Leprosy reactions are acute immunologically driven episodes in the chronic course of leprosy that result in significant functional morbidity. These may occur prior to, during or following the completion of multidrug therapy (MDT), with a reported lifetime prevalence of nearly 50% among leprosy patients.1,2 Two major types of leprosy reactions include reversal reactions (RRs) or type 1 reaction (T1R) and erythema nodosum leprosum (ENL) or type 2 reaction (T2R). RRs are seen in up to one-third of patients in the borderline spectrum of leprosy.3 RRs are type IV hypersensitivity reactions due to an increase in cell-mediated immune response (CMI) against Mycobacterium leprae (M. leprae). ENL occurs in 50% of patients with lepromatous leprosy (LL) and 10% of patients with borderline lepromatous (BL) leprosy.4 ENL can be classified based on its duration and recurrence. Acute ENL refers to an episode lasting less than six months, with treatment gradually tapered off and no recurrence while on therapy. Recurrent ENL involves a new episode occurring at least 28 days after completing treatment for a prior episode, while chronic ENL persists beyond six months, requiring continuous treatment or having treatment-free intervals shorter than 28 days. ENL is a type III hypersensitivity reaction with deposition of immune complexes, neutrophilic infiltrate and increased levels of circulating tumour necrosis factor-alpha (TNF-α). Lack of a satisfactory animal model that reproduces the whole spectrum of leprosy and reactional episodes is a major limitation in understanding the pathophysiology of the disease.
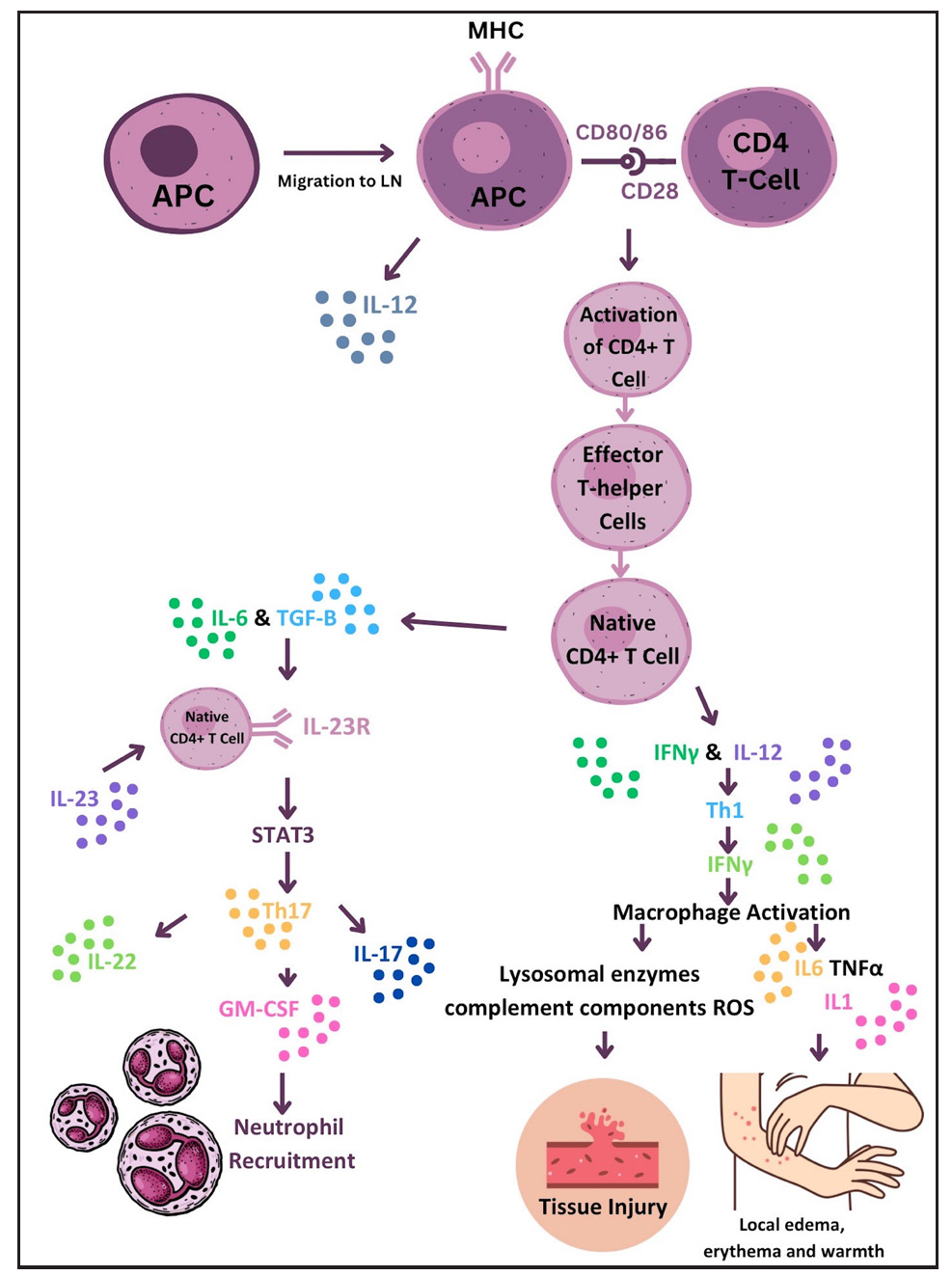
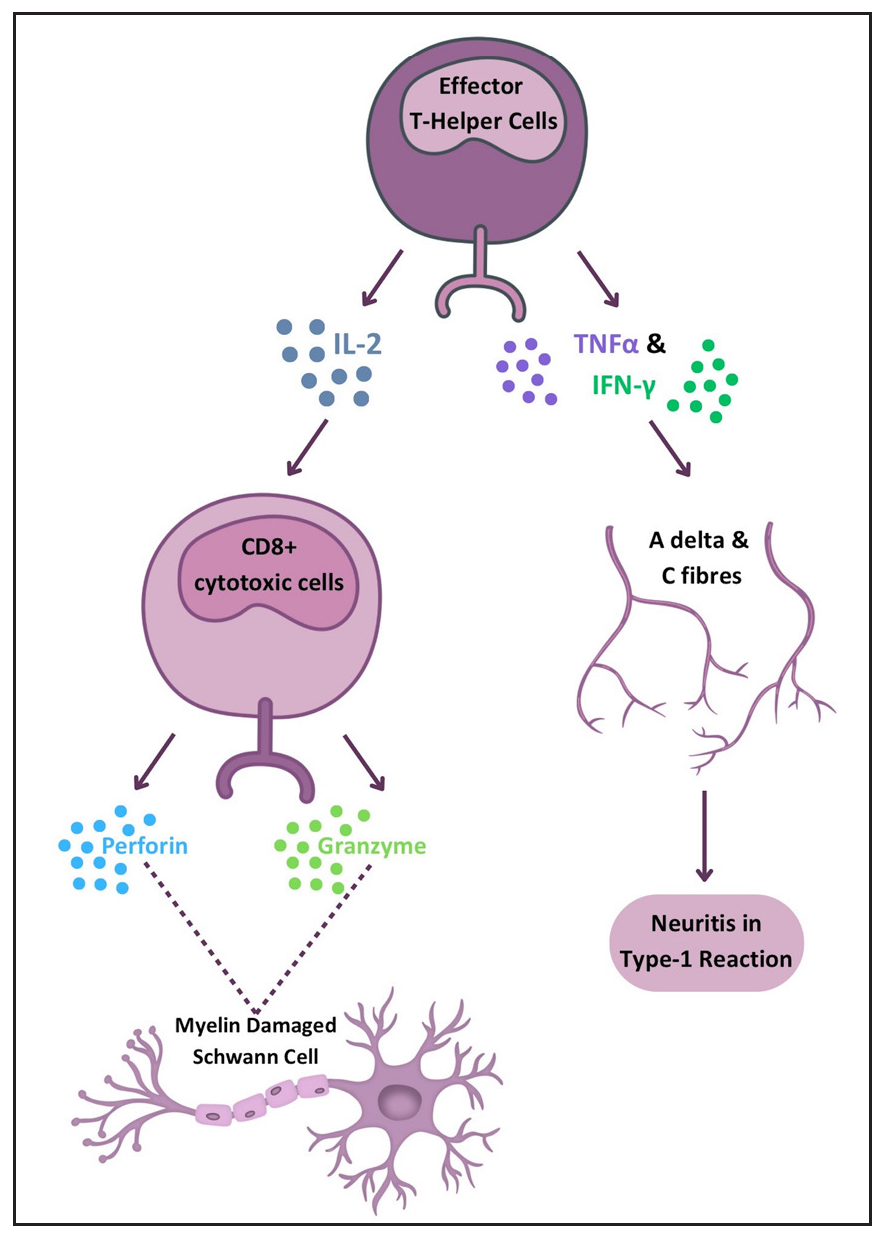
Pathophysiology Type 1 reactionT1R is a condition characterised by T-cell hypersensitivity against M. leprae in the borderline spectrum of leprosy. Upon M. leprae inoculation in the nasal mucosa, the immune response is initiated involving phagocytosis by antigen-presenting cells (APCs). These APCs capturing antigens from peripheral tissues migrate to lymph nodes, where they stimulate the adaptive immune response through major histocompatibility complex class II (MHC-II) molecules and two signals to naïve CD-4+ T helper (Th) cells. Interleukin (IL)-12 production by APCs promotes immune activation by differentiating CD4+ T-cells into effector Th cells. Effector Th cells release IL-2, stimulating the proliferation of Th cells and CD8+ T-cells. These activated T-cells migrate back to peripheral tissues, where CD8+ T-cells release perforin and granzymes, damaging myelin sheath and Schwann cells.3 Naïve T-cells differentiate into Th17cells due to IL-6 and tumour growth factor-beta (TGF-β) production by APCs. IL-17 from activated Th17 cells aids neutrophil recruitment. Interferon-gamma (IFN-γ) from APCs activates macrophages, leading to pro-inflammatory cytokine production (TNF-α, IL-1β, IL-6). IL-6 is thus produced by both APCs and activated macrophages and underlies the inflammatory process. Figure 1 illustrates the pathogenesis of T1R. TNF-α and IFN-γ directly stimulate nerve fibres, causing neuritis in type I reactions [Figure 2].5 This inflammatory environment, coupled with mycobacterial killing and antigenic spread, determines the progression of reactional episodes.
Export to PPT
Export to PPT
Heat shock proteins (HSPs), expressed during chronic inflammation and stress, may induce autoimmune reactions during mycobacterial infections due to molecular mimicry with host proteins. Studies have identified B-cell mimicking epitopes shared between M. leprae’s HSP65 and host keratin, finding significantly higher antibody levels against specific HSP peptides in T1R patients compared to non-reaction individuals and healthy controls.6,7 These findings suggest potential predictive biomarkers for T1R and highlight the role of molecular mimicry in leprosy-induced immune responses. Unconventional T-cells are proposed to mediate the interface between innate and adaptive immunity in T1Rs. Pathak et al. revealed decreased γδ T-cell numbers and elevated NKT-like and NK cells in T1R patients compared to non-reaction individuals.8
IFN-γ is pivotal in T1Rs, with studies identifying increased IL-15 production and autophagy activation as downstream effects.9 Comparative gene expression analysis between T1R skin lesions and M. leprae + IFN-γ stimulated macrophages revealed 13 common genetic elements, including the autophagy regulator translocated promoter region (TPR), significantly increased in macrophages from T1R patients.9 Autophagy is crucial for inflammasome component degradation, limiting exaggerated inflammatory responses. Tuberculoid leprosy patients exhibit upregulated autophagy genes compared to lepromatous cases.10 Autophagy is upregulated by dead bacilli, while live mycobacteria inhibit it, serving as an immune escape mechanism.10 Downregulation of autophagy genes, along with TLR3 and NLRP3-IL-1β pathway overexpression in multibacillary (MB) patients, increases RR risk.11 Blocking autophagy with 3-methyladenine in M. leprae-stimulated monocytes enhances NLRP3 inflammasome expression and subsequent IL-1β and IL-6 production.11 Pro-autophagic drugs may help control bacillary load and potentially treat T1Rs.
Th17 population increases and T-regulatory cell (Treg) population reductions are observed in leprosy reactions, with decreased TGF-β and increased IL-6 and IL-21 production.12,13 IL-21 is crucial in T1R pathogenesis by promoting the differentiation of T-regulatory cells into the Th17 pathway, evidenced by its higher levels in T1R patients’ blood and skin lesions, positive correlation with Th17 markers, and negative correlation with Treg markers.14Table 1 summarises novel mechanisms in T1R pathogenesis.
Table 1: Novel mechanisms implicated in the pathophysiology of type 1 leprosy reactions
Pathogenetic mechanism Role in type 1 reaction Evidence Molecular mimicry Monoclonal antibodies against M. leprae have been reported to cross-react with human nerve and skin components, suggesting a potential contribution to the development of autoimmune clinical manifestations in leprosyMolecular mimicry between cytokeratin-10 and HSP65 reported; elevated antibodies against HSP4 and HSP5 in type 1 reaction patients6,7
Differential antibody levels against mimicking peptides potentially predictive biomarkers for T1R development and leprosy disease
Unconventional T-cells Mediating interface between innate and adaptive immunitySignificant decrease in γδ T-cell numbers; elevated frequencies of NKT-like and NK cells in type 1 reactions8
Additionally, higher plasma levels of TNFα, IL1β, IL17 and CXCL10; increased gene expression of IFNγ, IP10, TNFα, IL6 and IL17A; upregulated chemokines like CCL3, CCR1, CCR5 and CXCR3 in Type 1 reaction patients8
AutophagyEssential for inflammasome component degradation, limiting exaggerated inflammatory responses
Downregulation of autophagy genes increases the risk of reversal reactions
Comparative gene expression analysis revealed common genetic elements, including autophagy regulator TPR9
Upregulated autophagy genes in tuberculoid leprosy10
Live mycobacteria inhibit autophagy10
Downregulation of autophagy genes and TLR3, NLRP3-IL-1β pathway overexpression increase risk of reversal reactions11
Blocking autophagy enhances NLRP3 inflammasome expression11
Th17 and Treg population alterations Increase in Th17 and reduction in Treg populations observedIL-21, a differentiating cytokine promoting T-regulatory cells to Th17 pathway, explored14
IL-21+ cells significantly higher in T1R patients; gene expression of IL-21 correlates positively with Th17 cell markers and negatively with Treg cell markers14
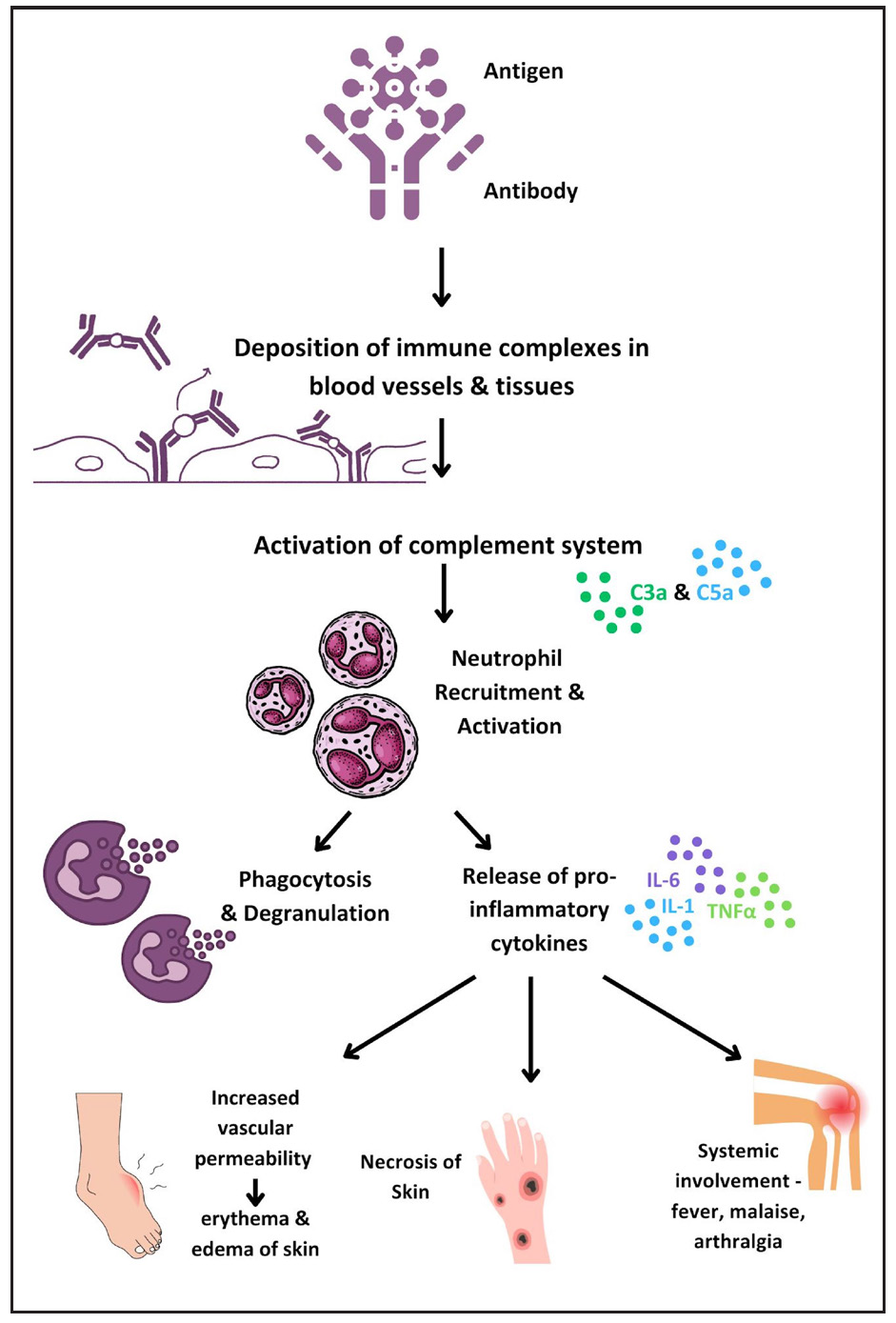
Type 2 reactionsThe pathogenesis of T2R involves type III hypersensitivity due to inadequate clearance of antigen-antibody complexes, resulting in inflammation and leukocyte chemotaxis. Skin biopsies of ENL patients show complement and immunoglobulin deposition in the dermis, similar to an Arthus reaction.15Figure 3 illustrates the current knowledge regarding immunopathogenesis of T2R.
Export to PPT
Neutrophils play a central role in ENL pathogenesis, with Tavares et al. highlighting elevated low-density neutrophils (LDNs) in ENL patients.16 Neutrophil extracellular traps (NETs), significant in severe autoimmune disorders, are abundant in ENL patients and reduced by thalidomide.17,18 The balance between IL-10 and TNF-α in neutrophils, along with Toll-like receptor 9 (TLR9) recognition of DNA, offers potential biomarkers for ENL.19,20 Further investigation revealed the upregulation of plasmacytoid dendritic cell/ type 1 interferon pathway, as a consequence to increase in TLR9 expression.21 B-cell subpopulation alterations22,23 and myeloid-derived suppressor cells with elevated annexin A1 levels may impact T-cell efficacy.24 Depleting CD25+ cells reversibly impairs immune response, highlighting Tregs as a potential therapeutic target.25 Neurotrophins, particularly nerve growth factor (NGF), showed decreased levels and the presence of autoantibodies modulated by cyclosporin A, suggesting targets for neuroimmune reaction control.26Table 2 summarises recent breakthroughs in ENL and their therapeutic implications.
Table 2: Summary of recent studies on pathogenesis of erythema nodosum leprosum
Study Salient findings Implications Tavares et al.16Flow cytometry of ENL patients demonstrated elevated frequency of LDN, which displayed a neutrophilic-activated phenotype
Higher CD11b and lower CD62L surface expressions on LDNs correlate with the activation status of LDNs
ENL patients under thalidomide treatment presented similar frequency of LDNs as observed before treatment but its activation status was lower
Potential biomarkers for diagnosis and monitoring of reactional states Pacheco et al.19Patients with ENL had a subpopulation of neutrophils that expressed IL-10R1 in both skin lesions and blood
Neutrophils found in the blood of ENL patients were able to secrete detectable levels of TNF-α, which could be blocked by the addition of IL-10
IL-10R1 is a possible biomarker in ENL
IL-10 pathway may be a therapeutic target for the management of ENL
Dias AA et al.20 DNA sensing via TLR-9 constitutes a major innate immunity pathway involved in the pathogenesis and evolution of ENL TLR-9 antagonists are potential alternative to more effectively treat ENL Da Silva et al.18Abundant NETs were found in T2R skin lesions and increased spontaneous NETs formation was observed in T2R peripheral neutrophils.
TLR9 expression was shown to be higher in T2R neutrophils
Treatment of T2R patients with thalidomide for seven consecutive days resulted in a decrease in all of the evaluated in vivo and ex vivo NETosis parameters.
DNA recognition via TLR9 may be one of the pathways triggering this process during T2R, thus it is a potential therapeutic target Nogueria et al.22Compared to uninfected subjects, an increase in mature B-cells and a decrease in memory B-cells was observed in MB disease and ENL
Decrease in atypical B-cells (CD27–CD21–) and an increase in activated B-cells (CD27+ CD21+) was noted during ENL episodes
First study to describe the different B-cell phenotypes in polar forms of leprosy and in ENL Negera et al.25Increase in activated memory B-cells and reduced number of tissue-like memory B-cells in untreated ENL patients as compared to LL patient
The percentage of total circulating B-cells was similar among ENL patients and non-reactional LL patients; however, proportion of B-cells was significantly reduced by treatment for ENL
B-cell depletion may have a role in management of ENL Da Silva et al.24An increased density of myeloid-derived suppressor cells has been observed in patients with lepromatous leprosy and T2R
Presence of annexin A1 was observed in all myeloid-derived suppressor cells. Monocytic myeloid-derived suppressor cell in the lepromatous patients had particularly higher levels of this protein when compared to the reactional patients
High annexin A1 expression in lepromatous patients may be responsible for reduction in the efficacy of T-cell action against M. leprae, rendering the patient susceptible to MB disease Jesus et al.26NGF is decreased in the course of leprosy and there is presence of autoantibodies against NGF in all clinical forms of leprosy and neuroimmune reactions
Levels of autoantibodies against NGF are decreased by the immunomodulatory activity of cyclosporin A, which mainly controls pain and improves motor function and sensitivity
Suppression of anti-NGF and the regulation of NGF levels can be attractive targets for immunomodulatory treatment and for controlling the neuroimmune reactions of leprosy Negera et al.25Tregs in ENL maintain suppressive function despite reduced numbers
Depletion of Tregs enhances TNFα and IFNγ responses, indicating a regulatory role
Tregs emerge as potential targets for immune modulation in leprosy Castro et al.13Decrease in CD4+TGF-β+ Treg and CD8+ TGF-β+ Treg cells
Upregulation of IL-17 and IL-6
Enhances our understanding of immune hyporesponsiveness in MB patients and hyperresponsiveness in reactions Rosa et al.21Increased type 1 IFN expression
Decreased frequency of peripheral pDC
pDC/type I IFN pathway may be utilised as potential biomarker for diagnosis
Targeting pDC may be a viable therapeutic approach in ENL
Infections are potential triggers for lepra reactions. Motta et al. found that 39% of patients with lepra reactions had concurrent infections, mainly oral.27 A thorough search based on patient history and clinical examination is advisable, especially in recurrent reactions. However, the impact of specific infections remains uncertain, as a scoping review did not indicate a higher incidence of reactions in patients with concurrent bacterial, fungal or parasitic infections.28
A recent study highlighted the different roles of dead and viable bacilli in the modulation of lepra reactions and nerve damage. While viable bacilli promote its own survival by exploiting the host cells, dead bacilli create a pro-inflammatory milieu and promote nerve damage.29
DiagnosisEarly diagnosis of reactions is essential to limit the resulting neural damage. Clinical examination remains the cornerstone of the diagnosis of a reaction once it manifests. Classically, T1R presents with erythema and oedema of pre-existing lesions which may or may not be associated with neuritis. ENL manifests as evanescent, tender nodules, more often associated with systemic complains like fever, joint pain, bone pain, and lymphadenopathy. Erythema Nodosum Leprosum International STudy (ENLIST) score is used frequently to classify it as mild, moderate or severe, which determines the management. There is an urgent need to identify biomarkers that may predict the course of the disease and identify patients who are at increased risk of developing reactional episodes. Currently, there is no diagnostic tool that reliably predicts the onset of reactions before clinical manifestations occur. Table 3 summarises the newer diagnostic modalities with a possible role in leprosy reactions.
Table 3: Newer diagnostic modalities with possible role in leprosy reactions
Type of diagnostic modality Specific investigation Results Role in leprosy reactions Non-invasive imaging Dermoscopy35T1R: intense erythema, large telangiectatic vessels, violaceous to brown periappendageal pigmentation, white globules, epidermal scaling, follicular plugging
T2R: erythema, vascular dilation, red dots, hypopigmented structureless areas
Diagnosis of leprosy reactions HRUS with colour Doppler30 Blood flow detected on colour Doppler in patients with nerve involvement in NCS and in those with minimal or no changes in NCS Diagnosis of neural involvement in leprosy reactions with minimal nerve involvement or those lacking a motor response in NCS Magnetic resonance imaging31–33Leprosy-associated peripheral nerve abscess usually reveals signal changes of an abscess with peripheral contrast enhancement of the abscess wall and inflammatory thickening of the whole nerve trunk
Rarely, CNS lesions may also be diagnosed by MRI
Differentiating many mass lesions of peripheral nerves such as a schwannoma, neurofibroma and more from leprosy-associated abscess or cranial nerve involvement Serology for M. leprae Anti-PGL-1 antibody37 Moderate sensitivity and specificity for predicting future reactions May predict risk of future ENL episodes, but not type 1 reaction Anti-NDO-LID-1 antibody37 Good sensitivity and specificity for predicting future reactions Anti LAM salivary antibody39 Similar odds of predicting reaction as anti-PGL-1 antibody Inflammatory markers NLR, PLR and LMR41 Fair sensitivity and specificity in diagnosis of leprosy reactions Diagnosis and possibly response to anti-inflammatory therapies C1q42 Lower level during ENL, with normalization after treatment Diagnosis of leprosy reactions and monitoring response to treatment Cyclooxygenase 2 and vascular endothelial growth factor expression in dermal macrophages and vascular endothelium43 Higher in type 1 followed by T2R compared to controls Potential targets for treatment of lepra reactions Pentraxin-344 Elevated prior to onset and during acute ENL, declined after treatment Prediction of predisposition to ENL, diagnosis of ENL and monitoring response to therapy Molecular diagnosis Dual colour reverse-transcription multiplex ligation-dependent probe amplification45 A transcriptomic signature of risk for reversal reactions consisting of five genes (CCL2, CD8A, IL2, IL15andMARCO) was identified based on cross-sectional comparison of RNAexpression Could predict reversal reactions at least two weeks before onsetNerve conduction study (NCS) is established for detecting neural dysfunction. Recent studies suggest high-resolution ultrasonography (HRUS) with colour Doppler (CD) can aid in diagnosing leprosy reactions, especially in patients with minimal nerve involvement or lacking a motor response in NCS. HRUS with CD is particularly useful for detecting reactions in nerves with minor changes or no motor response in NCS.30 Magnetic resonance imaging (MRI) helps diagnose nerve abscesses, distinguish leprosy reactions from other causes of nerve thickening and detect the central nervous system involvement.31–33 Dermoscopy is another easily available non-invasive imaging which has been utilised in leprosy reactions.34,35Table 3 outlines the dermoscopic features of lepra reactions.
Higher titres of antibodies against natural octyl disaccharide-leprosy IDRI diagnostic (anti-NDO-LID) at baseline have been associated with the risk of future ENL.36 An observational study showed that patients with ENL had 66.66% higher titres of antibodies against Phenolic Glycolipid-1 (anti-PGL-1) and 91.66% higher titres of anti-NOD-LID-1 as compared to those without reactions.37 Further, positivity for both antibodies was significantly associated with the likelihood of developing reactions in the future. Another study revealed anti-PGL1 to be an important prognostic factor for the prediction of leprosy reactions.38 Additionally, salivary antibodies to lipoarabinomannan (LAM) antigen may be used as a tool to monitor patients undergoing treatment to predict reactional episodes.39
Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) have been utilised to assess the inflammatory response in various diseases. Gomes et al. assessed the utility of assessing NLR in patients with leprosy reactions.40 The NLR cut-off for the diagnosis of any leprosy reaction was 2.75 (sensitivity 61.0%, specificity 92.0%, accuracy 77.0%) while the cut-off for T2R was 2.95 (sensitivity 81.0%, specificity 74.0%, accuracy 78.0%). Another study noted that NLR and PLR but not LMR were useful as a diagnostic biomarker for ENL.41
Complement component 1q (C1q), a key component of the classical complement pathway, has been studied as a diagnostic and monitoring parameter for ENL.42 C1q levels in peripheral blood were significantly lower in untreated ENL patients compared to LL controls. Additionally, increased genetic expression of C1q components was noted in ENL patients’ peripheral blood and skin biopsies, which normalised after treatment, suggesting C1q as a potential diagnostic marker and treatment response indicator for ENL. An immunohistochemical study also found significantly increased expression of cyclooxygenase 2 and vascular endothelial growth factor in dermal macrophages and vascular endothelium in T1Rs, followed by T2Rs, compared to controls.43
Pentraxin-3 (PTX-3), an inflammatory marker, is higher in MB patients before and during acute ENL and decreases within seven days of thalidomide therapy.44 A novel study used RNA expression analysis of 1090 whole blood samples to profile 103 target genes for innate and adaptive immune responses.45 A transcriptomic signature of five genes (CCL2, CD8A, IL2, IL15, MARCO) was identified, predicting RRs at least two weeks before onset.45 Other potential ENL diagnostic biomarkers include IL-6, IL-7, CCL-11, alpha-1 acid glycoprotein and CD-64.46 No single marker or a set of markers has shown to reliably and effectively prognosticate the occurrence of lepra reactions.
The Specialist System for Evaluation of Risk of Occurrence of Reactional States in Leprosy (SEPAREH) is an online tool that predicts leprosy reactions with up to 87.7% accuracy using socio-demographic details, family history, clinical, laboratory and genetic data.47
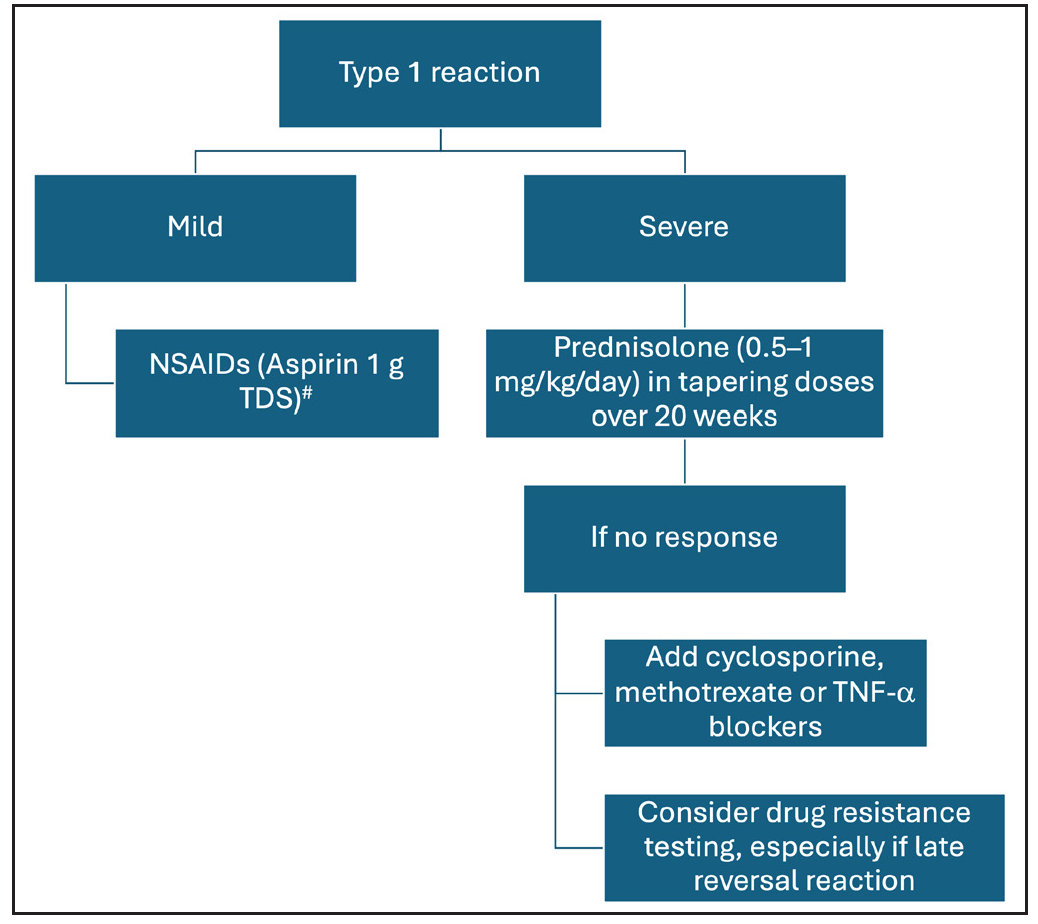
Treatment Type 1 reactionsThe objective of treatment for T1R is control of neural inflammation and prevention of further immune-mediated damage. For mild T1R, non-steroidal anti-inflammatory drugs may suffice, but severe reaction, especially with neuritis, requires medical and/or surgical treatment. Current evidence suggests that oral corticosteroids are the mainstay for severe T1Rs and should be started in a dose of 0.5–1 mg/kg/day and gradually tapered to zero over a span of 20 weeks [Figure 4]. A randomised trial (Treatment of Early Neuropathy in Leprosy or TENLEP) compared oral corticosteroids for 20 and 32 weeks in patients with recent onset nerve function impairment (less than six months duration), including T1R.48 No difference in clinical outcomes was noted between the groups at the end of the study period.
Export to PPT
Other immunosuppressive agents that have been used successfully for the management of T1R include cyclosporine and methotrexate.49-51A recent review and systematic analysis has highlighted the role of methotrexate in type 1 and 2 lepra reactions. The study recommends lower doses of methotrexate than the dose used in autoimmune diseases and its combination with low-dose steroids.52 A combination of azathioprine with prednisolone was not found to be superior over prednisolone alone.53 Further, incidence of anaemia was increased by a combination of azathioprine and dapsone.
Successful use of infliximab as a therapy for RR was reported recently in a patient with steroid-dependent neuritis.54 A dramatic and lasting remission was noted after three infliximab infusions at week 0, 1 and 6. However, it must be noted that infliximab has been implicated in causing neuritis and demyelination, and should be used with utmost caution in a patient with nerve function impairment. In cases of recalcitrant neuritis or nerve abscess, surgical decompression may be warranted, based on the physician’s discretion.55
Type II reactionsFigure 5 summarises the management of T2R. Rest and non-steroidal anti-inflammatory agents are the cornerstone therapy for mild ENL. Use of corticosteroids is recommended by WHO guidelines for severe ENL56; however, it is associated with several adverse effects, which may be minimised by splitting the dose of oral corticosteroids.57 Thalidomide is an effective and rapidly acting therapy for severe ENL that acts by inhibition of TNF-α. Its use is restricted by potential teratogenicity, neuropathy and limited availability. A recent real-world study demonstrated the low-dose thalidomide (25–150 mg/day) to be as efficacious as the high-dose regimen.58 Clofazimine is another important agent for the management of chronic ENL; however, the slow onset of action limits its use in cases of severe/acute ENL. Two pilot studies from Ethiopia analysed the role of cyclosporine in the management of ENL.49 Cyclosporine demonstrated potential benefits in acute ENL, reducing severity and prednisolone requirement, but showed less efficacy in chronic ENL, leading to earlier and more severe flare-ups requiring higher prednisolone doses. There is an unmet need for safe and effective therapies for the management of ENL.
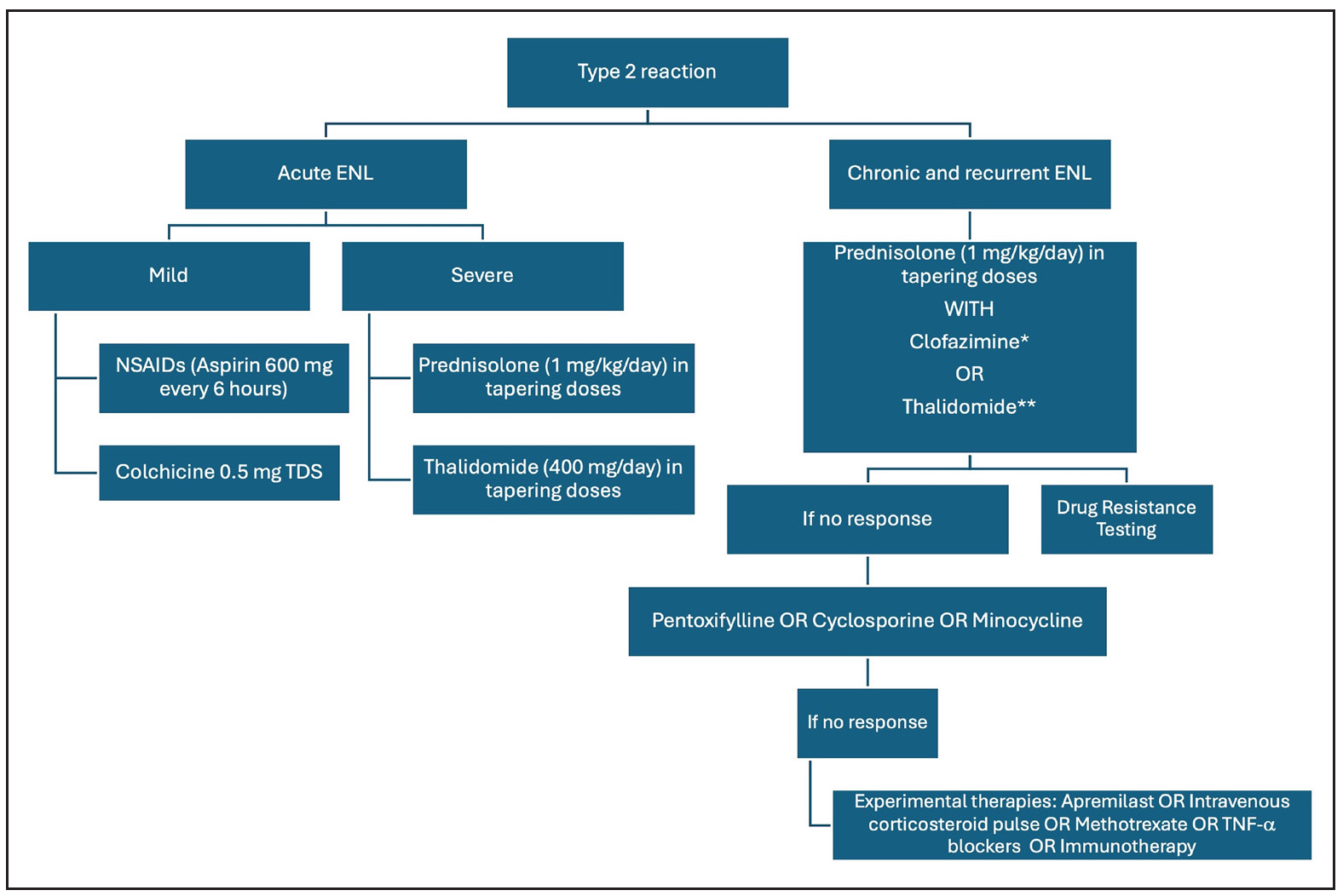
Export to PPT
Several therapies show promise in ENL, including methotrexate, apremilast, minocycline, colchicine and TNF-α inhibitors. A review of 21 patients treated with methotrexate (7.5–20 mg) found it safe and effective for leprosy reactions when combined with low-dose corticosteroids.59 Its role in ENL was highlighted in a recent trial, though the lack of controlled studies makes standard dosage protocols difficult.52 It is particularly useful for patients with steroid contraindications like diabetes. An ongoing trial (MaPs in ENL) is assessing methotrexate’s efficacy in ENL.60 However, careful patient selection is needed due to potential overlap with dapsone induced side effects and methotrexate side effects like anaemia and liver issues.61
In a case series of six ENL patients, etanercept combined with corticosteroids reduced steroid dosage by 42%.62 A systematic review found four reports of ENL successfully treated with infliximab or etanercept,63 but also noted ten cases of leprosy following TNF-α inhibitor use, suggesting a risk of leprosy infection or reactivation of subclinical infection.
留言 (0)