Psychosocial problems after aneurysmal subarachnoid hemorrhage (aSAH) are common, even in patients classified as rehabilitated and those with a favorable prognosis, thus affecting the patient's quality of life (1). Temporomesial atrophic changes might be associated with cognitive impairment after aSAH (2, 3). In addition, Wostrack et al. (4) reported that hippocampal atrophy was substantially greater after clipping than coiling. Aneurysm surgery may trigger neurocognitive outcomes that are less favorable than those triggered by endovascular approaches (5, 6).
The postoperative seizure rate for aSAH varies from 2% to 11%, and subarachnoid hematoma and craniotomy may contribute to its development (7–9). Therefore, the early evaluation of cognitive function and interictal epileptiform discharges (IEDs) in electroencephalography (EEG) after aSAH is important to patient outcomes. However, the relationships between clipping, hippocampal damage, cognitive dysfunction, and epilepsy remain unclear.
This study aimed to evaluate the surgical effect on hippocampal volume, cognitive function, and IEDs in patients in the subacute phase who underwent clipping for aSAH of anterior circulation.
2 Materials and methodsPatients with subarachnoid hemorrhage (SAH) who experienced their first anterior circulation aneurysm rupture between 2010 and 2018 and with modified Rankin Scale scores of 0–3 points at 1 month after undergoing clipping at our hospital were included. This study was approved by the Ethics Committee at Showa University (Approval No. 2972) and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants. We classified the locations of the ruptured aneurysm into the middle cerebral artery (MCA), internal carotid artery (ICA), and anterior cerebral artery (ACA) areas. Aneurysms in the MCA and ICA areas were clipped via the transsylvian approach (TSA) ipsilateral to the ruptured aneurysm, and those in the ACA area were clipped via the TSA or interhemispheric approach (IHA). Right craniotomy was performed for all patients undergoing the IHA. All patients underwent surgery immediately after diagnosis. Fluid management was implemented in all patients to maintain normovolemia 3 days to 2 weeks after craniotomy and clipping, in addition to fasudil hydrochloride administration via drip infusion. Cilostazol and statins were administered orally to prevent cerebral vasospasm. A ventriculoperitoneal shunt was placed in patients who developed hydrocephalus.
Patients with modified Rankin Scale scores of 0–3 points underwent magnetic resonance imaging (MRI) of the head, neuropsychological assessments, and interictal EEG evaluations postoperatively. The three-dimensional hippocampal volume was measured using MRI, and the volumes of the bilateral hippocampi were compared. The relationships of the hippocampal volume and neuropsychological assessments with the presence of intracerebral hematoma, intraventricular hematoma, delayed cerebral vasospasm, and hydrocephalus were also evaluated in the imaging studies. Furthermore, postoperative MRI fluid-attenuated inversion recovery (FLAIR) with high intensity on the brain cortex of the approach side was defined as FLAIR high-signal intensity. Thus, the FLAIR high-signal intensity would indicate the effects of SAH, circulatory disturbances in the brain, and cerebral damage by surgical manipulation. The relationships between the neuropsychological assessment and EEG findings and the presence of FLAIR high-signal intensity were evaluated.
2.1 Hippocampal volume measurementThe hippocampal volume was measured postoperatively using a 1.5-T MRI system (MPRAGE, FOV: 220/186/179; voxel size: 0.43 × 0.43 × 0.86 mm; TR/TE: 1220/4.29; repetition time: 1,160 ms; echo time: 4.27 ms; matrix: 256′256 pixels; MAGNETOM Advance, Siemens Healthcare, Erlangen, Germany) according to the method described by Pruessner et al. (10) Three-dimensional T1-weighted imaging (magnetization prepared rapid acquisition with gradient echo) was performed along the transverse, coronal, and sagittal planes. The hippocampal margin was traced manually in each plane, and the hippocampal volume was determined by reconstituting the data in three dimensions using an image analysis workstation (Ziostation2, Ziosoft, Inc., Tokyo). Measurements were performed by a neurosurgeon and radiologist, and the hippocampal volume was obtained.
2.2 Cognitive functionsNeuropsychological assessments were performed in accordance with the intelligence quotient measured using the Japanese version of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) (11). Memory testing was measured using the Japanese version of the Wechsler Memory Scale-Revised (WMS-R) (12). The WAIS-III assessed Verbal Intellectual Quotient, Performance Intellectual Quotient, Full-scale Intellectual Quotient, Verbal Comprehension, Perceptual Organization, Working Memory, and Processing Speed, and the WMS-R assessed verbal memory, visual memory, general memory, Attention/Concentration and Delayed Recall. The correlations among these scores and hippocampal atrophy findings were examined.
2.3 IEDs in EEGEEG assessments were performed interictally using a digital electroencephalograph (Nihon Kohden, Tokyo). The appearance of IEDs was examined at each aneurysm location using the spike index (SI) measurement method of Kessler et al. (13). The SI was expressed as the number of 1 s bins containing one or more epileptiform discharges (spike waves, sharp waves, and spike-and-slow wave complex), divided by the total number of seconds in the sleep recording and multiplied by 100 to calculate the percentage of the trace. The evaluation of SI was blinded for the aneurysmal location, the hippocampal volume, and the neuropsychological assessments. We measured SI on the approach and non-approach sides.
2.4 Statistical analysisStatistical analysis was performed using SPSS version 27 (IBM Corp., Armonk, NY). Wilcoxon's signed rank-sum test was performed to assess the bilateral hippocampal volumes and SI values on the approach and non-approach sides. The Mann−Whitney U-test was used to examine the relationships of radiological findings, such as intracerebral hemorrhage, intraventricular hematoma, delayed cerebral vasospasm, hydrocephalus, and FLAIR high-signal intensity with the hippocampal volume on the approach side, neuropsychological assessments, and SI. The SI and cognitive function findings were compared among aneurysm locations using univariate analysis. The correlation between hippocampal volume and cognitive function or SI was examined using Pearson's product-moment correlation coefficient in the presence of a normal distribution and Spearman's rank correlation coefficient in the absence of a normal distribution. A correlation coefficient of ≥|0.5| denoted a correlation, whereas a correlation coefficient of ≥|0.7| denoted a strong correlation. The threshold for statistical significance was set at p < 0.05.
3 ResultsThe flowchart of patients with aSAH and their clinical characteristics are presented in Figure 1 and Table 1, respectively. MRI, neuropsychological assessments, and EEG were performed in a median of 30 (27–46), 41 (27–80), and 32 (28–46) days, respectively.
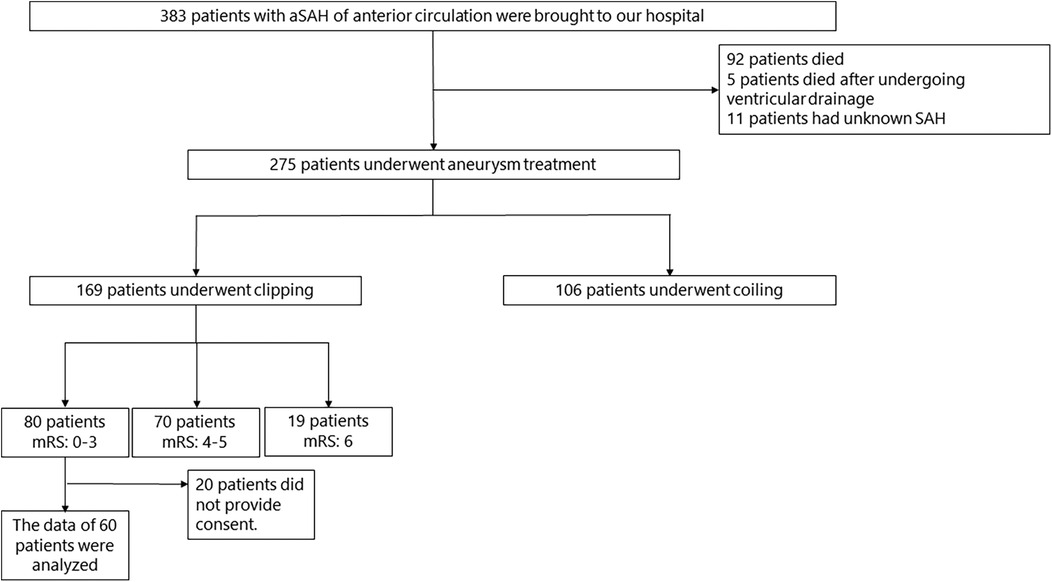
Figure 1. Flowchart of patients with aSAH. aSAH, aneurysmal subarachnoid hemorrhage; SAH, subarachnoid hemorrhage; mRS, modified rankin scale.
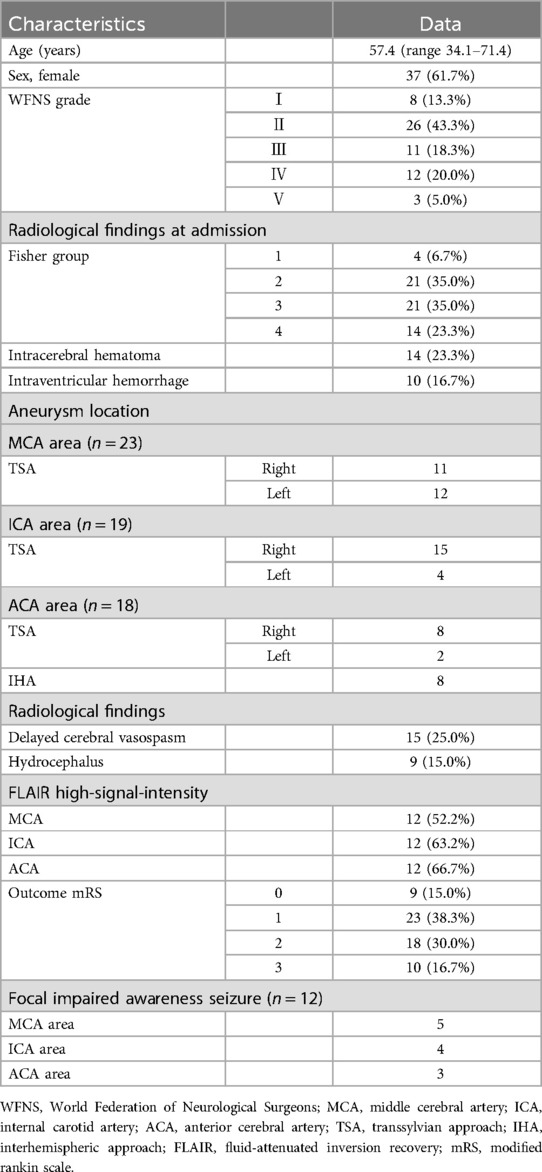
Table 1. Clinical characteristics.
3.1 Evaluation of the hippocampal volumeNo correlation was found between the number of clips and hippocampal volume at all aneurysm locations. Clipping was performed via the TSA ipsilateral to the ruptured aneurysm in all patients in the MCA and ICA groups. In patients with right aneurysm, the mean right and left hippocampal volumes were 3.11 ± 0.79 cm3 and 3.61 ± 0.52 cm3 and 3.19 ± 0.53 cm3 and 3.53 ± 0.46 cm3 in the MCA and ICA groups, respectively. In patients with left aneurysm, the mean right and left hippocampal volumes were 3.74 ± 0.77 cm3 and 3.30 ± 0.56 cm3 and 3.70 ± 0.29 cm3 and 2.80 ± 0.96 cm3 in the MCA and ICA groups, respectively. In patients in the ACA group who underwent surgery via the right TSA, the right and left hippocampal volumes were 2.89 ± 0.91 cm3 and 2.94 ± 0.89 cm3, respectively. In those who underwent surgery via the left TSA, the right and left hippocampal volumes were 2.55 ± 0.02 cm3 and 2.31 ± 0.11 cm3, respectively. In patients who underwent surgery via the IHA, the right and left hippocampal volumes were 3.48 ± 0.49 cm3 and 3.33 ± 0.60 cm3, respectively.
The hippocampal volume ipsilateral to the approach side was smaller than that ipsilateral to the non-approach side postoperatively in the MCA and ICA groups (MCA, p < .001; ICA, p < .001). No significant difference was observed between the left and right hippocampal volumes postoperatively in the ACA group (TSA, p = .753; IHA, p = .093) (Figure 2). The presence of radiological findings, such as intracerebral hemorrhage, intraventricular hematoma, delayed cerebral vasospasm, and hydrocephalus, was not related to the hippocampal volume on the approach side in the MCA, ICA, and ACA groups.
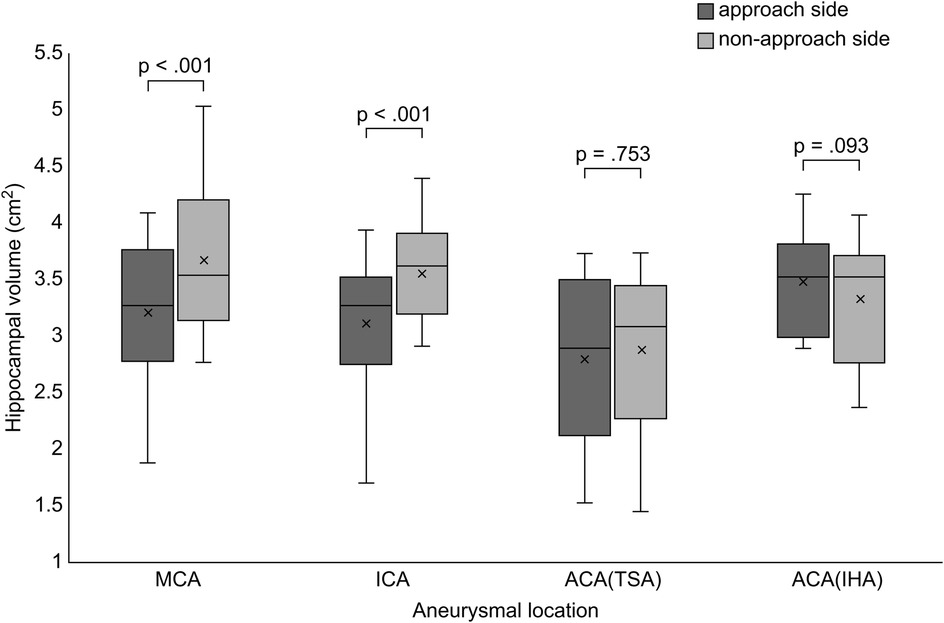
Figure 2. Comparison of hippocampal volume between the approach and non-approach sides after clipping surgery. Box and whisker plots show the distribution of hippocampal volume for each aneurysmal location. MCA, middle cerebral artery; ICA, internal cerebral artery; ACA, anterior cerebral artery; TSA, transsylvian approach; IHA, interhemispheric approach.
3.2 Evaluation of cognitive functionThere was no significant difference in the cognitive function among aneurysm locations. No correlations were observed between cognitive function and hippocampal volume in the MCA, ICA and ACA areas. The hippocampal volume in the left and right approach sides was not correlated with cognitive function at any aneurysmal location. ACA showed significant differences between FLAIR high-signal intensity in working memory (p = .04) and general memory (p = .036), but no other significant differences were found (Table 2).
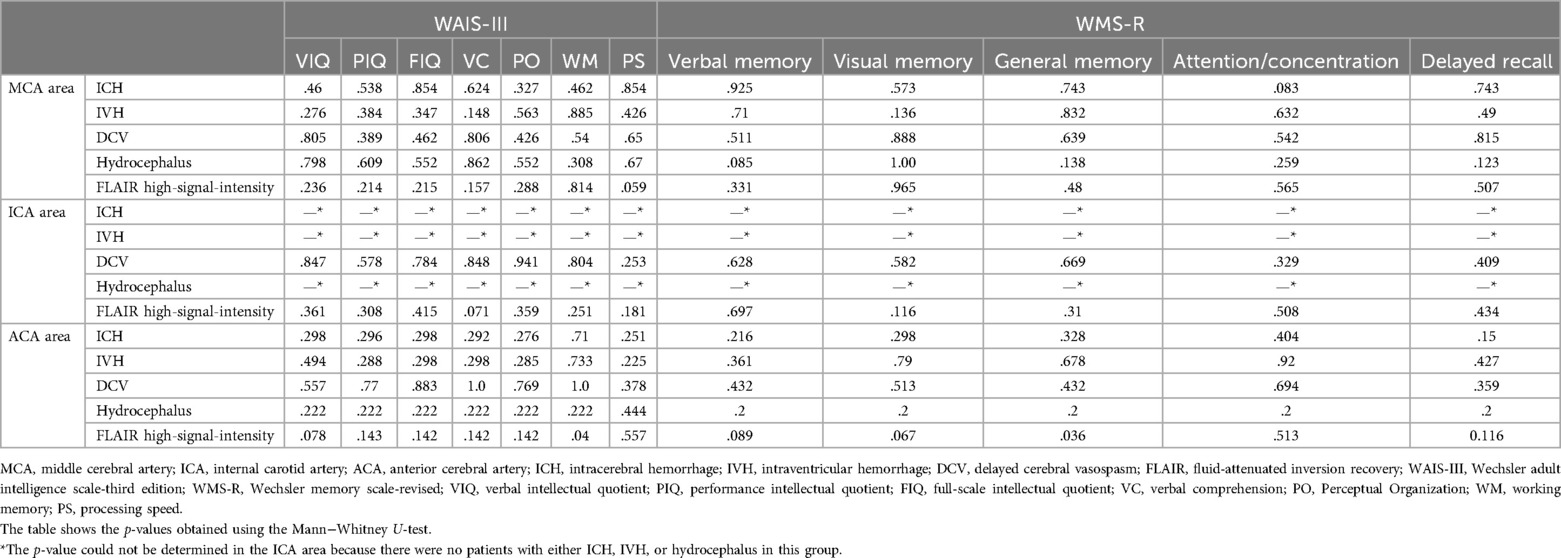
Table 2. Relationship between the radiological findings of aneurysmal location and neuropsychological assessment.
3.3 Evaluation of spike index and postoperative seizureThere was no significant difference in the SI of the approach side among aneurysm locations (p = .34). SI was significantly elevated on the approach side in the MCA group (p < .001), ICA group (p < .001), and ACA subgroup treated via the TSA (p < .001) (Figure 3). No significant correlation was found between the hippocampal volume and SI on the approach side (MCA, p = .997; ICA, p = .209; ACA, p = .207) (Table 3). The correlations between elevated SI values and cognitive function were found in working memory in the MCA (correlation coefficient: −0.642, p = .018) and visual memory in the ACA (correlation coefficient: −0.605, p = .084). Among all aneurysmal locations, no correlation was found between elevated SI values on the right approach side and cognitive function. However, elevated SI values on the left approach side showed some effects on performance intellectual quotient, perceptual organization, and working memory (correlation coefficients, 0.76, 0.718, and 0.891, respectively; p = .028, .045, .003, respectively) (Table 4). SI was not related to the presence of FLAIR high-signal intensity (Table 5). The FLAIR high-signal intensity was examined separately for frontal and temporal lobes in relation to SI, but no correlation was found. At 1 month postoperatively, there were 12 cases with focal impaired awareness seizure as the primary attack.
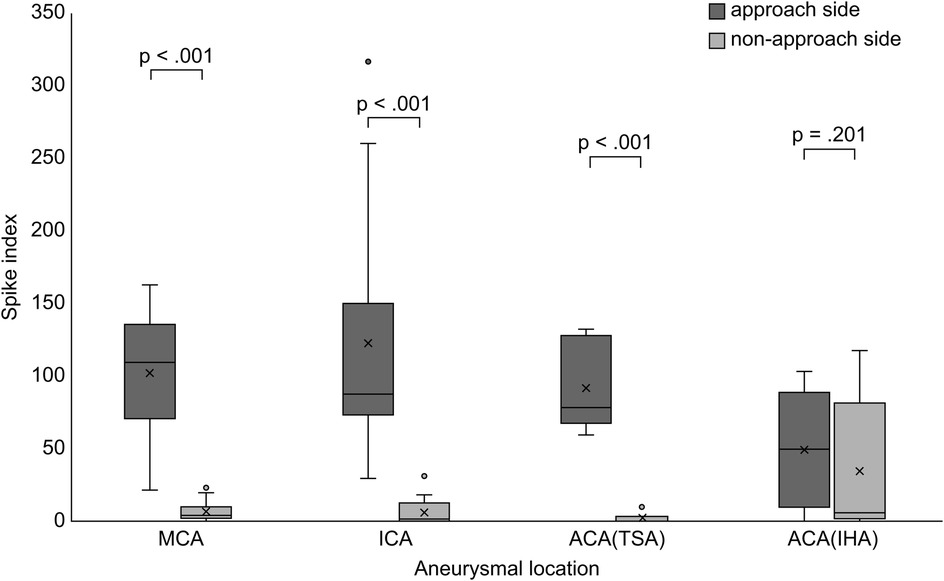
Figure 3. Comparison of the spike index between the approach and non-approach sides after clipping surgery. Box and whisker plots showing the distribution of the SI for each aneurysmal location. MCA, middle cerebral artery; ICA, internal cerebral artery; ACA, anterior cerebral artery; TSA, transsylvian approach; IHA, interhemispheric approach; SI, spike index.
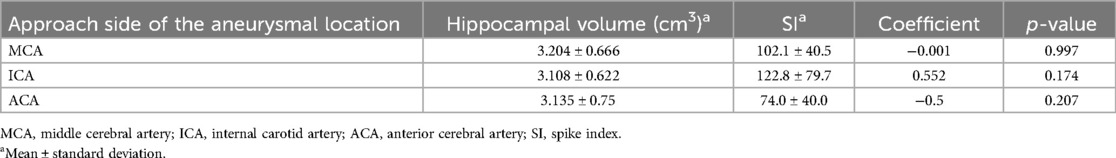
Table 3. Correlation between hippocampal volume and spike index on the approach side of the aneurysmal location.
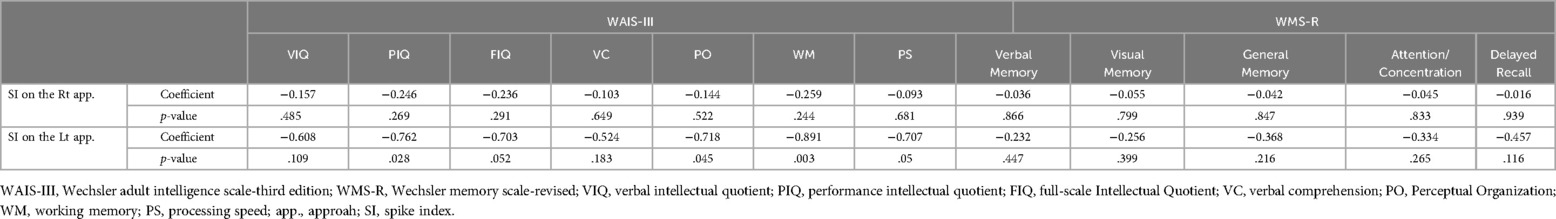
Table 4. Correlation between the spike index and neuropsychological assessments of all aneurysmal locations.

Table 5. Relationship between the spike index (SI) and the presence of FLAIR high-signal intensity.
4 DiscussionWe investigated the changes in brain structure, electrical activity, and cognitive function after clipping for aSAH of anterior circulation. In terms of brain structural changes, we focused specifically on the hippocampus and found that the hippocampal volume was reduced on the approach side compared to the non-approach side. Furthermore, regarding the relationship between hippocampal volume reduction and cognitive function, no correlations were observed in the MCA, ICA and ACA areas. SI was significantly elevated on the approach side in TSA, and elevated SI on the left approach was strongly correlated with a decline in cognitive function. There was no correlation between elevated SI and brain structural changes, such as hippocampal volume reduction and FLAIR high-signal intensity.
Hippocampal atrophy after clipping has been reported previously. Inoue et al. (14) reported that after surgery for an unruptured cerebral aneurysm, an enlarged inferior horn was observed on MRI due to intraoperative microvascular insufficiency and cerebral compression. Wostrack et al. (4) showed that patients had greater hippocampal atrophy after clipping than after endovascular treatment. Meanwhile, the relationship between intracerebral hemorrhage and hippocampal atrophy has been previously reported (15). The mechanism is that the medial structures of the temporal lobe are in contact with the tentorial incisura, making the hippocampus vulnerable to the effects of increased intracranial pressure (16). Therefore, local intracranial hypertension, insufficiency of the intracranial circulation, and the anatomical structure of the hippocampus and subarachnoid hemorrhage may lead to apoptosis of hippocampal neurons, thereby causing hippocampal volume reduction after aSAH. Surgery for aSAH is more difficult than surgery for unruptured aneurysms due to cerebral edema, which makes it more difficult to secure an adequate surgical field. Thus, microvascular insufficiency due to retraction of the temporal lobe may cause hippocampal volume reduction, and surgical manipulation/approach may be a factor that causes hippocampal volume reduction. The reduction in hippocampal volume on the operative side may also be caused by the force of the hemorrhage itself. The lack of left-right differences in hippocampal volume in ACA aneurysms may be related to the fact that the force of hemorrhage is not distinctly lateralized, as the force of hemorrhage often spreads anteriorly or posteriorly.
Regarding the timing of hippocampal atrophy, Wostrack et al. (4) assessed the extent of atrophy at 17 months postoperatively; herein, it was observed at 1 month postoperatively. This suggests that postoperative hippocampal volume reduction may occur earlier than previously thought.
The cause of cognitive dysfunction after aSAH remains unclear because of the diversity of neurological disorders. In general, mild to moderate cognitive dysfunction may reflect secondary brain damage due to intracranial circulatory disorder and subarachnoid hematoma (17, 18). Inoue et al. (14) reported no evidence of cognitive dysfunction in patients who presented with dilation of the inferior horns after clipping. However, sub-analyses of the International Subarachnoid Aneurysm Trial reported that cognitive dysfunction and epileptogenesis occur significantly less often after endovascular surgery (19, 20). These suggest that patients are more liable to epileptic seizures and cognitive dysfunction after clipping.
There was no correlation between elevated SI and brain structural changes on MRI in our study. Lüders et al. (21) reported on a network that develops epilepsy, suggesting that the elevated SI in the 1-month postoperative period of our study may complement interictal epileptiform discharges and capture the irritative zone they advocated. Jensen et al. (22) mentioned that morphological changes in the network reorganization that acquire epileptogenesis occur within weeks to months after injury. It is possible that the epileptogenesis was not acquired because the timing of our SI assessment was a subacute phase.
Lv et al. (23) reported that patients with an IEDs index of >10% showed a decline in cognitive function. Similarly in our study, cognitive dysfunction was associated with IEDs in EEG rather than with hippocampal volume reduction. Binnie et al. (24) reported that epileptiform discharges are not confined to epilepsy and that cognitive function improves when epileptiform discharges are suppressed with anti-seizure medications. Elevated SI is an early indicator of possible future epileptic seizures, and a decision to use anti-seizure medications to control elevated SI may prevent the worsening of cognitive function.
4.1 LimitationsThe main limitation of this study is the small sample size. The control group for hippocampal volume and SI was on the non-approach side, and future comparisons with the endovascular treatment group are desired. The study was limited to the subacute phase, and further investigation over time is needed to determine the structure and electrical activity of the cerebrum, cognitive function, and the occurrence of epileptic seizures in this population.
4.2 ConclusionHippocampal volume reduction and elevated SI were observed on the approach side in the subacute phase after clipping for the aSAH of anterior circulation. There was no association between cognitive dysfunction and hippocampal volume reduction. However, cognitive dysfunction was associated with IEDs.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Ethics Committee at Showa University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsKO: Writing – original draft, Writing – review & editing, Conceptualization. SS: Conceptualization, Writing – review & editing. HJ: Conceptualization, Writing – review & editing. YS: Data curation, Writing – review & editing. MK: Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe would like to thank Editage (https://www.editage.com) for the English language editing.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsACA, anterior cerebral artery; aSAH, aneurysmal subarachnoid hemorrhage; EEG, electroencephalography; FLAIR, fluid-attenuated inversion recovery; ICA, internal carotid artery; IEDs, interictal epileptiform discharges; IHA, interhemispheric approach; MCA, middle cerebral artery; MRI, magnetic resonance imaging; SI, spike index; TSA, transsylvian approach.
References1. Wermer MJ, Kool H, Albrecht KW, Rinkel GJ. Aneurysm screening after treatment for ruptured aneurysms study group. Subarachnoid hemorrhage treated with clipping: long-term effects on employment, relationships, personality, and mood. Neurosurgery. (2007) 60:91–7. doi: 10.1227/01.NEU.0000249215.19591.86
PubMed Abstract | Crossref Full Text | Google Scholar
2. Bendel P, Koivisto T, Hänninen T, Kolehmainen A, Könönen M, Hurskainen H, et al. Subarachnoid hemorrhage is followed by temporomesial volume loss: MRI volumetric study. Neurology. (2006) 67:575–82. doi: 10.1212/01.wnl.0000230221.95670.bf
PubMed Abstract | Crossref Full Text | Google Scholar
3. Bendel P, Koivisto T, Niskanen E, Könönen M, Aikiä M, Hänninen T, et al. Brain atrophy and neuropsychological outcome after treatment of ruptured anterior cerebral artery aneurysms: a voxel-based morphometric study. Neuroradiology. (2009) 51:711–22. doi: 10.1007/s00234-009-0552-5
PubMed Abstract | Crossref Full Text | Google Scholar
4. Wostrack M, Friedrich B, Hammer K, Harmening K, Stankewitz A, Ringel F, et al. Hippocampal damage and affective disorders after treatment of cerebral aneurysms. J Neurol. (2014) 261:2128–35. doi: 10.1007/s00415-014-7464-y
PubMed Abstract | Crossref Full Text | Google Scholar
5. Kreitschmann-Andermahr I, Poll E, Hutter BO, Reineke A, Kristes S, Gilsbach JM, et al. Quality of life and psychiatric sequelae following aneurysmal subarachnoid haemorrhage: does neuroendocrine dysfunction play a role? Clin Endocrinol (Oxf). (2007) 66:833–7. doi: 10.1111/j.1365-2265.2007.02821.x
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res. (2009) 170:245–51. doi: 10.1016/j.psychres.2008.07.006
PubMed Abstract | Crossref Full Text | Google Scholar
7. De Marchis GM, Pugin D, Lantigua H, Zammit C, Tadi P, Schmidt JM, et al. Tonic-clonic activity at subarachnoid hemorrhage onset: impact on complications and outcome. PLoS One. (2013) 8:e71405. doi: 10.1371/journal.pone.0071405
PubMed Abstract | Crossref Full Text | Google Scholar
8. Lanzino G, D’Urso PI, Suarez J. Participants in the international multi-disciplinary consensus conference on the critical care management of subarachnoid hemorrhage. Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2011) 15:247–56. doi: 10.1007/s12028-011-9584-x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Lin CL, Dumont AS, Lieu AS, Yen CP, Hwang SL, Kwan AL, et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J Neurosurg. (2003) 99:978–85. doi: 10.3171/jns.2003.99.6.0978
PubMed Abstract | Crossref Full Text | Google Scholar
10. Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. (2000) 10:433–42. doi: 10.1093/cercor/10.4.433
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wechsler D. Administration and Scoring Manual for the Wechsler Adult Intelligence Scale. San Antonio, TX: Harcourt Assessment, Inc. (2006).
12. Sugishita M. Manual for the Japanese Version of the Wechsler Memory Scale - Revised. Tokyo: Nihon Bunka Kagakusha (2001).
13. Kessler SK, Gallagher PR, Shellhaas RA, Clancy RR, Bergqvist AG. Early EEG improvement after ketogenic diet initiation. Epilepsy Res. (2011) 94:94–101. doi: 10.1016/j.eplepsyres.2011.01.012
PubMed Abstract | Crossref Full Text | Google Scholar
14. Inoue T, Ohwaki K, Tamura A, Tsutsumi K, Saito I, Saito N. Subtle structural change demonstrated on T2-weighted images after clipping of unruptured intracranial aneurysm: negative effects on cognitive performance. J Neurosurg. (2014) 120:937–44. doi: 10.3171/2013.12.JNS131790
PubMed Abstract | Crossref Full Text | Google Scholar
15. Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. (2007) 38:2861–3. doi: 10.1161/STROKEAHA.107.488015
PubMed Abstract | Crossref Full Text | Google Scholar
16. Hartzfeld P, Elisevich K, Pace M, Smith B, Gutierrez JA. Characteristics and surgical outcomes for medial temporal post-traumatic epilepsy. Br J Neurosurg. (2008) 22:224–30. doi: 10.1080/02688690701818901
PubMed Abstract | Crossref Full Text | Google Scholar
17. Hütter BO, Kreitschmann-Andermahr I, Mayfrank L, Rohde V, Spetzger U. Functional outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. (1999) 72:157–74. doi: 10.1007/978-3-7091-6377-1_13
PubMed Abstract | Crossref Full Text | Google Scholar
18. Ogden JA, Mee EW, Henning M. A prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery. (1993) 33(4):572–86; discussion 586–587. doi: 10.1227/00006123-199310000-00004
PubMed Abstract | Crossref Full Text | Google Scholar
19. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
PubMed Abstract | Crossref Full Text | Google Scholar
20. Scott RB, Eccles F, Molyneux AJ, Kerr RS, Rothwell PM, Carpenter K. Improved cognitive outcomes with endovascular coiling of ruptured intracranial aneurysms: neuropsychological outcomes from the international subarachnoid aneurysm trial (ISAT). Stroke. (2010) 41:1743–7. doi: 10.1161/STROKEAHA.110.585240
PubMed Abstract | Crossref Full Text | Google Scholar
21. Lüders HO, Awad I. Chapter 7. Conceptual considerations. In: Lüders HO, editor. Epilepsy Surgery. New York: Raven Press (1992). p. 51–73.
22. Jensen FE. Introduction. Posttraumatic epilepsy: treatable epileptogenesis. Epilepsia. (2009) 50:1–3. doi: 10.1111/j.1528-1167.2008.02003.x
Crossref Full Text | Google Scholar
23. Lv Y, Wang Z, Cui L, Ma D, Meng H. Cognitive correlates of interictal epileptiform discharges in adult patients with epilepsy in China. Epilepsy Behav. (2013) 29:205–10. doi: 10.1016/j.yebeh.2013.07.014
留言 (0)