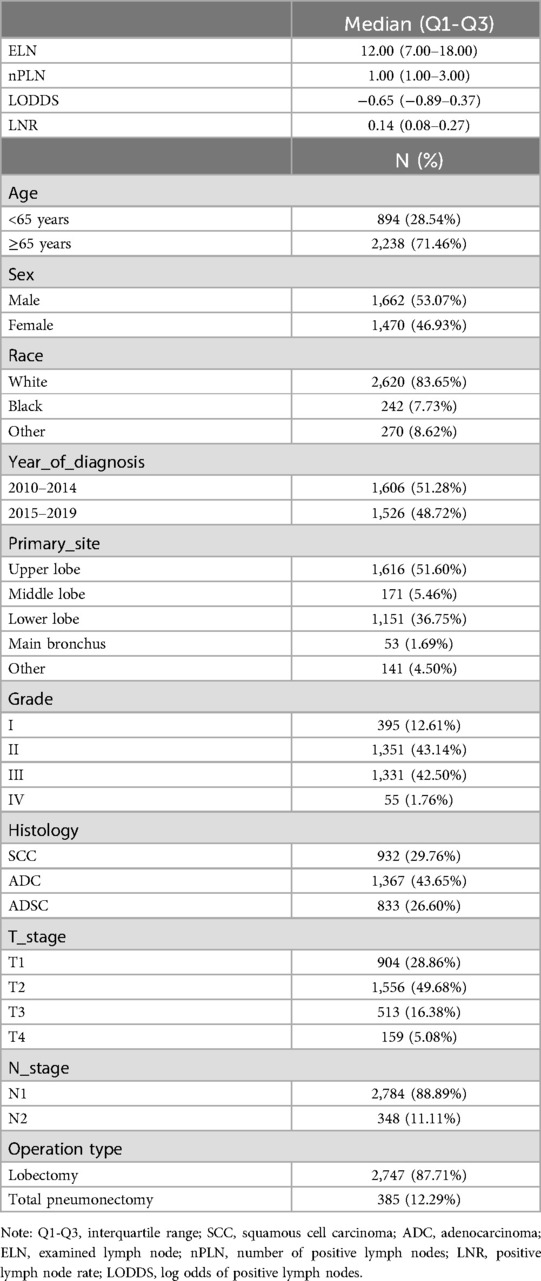
Table 1. Baseline characteristics of the population.
3.2 Association of LODDS, number of positive lymph nodes, and lymph node ratio with survival outcomesFigure 1 illustrates the relationships among the nPLN, LNR, and LODDS. Notably, when the number of positive lymph nodes reaches 22, LODDS ceases to increase with the number of positive lymph nodes, whereas LODDS continues to rise in conjunction with LNR.
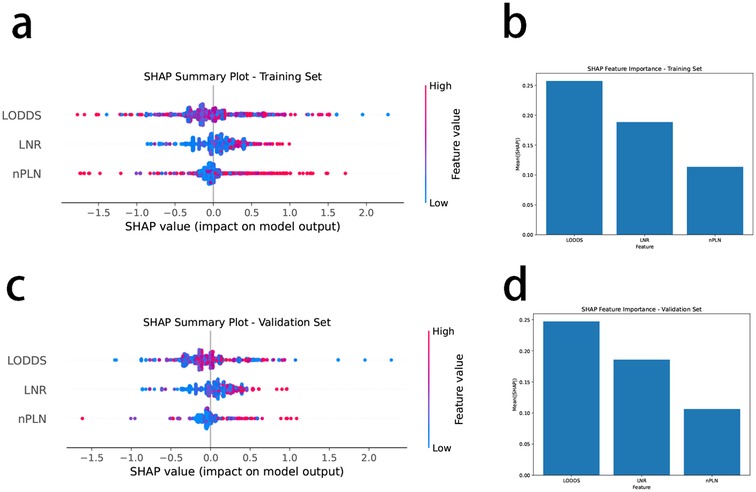
Figure 1. Smooth fitting curves for LODDS and LNR, nPLN. (a) LODDS and PLN: The red curve illustrates the relationship between LODDS (log odds of positive lymph nodes) and PLN (number of positive lymph nodes). As PLN increases, LODDS also rises; however, when PLN reaches around 22, the increase in LODDS plateaus. This suggests that beyond a high number of positive lymph nodes, additional PLN may have limited predictive value for survival. The blue dotted lines represent the confidence interval. (b) LODDS and LNR: The red curve shows the relationship between LODDS and LNR (lymph node ratio). As LNR increases, LODDS also rises, demonstrating a linear trend. The black tick marks along the x-axis indicate the distribution of LNR among patients, illustrating the range of LNR values in the sample.
The univariate Cox regression analysis revealed that age, gender, histology, primary site, grade, histologic type, T stage, and surgical approach were significantly relatived with both OS and CSS (Table 2).

Table 2. Univariate analyses of cohort.
Following these findings, three distinct multivariate models were developed to assess the predictive capacity of npLN, LNR, and LODDS. In the fully adjusted model, using OS as the outcome measure for the entire cohort, LNR and LODDS as continuous variables demonstrated predictive value for both OS and CSS (P < 0.05), whereas npLN did not show predictive significance (P = 0.0924). In the fully adjusted model, each unit increase in LODDS was associated with a 44% higher risk of overall mortality (OS) (HR = 1.44, 95% CI: 1.29–1.60, p < 0.0001), while increases in LNR and PLN were linked to 4% (HR = 1.04, 95% CI: 1.01–1.07, p = 0.0180) and 1% (HR = 1.01, 95% CI: 1.00–1.01, p = 0.0924) increases in risk, respectively. For cancer-specific survival (CSS), each additional unit of LODDS corresponded to a 45% higher risk of cancer-specific mortality (HR = 1.45, 95% CI: 1.28–1.64, p < 0.0001), while changes in LNR and PLN were associated with modest increases of 2% (HR = 1.02, 95% CI: 0.98–1.07, p = 0.3352) and 1% (HR = 1.01, 95% CI: 1.00–1.01, p = 0.1310) in risk, respectively (Table 3). Significant survival differences were observed across different groups. For patients with PLN ≤ 2, the 1-year, 3-year, and 5-year survival rates were 72.19%, 40.79%, and 21.53%, respectively, with a median survival time of 27 months. In contrast, patients with PLN > 2 had reduced survival rates of 59.42% at 1 year and 11.76% at 5 years, with a shorter median survival time of 17 months. Similar trends were observed for LNR and LODDS groups, with patients in the LNR ≤ 0.5 and LODDS ≤ 0.26 categories showing higher survival rates and longer median survival times. Overall, lower PLN, LNR, and LODDS values were associated with better survival outcomes. When CSS was used as the outcome, LODDS as a continuous variable was a significant predictor of both OS and CSS (P < 0.0001), while LNR (P = 0.3352) and nPLN (P = 0.1310) did not demonstrate predictive capability. The respective HR and CI are detailed in Table 3.
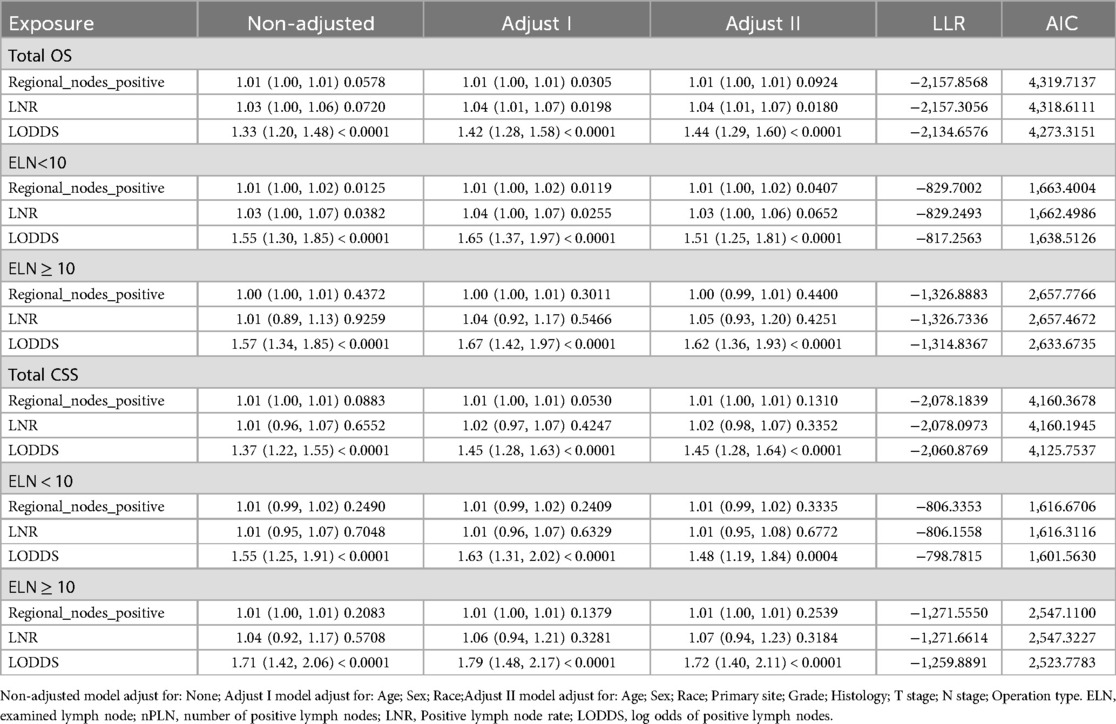
Table 3. Cox proportional hazards model analysis.
In stratified analysis, the LODDS metric demonstrated a significant prognostic value for both lung cancer-specific mortality and all-cause mortality. Patients with LODDS > 0.26 had substantially higher lung cancer-specific mortality in SCC and ADC subgroups (SCC HR = 1.9, 95% CI: 1.1–3.3, p = 0.018; ADC HR = 1.7, 95% CI: 1.3–2.4, p < 0.001). Furthermore, among patients with N2 staging, LODDS > 0.26 was also associated with a markedly increased mortality risk (HR = 1.9, 95% CI: 1.1–3.4, p = 0.025). Consistent with these findings, all-cause mortality was similarly elevated in SCC and ADC patients with LODDS > 0.26 (SCC HR = 1.7, 95% CI: 1.1–2.7, p = 0.023; ADC HR = 1.7, 95% CI: 1.3–2.2, p < 0.001). Across various histologic subtypes and staging groups, LODDS > 0.26 was closely associated with poorer prognosis, underscoring its potential clinical value in risk stratification and outcome prediction (Table 4).
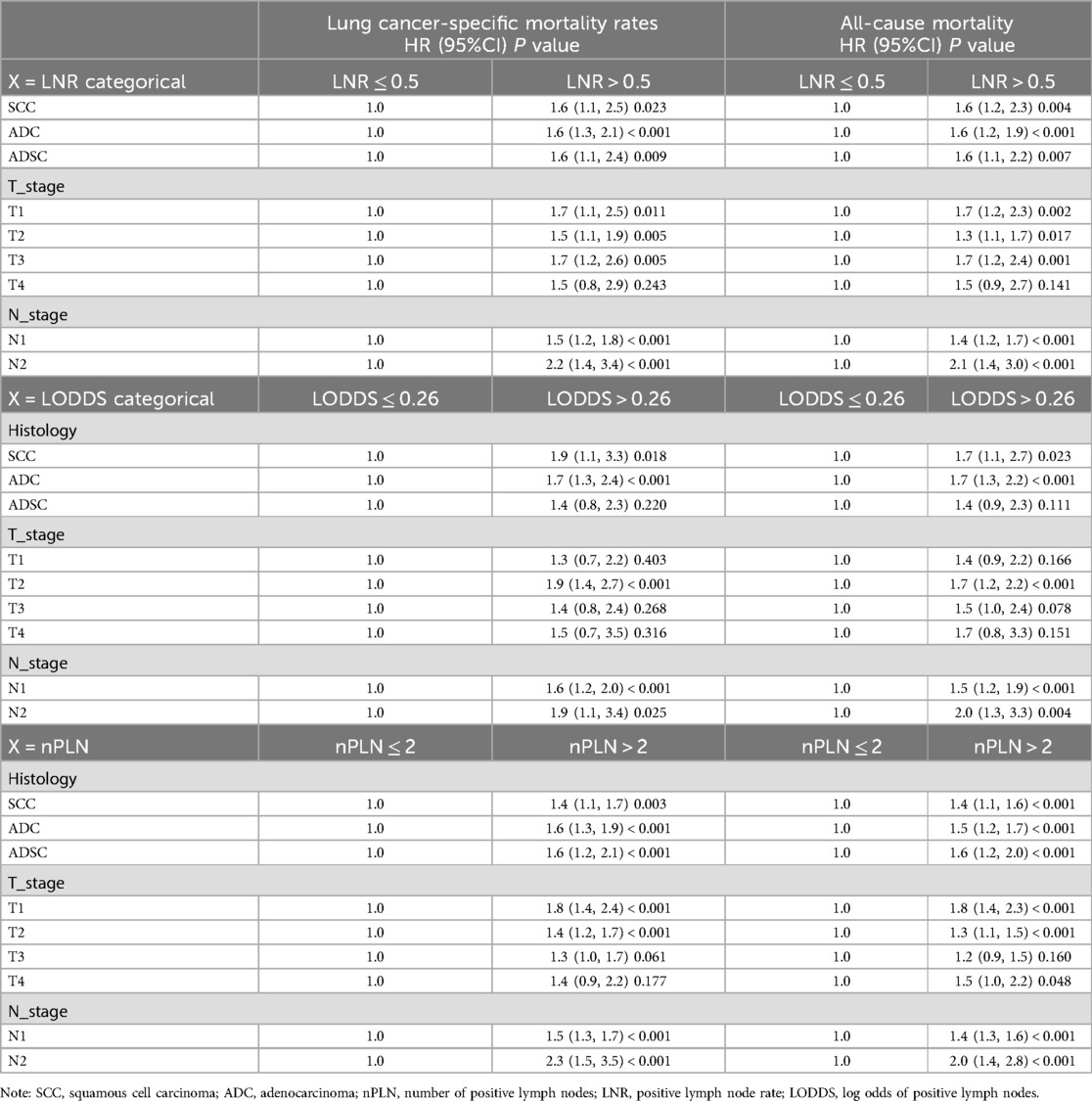
Table 4. Stratified analyses of cohort
3.3 The relative validity of LNR, LODDS, and npLN in CSS and OS predictionAmong all patients, the LODDS model exhibited superior fit compared to both npLN and LNR (Table 3). Specifically, LODDS demonstrated the most accurate predictive capacity in cohorts with fewer than 10 ELN, characterized by a higher Log Likelihood Ratio (LLR) and a lower Akaike Information Criterion (AIC) which best predicted both OS and CSS. Similarly, in patients with 10 or more examined lymph nodes, LODDS continued to provide superior predictive value for OS and CSS in comparison to both LNR and npLN (Table 3). Higher scores on all three measures—nPLN, LNR, and LODDS—were substantially (P < 0.001) related with a worse prognosis, according to Kaplan-Meier analyses (Figures 2, 3).
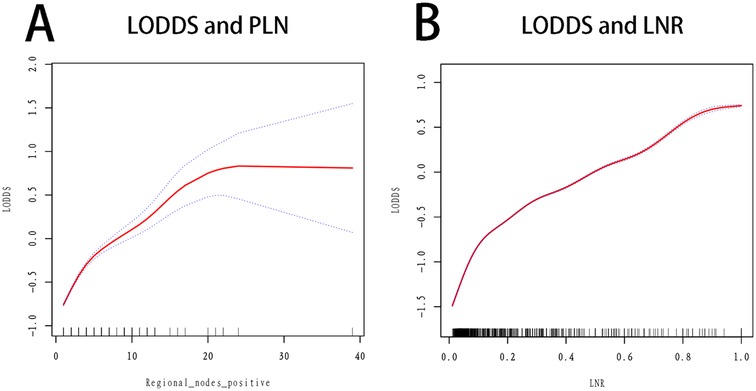
Figure 2. Km curves grouped by LODDS, LNR, nPLN (OS as an indicator of outcome). (A) OS by PLN: This Kaplan-Meier curve shows overall survival based on the number of positive lymph nodes (PLN). Patients with PLN ≤ 2 (red line) have a higher survival probability compared to those with PLN > 2 (blue line). The survival difference is statistically significant with p < 0.0001. (B) OS by LNR: The curve displays overall survival stratified by lymph node ratio (LNR). Patients with LNR ≤ 0.5 (red line) show better survival outcomes than those with LNR > 0.5 (blue line), with a significant survival difference of p < 0.0001. C) OS by LODDS: This plot illustrates overall survival categorized by log odds of positive lymph nodes (LODDS). Patients with LODDS ≤ 0.26 (red line) have a significantly better survival probability than those with LODDS > 0.26 (blue line), with p < 0.0001.

Figure 3. Km curves grouped by LODDS, LNR, nPLN (CSS as an indicator of outcome). (A) CSS by PLN: This Kaplan-Meier curve illustrates cancer-specific survival based on the number of positive lymph nodes (PLN). Patients with PLN ≤ 2 (red line) have a higher cancer-specific survival probability compared to those with PLN > 2 (blue line), with a statistically significant difference (p < 0.0001). (B) CSS by LNR: The curve shows cancer-specific survival stratified by lymph node ratio (LNR). Patients with LNR ≤ 0.5 (red line) display better cancer-specific survival outcomes than those with LNR > 0.5 (blue line), with a significant difference (p < 0.0001). (C) CSS by LODDS: This plot presents cancer-specific survival categorized by log odds of positive lymph nodes (LODDS). Patients with LODDS ≤ 0.26 (red line) have a significantly better cancer-specific survival probability than those with LODDS > 0.26 (blue line), with p < 0.0001.
3.4 Feature importance analysisThe XGBoost model was developed to predict OS using a cohort of 2,514 patients in the training set and 618 patients in the validation set. The prognostic relevance of three lymph node-associated features—log odds of positive lymph nodes LODDS, LNR, and the number of positive lymph nodes PLN—was systematically evaluated.
In both the training and validation sets, LODDS emerged as the most critical feature for OS prediction, exhibiting the highest mean SHAP values (0.25), followed by LNR, while PLN demonstrated the least importance (Figures 4A,C). This hierarchy of feature importance was consistently observed across datasets (Figures 4B,D), underscoring the superior predictive capacity of LODDS.

Figure 4. SHAP analysis of feature importance and impact on overall survival prediction using XGBoost. (A) SHAP summary plot for the training set. Each dot represents the SHAP value for a single patient, indicating the contribution of the corresponding feature to the predicted overall survival (OS). Features include log odds of positive lymph nodes (LODDS), lymph node ratio (LNR), and the number of positive lymph nodes (nPLN). The horizontal axis shows the SHAP value, with positive values indicating an increased risk of mortality and negative values indicating improved survival. The color gradient represents the feature value, with red indicating higher feature values and blue indicating lower feature values. LODDS exhibits the widest SHAP value distribution, highlighting its dominant role in influencing model predictions. (B) Bar chart of mean SHAP values for the training set, showing the average contribution of each feature to the model's predictions. LODDS has the highest mean SHAP value (0.25), followed by LNR, with nPLN contributing the least. This ranking demonstrates the superior importance of LODDS in predicting OS within the training dataset. (C) SHAP summary plot for the validation set, using the same feature and SHAP value conventions as in panel (A) The validation set results confirm the findings from the training set, with LODDS maintaining the highest impact on model predictions. (D) Bar chart of mean SHAP values for the validation set. Similar to the training set, LODDS remains the most important feature, with LNR contributing moderately and nPLN showing minimal importance. This consistency across both datasets underscores the robustness and generalizability of LODDS as a critical predictor of OS.
3.5 Feature impact on model predictionsSHAP summary plots further delineated the influence of individual features on model output (Figures 4A,C). Among the features, LODDS displayed the widest distribution of SHAP values, signifying its pronounced impact on survival prediction. Elevated LODDS values were strongly correlated with increased mortality risk, while LNR exhibited a moderate contribution. Conversely, PLN had a negligible effect on model predictions.
These results collectively establish LODDS as the most robust and reliable predictor of OS among the three evaluated features. Its consistent superiority across training and validation cohorts highlights its potential as a pivotal prognostic marker for NSCLC patients undergoing survival risk stratification.
4 DiscussionAlthough the traditional TNM staging system serves as a valuable guide for treatment and prognosis, some early-stage patients who receive standard care still succumb to postoperative recurrence (25). This discrepancy has driven many researchers to explore novel methods to delineate patient heterogeneity within the same TNM stage, aiming to develop a staging system that more accurately forecasts prognosis and directs treatment strategies. LNR has been reported to outperform the conventional N staging system for NSCLC and other malignancies (26). In this study, we sought to further refine the staging paradigm by focusing on the LODDS, which has demonstrated superior prognostic capability compared to TNM-N stage and LNR in breast, colon, and gastric cancers (27–29), though it has yet to be assessed in NSCLC.
The results from the XGBoost model (as shown in Figure 4) clearly demonstrate the superior prognostic value of the log odds of positive lymph nodes compared to the lymph node ratio and the number of positive lymph nodes in predicting overall survival in NSCLC patients. SHAP analysis revealed that log odds of positive lymph nodes consistently exhibited the highest feature importance across both the training and validation datasets, with significantly higher mean SHAP values and broader distributions than the other two lymph node-related parameters. These findings are consistent with previous studies, further validating its prognostic capability. By incorporating both positive and negative lymph nodes, log odds of positive lymph nodes provides a more comprehensive assessment of lymph node burden. Furthermore, the consistent predictive performance in both datasets highlights its potential as a universally applicable prognostic marker. In contrast, lymph node ratio demonstrated moderate utility, while the number of positive lymph nodes contributed minimally, reflecting the limitations of simpler metrics in accurately stratifying survival risk. Collectively, these results support the adoption of log odds of positive lymph nodes as an important complement to, or even a potential replacement for, traditional lymph node evaluation metrics in prognostic models for NSCLC.
Unlike LNR, which is calculated solely from the ratio of positive lymph nodes, LODDS incorporates both positive and negative lymph nodes, thus potentially offering more nuanced prognostic insights for patients with p-N0 NSCLC—insights that LNR may not capture. While LNR and LODDS may appear as alternative transformations of similar data, LODDS can provide more detailed prognostic information when LNR values are equivalent. Wang and colleagues posed an intriguing question (30): Is the prognosis of Patient A, who has 4 PLN with 4 lymph nodes removed, the same as Patient B, who has 20 PLN with 20 removed? By extension, we might ask: Does Patient C, with 4 PLN and 0 lymph nodes examined, share the same prognosis as Patient D, with 20 PLN and 0 examined lymph nodes? Intuitively, one would expect Patient A to have a better prognosis than Patient B, and Patient C to fare worse than Patient D. Despite identical LNR values for Patients A and B, and C and D, their respective LODDS values differed (LODDS = 0.95 for Patient A, 1.61 for Patient B, 0.95 for Patient C, and 1.61 for Patient D). Accordingly, our grouping strategy categorized Patients A and B in the LODDS4 group, Patient C in the LODDS2 group, and Patient D in the LODDS1 group. This demonstrates that patients with identical LNR values, particularly zero, can exhibit differing LODDS and thus belong to distinct prognostic groups. Furthermore, an LNR of 0 correlates with N0 status, yet patients classified as N0 may still exhibit varying LODDS and prognoses. The LODDS system, by classifying N0 patients into distinct prognostic categories, holds significant value in shaping treatment strategies. For instance, a patient with N0 disease but a high LODDS should be closely monitored for potential false negatives and subjected to vigilant follow-up.
Staging based on the log odds of positive lymph nodes not only provides superior prognostic predictions for patients with N1 and N2 stages but also differentiates among patients with N0 status. Similar advantages have been documented in studies of breast, colon, and gastric cancers (30). Unlike LNR, which only accounts for positive lymph nodes, LODDS also considers the number of negative lymph nodes, a crucial factor for patients with N0 NSCLC. Previous research indicates that a greater number of examined lymph nodes correlates with higher survival rates, plateauing at approximately 11–16 nodes (31). Since LODDS includes the number of negative lymph nodes, it offers a more comprehensive and effective prognostic indicator compared to LNR.
Extensive research has examined the LODDS, with analyses of 80,000 + breast cancer patients in the SEER database suggesting that LODDS estimates closely align with LNR results (32). A study by the Polish Lung Cancer Group found that LODDS was superior to other classifications involving lymph nodes or LNR for patients with radically resected NSCLC, although it did not specify the number of negative lymph nodes or stratify by the total number of retrieved nodes (33). Our study found that the LODDS model was superior to both npLN and LNR models in Cox regression analysis, suggesting the former's enhanced utility for patients with resected lymph node-positive NSCLC.
Nevertheless, this study has limitations. Lymph node tissue is often fragmented during extraction, which can lead to an overestimation of the number of resected nodes (3, 4, 34). Conversely, difficulties in distinguishing individual lymph nodes from anatomical tissue may result in underestimation. Accurately calculating the number of nodes remains a challenge. The LODDS model, developed to account for both positive and negative lymph nodes, addresses this issue by incorporating a variable that adjusts for the number of nodes collected. Additionally, retrospective studies inherently carry selection bias. Factors not covered by SEER, such as patient comorbidities, performance status, and chemotherapy usage, may also influence our findings. The LODDS model in this study demonstrated potential value in prognostic assessment. However, the lack of external validation may limit its generalizability. Future research should aim to validate the LODDS model using different patient datasets to further confirm its robustness and applicability. Such validation would help ensure the model's utility across diverse populations, thereby improving its clinical applicability.Thus, external validation through large-scale databases is necessary to confirm the predictive accuracy of LODDS for OS and CSS before it can be endorsed for clinical application.
5 ConclusionsCompared to the nPLN and LNR staging systems, LODDS demonstrates superior prognostic power for patients with stage I–IIIA NSCLC undergoing lobectomy or pneumonectomy. By integrating both positive and negative lymph node information, LODDS offers a refined risk stratification that is particularly valuable in cases with high lymph node heterogeneity. Clinically, LODDS can serve as a primary tool for identifying high-risk patients, supporting the development of individualized treatment strategies. Incorporating LODDS into routine clinical practice may enhance decision-making processes and improve patient outcomes.
Data availability statementPublicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Ethics statementThe studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Nanchang University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsQH: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Investigation, Methodology, Writing – original draft. LW: Data curation, Formal Analysis, Methodology, Writing – original draft. DY: Data curation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft. LX: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Scientific and Technological Research Project by the Jiangxi Provincial Department of Education (No. GJJ2200185).
AcknowledgmentsWe would like to gratefully acknowledge to the the patients who participated in the cohort for their contribution to data collection.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1530250/full#supplementary-material
Supplementary Figure S1 | The best cut-off values for the nPLN and LNR by using X-tile. a) Distribution of PLN: This histogram shows the distribution of patients based on the number of positive lymph nodes (PLN). Most patients have a low PLN value, with a peak near the lower end of the axis. b) X-tile plot for PLN: This plot visualizes the cut-off analysis for PLN, where different shades represent the statistical significance of survival differences between groups. Red indicates a high-risk group, and green indicates a low-risk group. The optimal cut-off point, marked by a white dot, is PLN = 2. c) Risk plot for PLN: This line graph shows the relative risk for different PLN cut-off values. The risk level peaks at PLN = 2, suggesting it as the most significant threshold for prognostic purposes. d) Distribution of LNR: This histogram displays the distribution of patients based on lymph node ratio (LNR). Similar to PLN, most patients have a low LNR, as shown by the concentration at the left end of the axis. e) X-tile plot for LNR: This plot illustrates the optimal cut-off analysis for LNR, with red and green indicating high- and low-risk groups, respectively. The optimal cut-off point, marked by a white dot, is LNR = 0.5, representing the threshold with the most distinct survival separation. f) Risk plot for LNR: This plot displays the relative risk for various LNR cut-off values. The identified cut-off point aligns with a peak in risk at LNR = 0.5, indicating it as a significant threshold for distinguishing prognostic groups.
References3. Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. (2005) 128(3):1545–50. doi: 10.1378/chest.128.3.1545
PubMed Abstract | Crossref Full Text | Google Scholar
4. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. (2017) 35(11):1162–70. doi: 10.1200/JCO.2016.67.5140
PubMed Abstract | Crossref Full Text | Google Scholar
5. Saji H, Tsuboi M, Shimada Y, Kato Y, Yoshida K, Nomura M, et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest. (2013) 143(6):1618–25. doi: 10.1378/chest.12-0750
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wei S, Asamura H, Kawachi R, Sakurai H, Watanabe S. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol. (2011) 6(2):310–8. doi: 10.1097/JTO.0b013e3181ff9b45
PubMed Abstract | Crossref Full Text | Google Scholar
7. Wisnivesky JP, Arciniega J, Mhango G, Mandeli J, Halm EA. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax. (2011) 66(4):287–93. doi: 10.1136/thx.2010.148601
PubMed Abstract | Crossref Full Text | Google Scholar
8. Jonnalagadda S, Arcinega J, Smith C, Wisnivesky JP. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer. (2011) 117(20):4724–31. doi: 10.1002/cncr.26093
PubMed Abstract | Crossref Full Text | Google Scholar
9. Taylor MD, LaPar DJ, Thomas CJ, Persinger M, Stelow EB, Kozower BD, et al. Lymph node ratio predicts recurrence and survival after R0 resection for non-small cell lung cancer. Ann Thorac Surg. (2013) 96(4):1163–70. doi: 10.1016/j.athoracsur.2013.04.031
PubMed Abstract | Crossref Full Text | Google Scholar
10. Huang B, Chen C, Ni M, Mo S, Cai G, Cai S. Log odds of positive lymph nodes is a superior prognostic indicator in stage III rectal cancer patients: a retrospective analysis of 17,632 patients in the SEER database. Int J Surg. (2016) 32:24–30. doi: 10.1016/j.ijsu.2016.06.002
PubMed Abstract | Crossref Full Text | Google Scholar
12. Allaix ME, Arezzo A, Cassoni P, Mistrangelo M, Giraudo G, Morino M. Metastatic lymph node ratio as a prognostic factor after laparoscopic total mesorectal excision for extraperitoneal rectal cancer. Surg Endosc. (2013) 27(6):1957–67. doi: 10.1007/s00464-012-2694-5
PubMed Abstract | Crossref Full Text | Google Scholar
13. Chen S, Zhao BW, Li YF, Feng XY, Sun XW, Li W, et al. The prognostic value of harvested lymph nodes and the metastatic lymph node ratio for gastric cancer patients: results of a study of 1,101 patients. PLoS One. (2012) 7(11):e49424. doi: 10.1371/journal.pone.0049424
PubMed Abstract | Crossref Full Text | Google Scholar
14. Shamseddine AI, Mukherji D, Melki C, Elias E, Eloubeidi M, Dimassi H, et al. Lymph node ratio is an independent prognostic factor after resection of periampullary malignancies: data from a tertiary referral center in the middle East. Am J Clin Oncol. (2014) 37(1):13–8. doi: 10.1097/COC.0b013e31826b9c74
PubMed Abstract | Crossref Full Text | Google Scholar
15. Gohari MR, Khodabakhshi R, Shahidi J, Fard ZM, Foadzi H, Soleimani F, et al. The impact of multiple recurrences in disease-free survival of breast cancer: an extended cox model. Tumori. (2012) 98(4):428–33. doi: 10.1177/030089161209800405
PubMed Abstract | Crossref Full Text | Google Scholar
16. Polterauer S, Grimm C, Hofstetter G, Concin N, Natter C, Sturdza A, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. (2012) 107(6):918–24. doi: 10.1038/bjc.2012.340
PubMed Abstract | Crossref Full Text | Google Scholar
17. Bhamidipati CM, Stukenborg GJ, Thomas CJ, Lau CL, Kozower BD, Jones DR. Pathologic lymph node ratio is a predictor of survival in esophageal cancer. Ann Thorac Surg. (2012) 94(5):1643–51. doi: 10.1016/j.athoracsur.2012.03.078
PubMed Abstract | Crossref Full Text | Google Scholar
18. Cao J, Yuan P, Ma H, Ye P, Wang Y, Yuan X, et al. Log odds of positive lymph nodes predicts survival in patients after resection for esophageal cancer. Ann Thorac Surg. (2016) 102(2):424–32. doi: 10.1016/j.athoracsur.2016.03.030
PubMed Abstract | Crossref Full Text | Google Scholar
19. Jian-Hui C, Shi-Rong C, Hui W, Si-le C, Jian-Bo X, Er-Tao Z, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol. (2016) 37(8):11105–13. doi: 10.1007/s13277-015-4191-7
PubMed Abstract | Crossref Full Text | Google Scholar
20. Kwon J, Eom KY, Kim IA, Kim JS, Kim YB, No JH, et al. Prognostic value of log odds of positive lymph nodes after radical surgery followed by adjuvant treatment in high-risk cervical cancer. Cancer Res Treat. (2016) 48(2):632–40. doi: 10.4143/crt.2015.085
PubMed Abstract | Crossref Full Text | Google Scholar
21. La Torre M, Nigri G, Petrucciani N, Cavallini M, Aurello P, Cosenza G, et al. Prognostic assessment of different lymph node staging methods for pancreatic cancer with R0 resection: pN staging, lymph node ratio, log odds of positive
留言 (0)