Despite significant progress in spinal fusion surgery over the past several years, the pursuit of minimally invasive spine surgery is ongoing. Lumbar spine fusion remains an efficient surgical method for treating various lumbar degenerative diseases, including spondylolisthesis and spinal stenosis (1–3). The percutaneous endoscopic transforaminal lumbar interbody fusion technique, also known as Endo-TLIF, has emerged significantly in recent years, drawing the attention of spine surgeons owing to its benefits, such as reduced trauma, less bleeding, conservation of soft tissues, quicker recovery rate, short duration of hospital stay, and better clinical results (4–6). In 2000, Sehat et al. were the first to describe the notion of hidden blood loss (HBL). They discovered that it comprised 26% and 49% of total blood loss (TBL) following total knee and hip replacement operations, respectively (7). Subsequent studies have shown that HBL contributes to increased TBL and risk of postoperative blood transfusion if not properly managed (8, 9). Furthermore, elevated HBL levels can result in extended periods of hospital stay and recuperation, slow progress in wound recovery, heightened susceptibility to infections, and increased risk of cardiovascular and cerebrovascular accidents due to augmented bleeding volume (10, 11). Given the growing concern among spinal surgeons regarding HBL, an increasing number of researchers have investigated this issue (12–14). However, despite the expanding literature on HBL and minimally invasive spine surgery, no study has yet been conducted to quantify HBL and analyze its risk factors specifically in the context of Endo-TLIF. Ignorance of hidden blood loss after Endo-TLIF can lead to several complications in patients due to increased blood loss, such as anemia, increased rates of postoperative infections, prolonged hospital stays, and slow incision recovery. To address this, we carried out a retrospective study to determine the amount of HBL in Endo-TLIF procedures and investigate the associated risk factors. Our aim was to provide theoretical guidance to spinal surgeons performing Endo-TLIF to prevent related complications.
2 Materials and methods 2.1 PatientsBetween Oct 2017 and Mar 2021, 83 individuals with lumbar degenerative disease underwent surgery using the Endo-TLIF technique at the Spine Surgery Department of the hospital, where the primary author worked. This study included 42 males and 41 females. The inclusion criteria were as follows: patients at least 18 years old; confirmed lumbar spinal stenosis; mild lumbar spondylolisthesis (Meyerding grades I and II) with symptoms of recurrent lumbar disc herniation; symptoms that did not improve or worsened after receiving 6 mo or more of conservative treatment; and patients with segmental instability. The exclusion criteria included patients unable to undergo surgery due to severe systemic disease; those with severe lumbar spondylolisthesis (Meyerding grade Ⅲ and Ⅳ); patients with hematologic diseases; patients using antiplatelet drugs or anticoagulants; and those with lumbar spine tumor, tuberculosis, or infection. In this research, a single senior surgeon, who had already reached the pinnacle of his learning curve, performed all the surgeries. The ethics committee of the hospital approved this study and ensured that all procedures adhered to the necessary regulations and guidelines.
2.2 Data collectionsThe authors’ Hospital provided all patient information through a digital health database. This included demographic characteristics, such as age, sex, body mass index (BMI), height, and weight. Details of the duration of symptoms, smoking and drinking habits, hypertension, diabetes, osteoporosis, thickness of preoperative subcutaneous fat tissue in the lumbar region (as measured using midsagittal T2-weighted MRI) (15), and grade of lumbar disc degeneration (according to Pfirrmann grades) were also included (16). The parameters associated with blood loss, such as the patient blood volume (PBV), TBL, preoperative fibrinogen (FIB), activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), platelet count (Plts), intraoperative blood loss, and pre- and postoperative hematocrit (Hct) and hemoglobin (Hb) levels, were important considerations. Furthermore, operative data such as the number of fusion levels, duration of the surgery, and intraoperative blood loss were recoded. The World Health Organization defines anemia as hemoglobin (Hb) levels below 12.0 g/dl in women and below 13.0 g/dl in men (17, 18). All data were collected from the electronic medical record system.
2.3 Surgical procedures for endo-TLIFAll patients underwent general anesthesia and were placed in the prone position, with their abdomens lifted to minimize lumbar lordosis. The lesion segment was identified using C-arm fluoroscopy, and the locations of the surgical channel of the intervertebral foramen and skin cut of the pedicle screw were marked. A decompression cut approximately 1 cm in length was created approximately 4.5–5 cm from the midline of the surgery area. This incision is utilized for decompression, fusion, and pedicle screw placement. Its length can be adjusted to allow for parallel interbody decompression and cage placement, and from the perspective of the C-arm, the working cannula is positioned within the intervertebral foramen, while the display and light source are linked together. The joint capsule was treated with endoscopic electrocoagulation and the facet joint and part of the vertebral plate were gradually removed under endoscopic guidance using a circular saw.
Upon removal of the ligamentum flavum, the nerve roots and dural sac of the responsible segment were exposed. The large cannula was replaced, and the intervertebral space was treated with a reamer to scrape the cartilage endplate of the diseased segment. This method prepares the surface for the implant fusion under endoscopic guidance. A test mold is used to select the fusion cage of appropriate size. A homemade iliac bone extractor is used to collect cancellous bone from the posterior superior iliac spine. This procedure was performed after a cut was made in the contralateral distal arch of the nail tract. The bone is then implanted into the vertebral space of the responsible segment along with decompressed bone from the articular eminence and vertebral plate. When enough bone graft has been obtained, a fusion cage filled with autogenous bone is inserted parallel to the vertebral space using an expanded transforaminal method. C-arm fluoroscopy was performed to ensure that the cage remained in satisfactory position. The nerve roots on the affected side of the responsible segment were reexamined under endoscopic guidance. Finally, pedicle screws and bilateral connecting rods were placed percutaneously, and the skin was sutured and covered with a sterile dressing after confirming the proper position for internal fixation using C-arm fluoroscopy.
2.4 Postoperative managementsPostoperatively, all patients were on bed rest and received a rehydration regimen comprising of hormones, pain medications, and electrolytes. No anticoagulants were administered; however, the patients were instructed to move and raise their legs in bed and receive pneumatic pump therapy to prevent thrombosis. The patients were allowed to walk in the ground after confirming that the implant was in a satisfactory position via an imaging review. No drains were inserted; therefore, monitoring of drainage was not required.
2.5 Calculation of hidden blood lossHBL is equal to TBL minus visual blood loss (VBL) plus transfusion, that is, HBL (ml) = TBL−VBL + transfusion. Therefore, to calculate HBL, we need to calculate TBL and VBL. For calculating TBL, formula by Gross et al.is used, which is given by TBL (ml) = [PBV (L) × (Hctpre−Hctpost)]/[(Hctpre + Hctpost)/2] × 1,000 (19). According to the formula guiding the patient's Hct, we also choose the preoperative and Hct after blood volume stabilization for 23 d postoperatively. Nadler et al. considered the patient's complete blood volume, suggesting that it can be calculated based on the patient's sex, height, and weight. The formula PBV (L) is equal to k1 multiplied by the cube of height (m) plus k2 multiplied by weight (kg) plus k3. For males, the values of k1, k2, and k3 were 0.3669, 0.03219, and 0.6041, respectively. For females, these constants were 0.3561, 0.03308, and 0.1833, respectively. It is important to mention that all patients did not have drains postoperatively, hence, the VBL is approximately equal to the intraoperative blood loss. The calculation of intraoperative blood loss considers the weight of blood found in the suction bottle (while eliminating the weight of the irrigation fluid used during surgery), as well as the blood in the gauze and gauze strips (eliminating the weight of the dry gauze and gauze strips used during surgery). Additionally, none of the patients required intraoperative or postoperative transfusion, leading to the equation as follows: HBL = TBL—intraoperative blood loss. From the above statement, to obtain the amount of HBL, we only needed to calculate the TBL based on the change in Hct and calculate the VBL based on the intraoperative blood loss. The difference between the TBL and intraoperative blood loss equal to the HBL.
2.6 Statistical analysisWe utilized SPSS v26.0 for Windows (IBM Corp., Armonk, NY, USA) for data analysis. All quantities were derived as mean ± SD. The level of statistical significance was set at P < 0.05. All variables that might be related to HBL were screened using single-factor correlation analysis. It included 14 quantitative variables (age, BMI, duration of symptoms, lumbar subcutaneous fat tissue thickness, time of surgery, preoperative FIB, APTT, PT, TT, Plts, preoperative Hct and HB, and postoperative Hct and HB two days after surgery) and 9 qualitative variables (sex, hypertension, diabetes, osteoporosis, smoking, drinking, anemia, disk degeneration grade, and number of fusion levels). Sex, hypertension, diabetes, osteoporosis, smoking, and drinking were analyzed using the independent sample t test, and disk degeneration grade and number of fusion levels were analyzed using one-way analysis of variance test. Age, BMI, duration of symptoms, time of surgery, lumbar subcutaneous fat tissue thickness, preoperative FIB, APTT, PT, TT, and Plts were analyzed by Pearson or Spearman correlation analysis. Variables with a significant correlation were selected, and multivariate linear regression analysis was used to identify independent risk factors associated with HBL. A positive correlation coefficient implies a direct relationship with HBL, whereas a negative coefficient indicates the same relationship. Discrepancies in preoperative and postoperative Hct and Hb levels were examined using Student's t-test. Differences between pre- and postoperative anemia were assessed using the chi-square test.
3 ResultsIn our study, 83 patients (42 males and 41 females) underwent Endo-TLIF surgery. The average age was 58.6 ± 9.9 years (35–79) and average BMI was 24.5 ± 2.8 (18.5–31.3). The demographic information is presented in Table 1. The Hct and Hb preoperative readings were 0.412 ± 0.04 and 140 ± 15 g/L respectively, whereas the postoperative readings were 0.338(P < 0.001) and 110 ± 16(P < 0.001), respectively. Meanwhile, 58 patients developed anemia after surgery (P < 0.001, Table 2). The findings of the single-factor correlation analyses are presented in Table 3. Hypertension (i.e., blood pressure ≥140/90 mmHg) (P = 0.003), fusion levels (P = 0.003), and surgery time (P < 0.001) were significantly correlated with HBL through single-factor correlation analysis. However, the factors such as sex (P = 0.597), age (P = 0.420), BMI (P = 0.860), diabetes (i.e., fasting blood-glucose ≥6.1 mmol/L) (P = 0.809), osteoporosis (i.e., Dual-emission x-ray absorptiometry, T value ≤–2.5) (P = 0.219), smoking (P = 0.103), drinking (P = 0.978), Pfirrmann grades (P = 0.150), duration of symptoms (P = 0.795), preoperative lumbar subcutaneous fat tissue thickness (P = 0.867), preoperative FIB (P = 0.666), APTT (P = 0.778), TT (P = 0.650), PT (P = 0.820), and Plts (P = 0.373) were not correlated with HBL. Multiple linear regression analysis revealed a significant correlation between fusion level (P < 0.001) and surgery time (P < 0.001) with HBL (Table 4).

Table 1. Patients demographics.
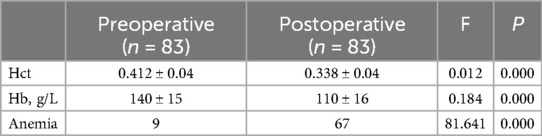
Table 2. Changes in Hct, Hb, and Anemia level following endo-TLIF spine surgery.
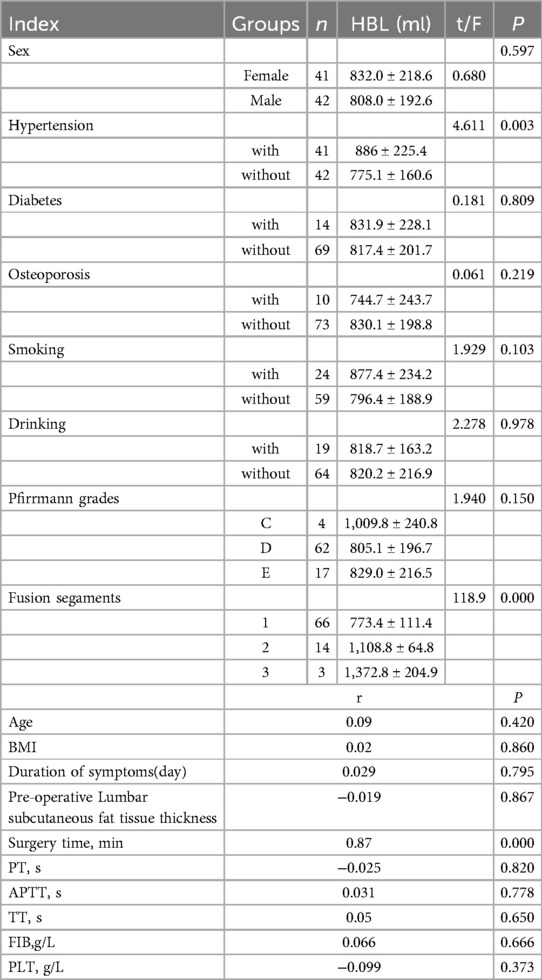
Table 3. Results of single factor correlation analysis for HBL.
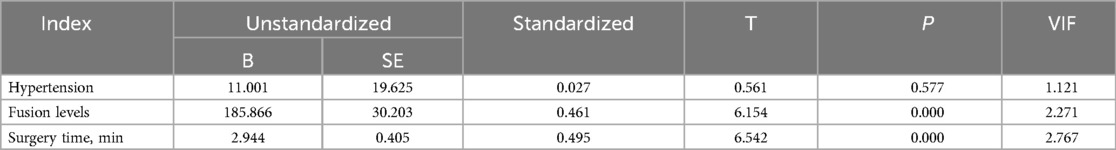
Table 4. Results of multiple line regression analysis for HBL.
4 DiscussionDegenerative lumbar spine disease is common in older patients and frequently causes symptoms such as lower back pain, shooting pain in the lower limbs, and periodic limping. These can lead to decreased movement, possible disability, and substantial reduction in the patient's quality of life. An efficient method to treat such degenerative conditions of the lumbar spine is through a procedure known as lumbar interbody fusion. It directly decompresses the nerve roots, corrects lumbar lordosis, and indirectly restores the height of the lumbar spinal space, ultimately achieving cure.
While traditional open procedures, such as PLIF and TLIF, yield positive results, they are often poorly tolerated by many older patients with underlying health conditions. Open surgery requires large incisions, muscle stripping, and pulling, increasing the risk of complications such as excessive blood loss, extended postoperative recovery time, and higher chances of postoperative infections. Recently, more spine surgeons have turned to Endo-TLIF surgery, a less invasive operation that causes less bleeding and involves small incisions with a cannula-protected operation, offering improved protection for the soft tissues, reducing postoperative infection risk, and promoting quick recovery. Existing literature suggests that Endo-TLIF effectively alleviates pain in older patients with poor underlying conditions who struggle with open surgery, significantly improving their quality of life (14, 20–22).
HBL is a condition where bleeding is undetectable during surgery. Traditionally, physicians have relied on estimates and formula calculations to determine blood loss. However, research conducted by Sehat et al. indicated that this technique is susceptible to a considerable number of errors. He demonstrated how postoperative blood can seep into muscle gaps and underlying compartments, leading to hidden bleeding that cannot be measured accurately, referred to as HBL (7).
HBL poses serious risks to patient recovery and safety after surgery. Underestimating blood loss can result in postoperative anemia, which in turn can weaken the patient's strength and immunity and increase the risk of infection and complications. Moreover, the healing process of wounds may be hindered by covert blood loss, which contributes to leakage of blood into tissue areas, consequently increasing the possibility of infection and development of scars. Furthermore, it can lead to prolonged postoperative hospitalization and increased utilization of healthcare resources.
Consequently, surgeons have begun to focus on addressing concealed blood loss. An increasing number of scholars have discovered that even in minimally invasive surgery, HBL cannot be ignored (Zhou et al. noted a substantial HBL (488.4 ± 294.0 ml, 52.5% of TBL) post MIS-TLIF treatment (12). Similarly, an average hidden blood loss of (317 ± 156) ml was discovered in 125 osteoporotic vertebral compression fracture patients treated with percutaneous vertebroplasty (23). Wang et al. reported notable HBL of approximately 469.5 ± 195.3 ml following UBE surgery, constituting 57.6% of TBL (13). Additionally, patients who underwent anterior cervical discectomy fusion experienced a substantial HBL of approximately 487.98 ± 255.96 ml, making up 67.61 ± 5.20% of the TBL (24). However, there is currently a lack of research on HBL in Endo-TLIF, preventing the calculation of HBL and analysis of risk factors. This knowledge gap hinders the effective management of patients undergoing Endo-TLIF. To resolve this problem, we conducted a retrospective study on HBL and its risk factors in patients who underwent Endo-TLIF. Through this, we intended to offer theoretical assistance for postoperative care and minimize the complications caused by excessive blood loss. This study included various risk factors such as sex, age, BMI, hypertension, diabetes, osteoporosis, smoking, drinking, Pfirrmann grades, fusion segments, symptom duration, preoperative lumbar subcutaneous fat tissue thickness, operation time, preoperative FIB, PT, APTT, TT, and Plts. In this study, we found that the HBL in patients treated with Endo-TLIF was 819.8 ± 204.9 ml. Single-factor correlation analysis revealed that increased blood pressure, operation time, and fusion segment were associated with high HBL. Multiple linear regression analysis confirmed that the duration of operations and fusion segments independently contributed to the risk of HBL, consistent with the findings of Zhang (25). Long operation times may result in increased HBL, possibly owing to prolonged washing time, extended wear time of the wound surface, and more severe inflammatory reactions, leading to increased hemolysis. This finding is consistent with the conclusions of Wang et al. and Lei et al (13, 26). Prolonged surgery may involve greater tissue cutting and incision widening, leading to increased blood vessel damage and HBL. It can also cause localized tissue edema, further increasing the risk of HBL (26). Analysis of the increase in HBL associated with the fusion segments revealed that an increase in the fusion segments led to greater soft tissue damage and bone destruction. With more fusion segments, additional tissue spaces and surgical cavities were created, increasing the blood storage capacity of HBL. Furthermore, bone debris from multi-level decompression and intervertebral space treatment may enter the bloodstream, causing abnormal capillary bed opening and blood extravasation, thereby increasing HBL. Removing the joints and ligaments located between the disc and vertebral body affects the blood flow to the fused area, reducing blood supply and leading to ischemia and concealed hemorrhage within the fused section. This finding aligns with the conclusions of Yu et al. (27). In our study, we discovered that factors such as sex, age, BMI, hypertension, diabetes, osteoporosis, smoking or drinking habits, Pfirrmann grades, symptom duration, prior lumbar subcutaneous fat tissue density, preoperative FIB, PT, APTT, TT, and Plts examinations had no substantial correlation with HBL. These results contradict those of the previous studies. Wang et al. identified age and preoperative FIB as risk factors in patients undergoing UBE surgery (13), while Zhou et al. revealed that age, muscle thickness, and preoperative FIB were independent risk factors for HBL in MIS-TLIF (12). Dai found that diabetes and hypertension are risk factors for HBL, which may be due to varying surgical procedures. Our Endo-TLIF procedure involves four small incisions of approximately 1 cm for decompression, fusion, and screw placement. This approach minimizes soft tissue damage and handles cartilage endplates under direct endoscopic visualization to reduce bone damage, thus decreasing HBL and mitigating risk factors associated with it. Moreover, the variety of patient populations with differing types and severity of conditions that various studies may incorporate can cause disparities in outcomes. In our study, the TBL of Endo-TLIF was 880.7 ± 219.8 ml. Some scholars have suggested that the use of new bone materials, such as biologics or allografts, may reduce TBL (28). Nevertheless, some studies have suggested that autologous iliac bone is the preferred graft for spinal fusion surgery. Consequently, we continued to extract the iliac bone during the surgery. However, this procedure can lead to concealed blood loss, and an overall increase in blood loss (29–31). Therefore, it is crucial to use bone wax and other techniques to effectively control bleeding after bone removal.
Our study has several limitations. Primarily, this was a retrospective study that focused on a single center and involved a small number of patients. The included risk factors are not comprehensive enough. To mitigate the risk of false positives and bias, we need to execute a prospective, multicenter study with a large sample size in the future to enhance the accuracy of the results. These factors likely influenced our findings. Secondly, we used Hct levels measured 2–3 d postoperatively, but it was still not possible to determine the balance of blood circulation, potentially causing errors in the amount of HBL calculated using the formula. Lastly, all our surgical cases were performed during the platform stage of the surgeons’ operations, ignoring the surgery-related situation of spine surgeons at the rising stage of the learning curve; therefore, the correlation between surgical experience and HBL was not addressed.
5 ConclusionTo summarize, a significant percentage of the TBL in patients with lumbar degenerative diseases treated with Endo-TLIF can be attributed to HBL. An increased number of fusion segments and prolonged operation time are risk factors for increased perioperative HBL during Endo-TLIF. Attention to HBL is critical for ensuring patient safety during perioperative care.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Hangzhou Xiaoshan Hospital of Traditional Chinese Medicine Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsMG: Writing – original draft. FZ: Investigation, Writing – original draft. WD: Data curation, Investigation, Writing – original draft. ZY: Data curation, Funding acquisition, Writing – original draft. ZX: Visualization, Writing – original draft. LZ: Formal Analysis, Writing – original draft. HZ: Formal Analysis, Writing – original draft. JY: Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from The Zhejiang Provincial Medical and Health Science and Technology Plan (2024KY1446 to ZY).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Lan T, Hu SY, Zhang YT, Zheng YC, Zhang R, Shen Z, et al. Comparison between posterior lumbar interbody fusion and transforaminal lumbar interbody fusion for the treatment of lumbar degenerative diseases: a systematic review and meta-analysis. World Neurosurg. (2018) 112:86–93. doi: 10.1016/j.wneu.2018.01.021
PubMed Abstract | Crossref Full Text | Google Scholar
2. Umeta RS, Avanzi O. Techniques of lumbar-sacral spine fusion in spondylosis: systematic literature review and meta-analysis of randomized clinical trials. Spine J. (2011) 11:668–76. doi: 10.1016/j.spinee.2011.04.026
PubMed Abstract | Crossref Full Text | Google Scholar
3. Patel PD, Canseco JA, Houlihan N, Gabay A, Grasso G, Vaccaro AR. Overview of minimally invasive spine surgery. World Neurosurg. (2020) 142:43–56. doi: 10.1016/j.wneu.2020.06.043
PubMed Abstract | Crossref Full Text | Google Scholar
5. Wu J, Liu H, Ao S, Zheng W, Li C, Li H, et al. Percutaneous endoscopic lumbar interbody fusion: technical note and preliminary clinical experience with 2-year follow-up. Biomed Res Int. (2018) 2018:5806037. doi: 10.1155/2018/580603
PubMed Abstract | Crossref Full Text | Google Scholar
6. Yang J, Liu C, Hai Y, Yin P, Zhou L, Zhang Y, et al. Percutaneous endoscopic transforaminal lumbar interbody fusion for the treatment of lumbar spinal stenosis: preliminary report of seven cases with 12-month follow-up. Biomed Res Int. (2019) 2019:3091459. doi: 10.1155/2019/3091459
PubMed Abstract | Crossref Full Text | Google Scholar
8. Ogura Y, Dimar Ii JR, Gum JL, Crawford CH 3rd, Djurasovic M, Glassman SD, et al. Hidden blood loss following 2- to 3-level posterior lumbar fusion. Spine J. (2019) 19:2003–6. doi: 10.1016/j.spinee.2019.07.010
PubMed Abstract | Crossref Full Text | Google Scholar
10. Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J. (2013) 13:877–81. doi: 10.1016/j.spinee.2013.02.008
PubMed Abstract | Crossref Full Text | Google Scholar
12. Zhou Y, Fu X, Yang M, Ke S, Wang B, Li Z. Hidden blood loss and its possible risk factors in minimally invasive transforaminal lumbar interbody fusion. J Orthop Surg Res. (2020) 15:445. doi: 10.1186/s13018-020-01971-5
PubMed Abstract | Crossref Full Text | Google Scholar
13. Wang H, Wang K, Lv B, Li W, Fan T, Zhao J, et al. Analysis of risk factors for perioperative hidden blood loss in unilateral biportal endoscopic spine surge-ry: a retrospective multicenter study. J Orthop Surg Res. (2021) 16(16):559. doi: 10.1186/s13018-021-02698-7
PubMed Abstract | Crossref Full Text | Google Scholar
14. Wu YS, Zhang H, Zheng WH, Feng ZH, Chen ZX, Lin Y. Hidden blood loss and the influential factors after percutaneous kyphoplasty surgery. Eur Spine J. (2017) 26:1878–83. doi: 10.1007/s00586-017-4950-9
PubMed Abstract | Crossref Full Text | Google Scholar
15. Atalay B, Saritepe F, Topcam A, Guclu H, Gurbuz MS. Evaluation of the association between lumbar spinal stenosis and lumbar subcutaneous fat tissue thickness by MRI: a novel perspective. J Coll Physicians Surg Pak. (2022) 32:147–51. doi: 10.29271/jcpsp.2022.02.147
PubMed Abstract | Crossref Full Text | Google Scholar
16. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. (2001) 26:1873–8. doi: 10.1097/00007632-200109010-00011
PubMed Abstract | Crossref Full Text | Google Scholar
17. Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: does hemoglobin change with aging? Semin Hematol. (2015) 52:261–9. doi: 10.1053/j.seminhematol.2015.07.006
PubMed Abstract | Crossref Full Text | Google Scholar
18. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. (1968) 405:5–37.4975372
PubMed Abstract | Google Scholar
20. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. (1962) 51:224–32.21936146
PubMed Abstract | Google Scholar
21. Lv Y, Chen M, Wang SL, Qin RJ, Ma C, Ding QR, et al. Endo-TLIF versus MIS-TLIF in 1-segment lumbar spondylolisthesis: a prospective randomized pilot study. Clin Neurol Neurosurg. (2022) 212:107082. doi: 10.1016/j.clineuro.2021.107082
PubMed Abstract | Crossref Full Text | Google Scholar
22. Zhu JM, He XP, Li KY, Huang DK, Cao XD. Change of preoptic opiate receptors after injection of 6-OHDA into sublocus coeruleus in the rabbit. Sheng Li Xue Bao. (1990) 42:114–20.2165279
PubMed Abstract | Google Scholar
23. Lu HL, Tao ZS, Ma JM, Zhu XF, Yang M, Ding GZ. Analysis of hidden blood loss and its risk factors in the treatment of osteoporotic vertebral compression fractures with PVP. Zhongguo Gu Shang. (2020) 33:445–8. doi: 10.12200/j.issn.1003-0034.2020.05.011
PubMed Abstract | Crossref Full Text | Google Scholar
24. Cai T, Chen D, Wang S, Shi P, Wang J, Wang P, et al. Perioperative hidden blood loss in elderly cervical spondylosis patients with anterior cervical discectomy fusion and influencing factors. Geriatr Orthop Surg Rehabil. (2021) 12:21514593211002164. doi: 10.1177/21514593211002164
PubMed Abstract | Crossref Full Text | Google Scholar
25. Zhang R, Xing F, Yang Z, Lin G, Chu J. Analysis of risk factors for perioperative hidden blood loss in patients undergoing transforaminal lumbar interbody fusion. J Int Med Res. (2020) 48:300060520937848. doi: 10.1177/0300060520937848
PubMed Abstract | Crossref Full Text | Google Scholar
26. Lei F, Li Z, He W, Tian X, Zheng L, Kang J, et al. Hidden blood loss and the risk factors after posterior lumbar fusion surgery. Medicine. (2020) 99:e20103. doi: 10.1097/MD.0000000000020103
PubMed Abstract | Crossref Full Text | Google Scholar
28. Dai Z, Feng DP, Wu KL, Zhu JY, Li ZW. Hidden blood loss of minimally invasive hybrid lumbar interbody fusion: an analysis of influencing factors. BMC Musculoskelet Disord. (2022) 23(1):1099. doi: 10.1186/s12891-022-06079-x
PubMed Abstract | Crossref Full Text | Google Scholar
29. Kasis AG, Jensen C, Dharmadhikari R, Emmerson BR, Mawdsley M. Novel bone grafting technique in stand-alone ALIF procedure combining allograft and autograft (‘northumbria technique’)-fusion rate and functional outcomes in 100 consecutive patients. Eur Spine J. (2021) 30:1296–302. doi: 10.1007/s00586-021-06758-8
PubMed Abstract | Crossref Full Text | Google Scholar
30. Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. (2002) 84:716–20. doi: 10.2106/00004623-200205000-00003
PubMed Abstract | Crossref Full Text | Google Scholar
31. Michielsen J, Sys J, Rigaux A, Bertrand C. The effect of recombinant human bone morphogenetic protein-2 in single-level posterior lumbar interbody arthrodesis. J Bone Joint Surg Am. (2013) 95:873–80. doi: 10.2106/JBJS.L.00137
留言 (0)