Dear Editor,
Actinic Granuloma (AG) is a rare, noninfectious granulomatous disorder primarily affecting skin exposed to sunlight. It is often compared to Granuloma Annulare (GA) and is thought to be triggered by solar-damaged elastic fibers. First described by O’Brien in 1975, AG is characterised by granulomatous inflammation resulting from ultraviolet radiation-induced alterations in elastic fibers. This condition predominantly affects the skin of middle-aged individuals. Although the exact pathogenesis of AG remains unclear, current theories suggest that UV exposure may initiate a cellular immune response to altered antigenic determinants on these fibers.1 We present a case of intractable AG, whose symptoms significantly improved with treatment using the JAK1/2 inhibitor baricitinib.
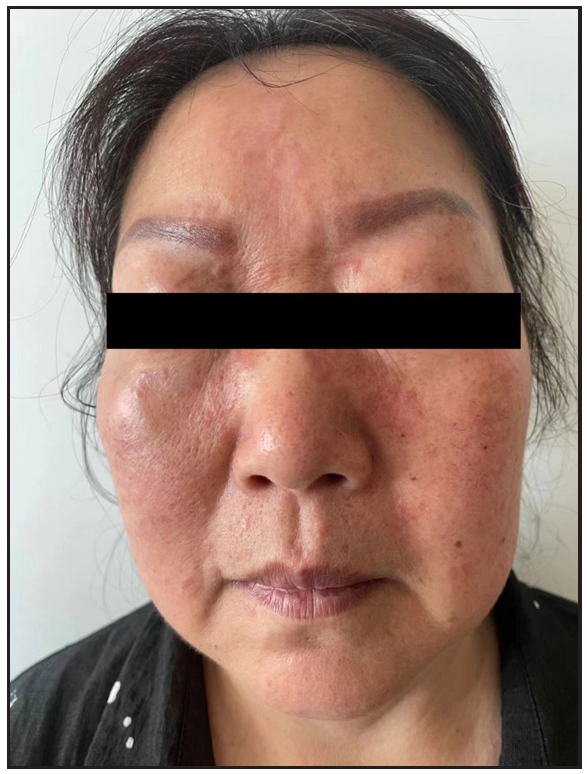
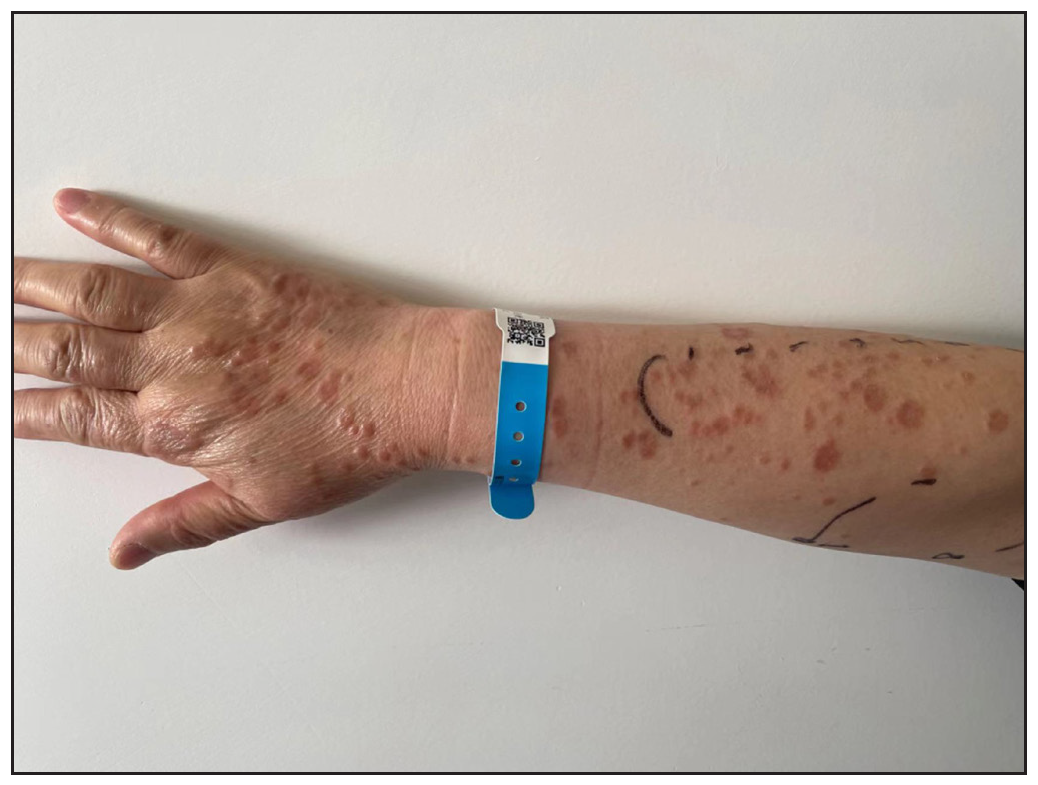
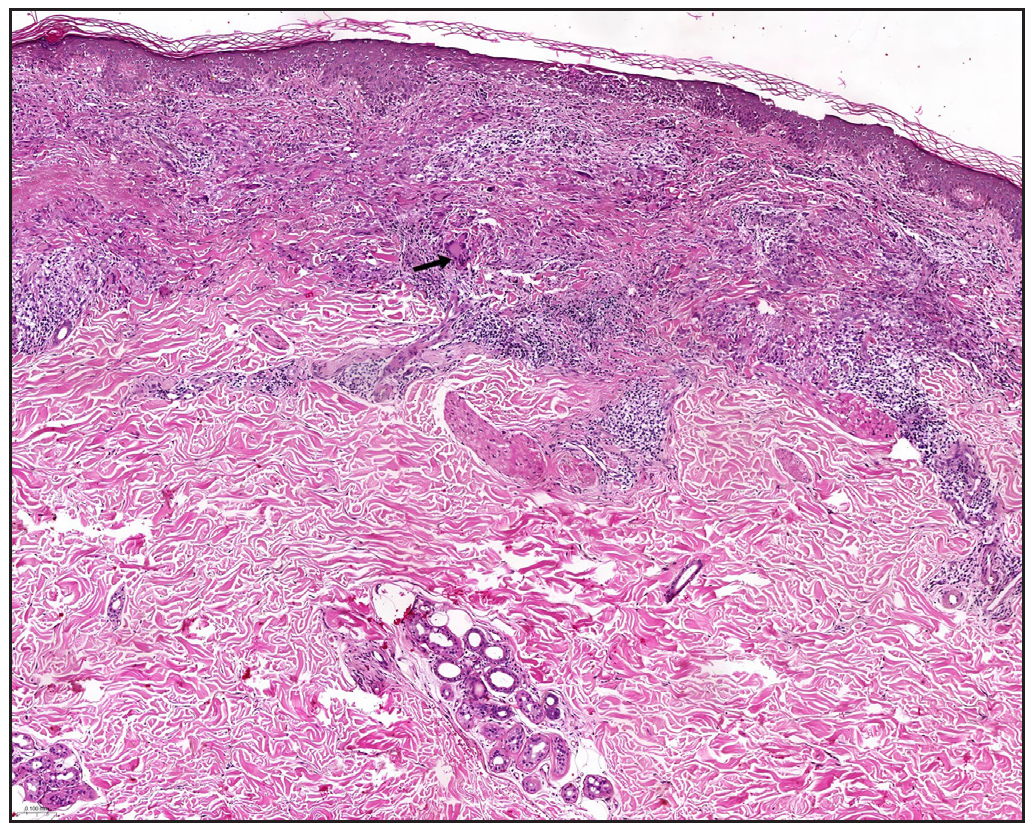
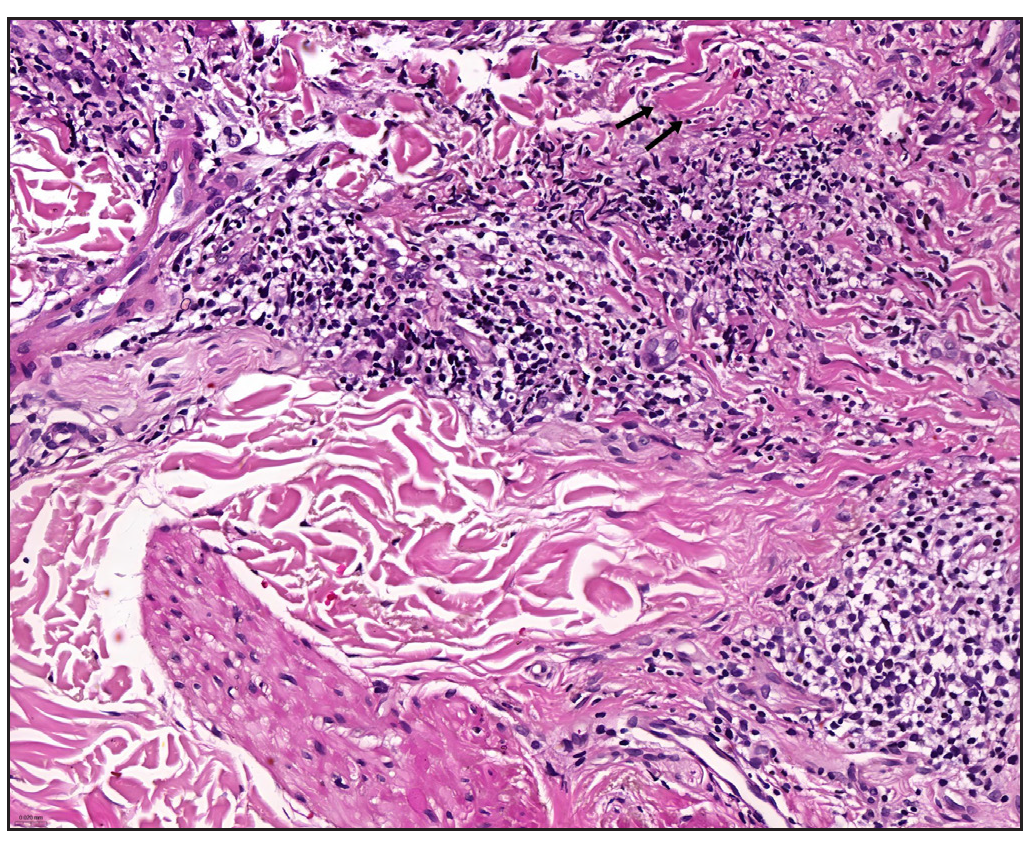
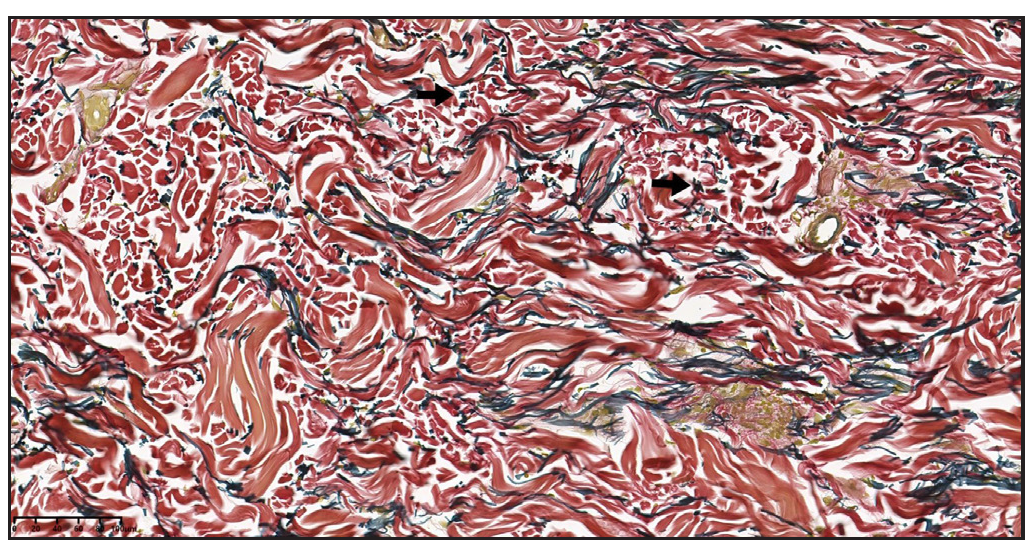
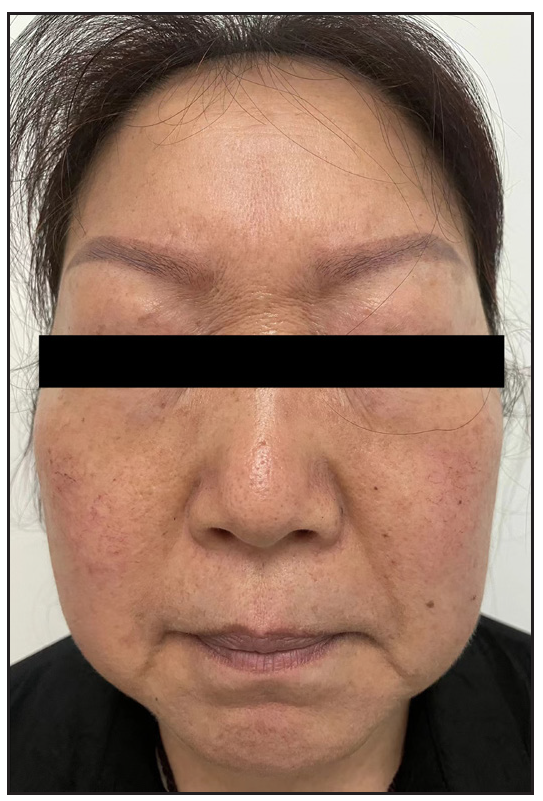
In 2023, a 57-year-old female was admitted due to a persistent two-year history of chronic erythematous and pruritic annular plaques localised on her face, neck, and upper extremities [Figures 1a-1b]. She was initially diagnosed with annular granuloma based on a histopathological evaluation from an external facility. However, treatment attempts with oral hydroxychloroquine and topical halometasone were minimally effective. Her clinical profile was noted for sun-induced dermatological disorders, with symptoms exacerbated by UV exposure. A subsequent dermatopathological examination at our centre reaffirmed the diagnosis of actinic granuloma [Figures 2a–2c]. Based on her clinical presentation and histopathological evidence, she was definitively diagnosed with AG. Although initial treatments with oral prednisolone showed limited efficacy, transitioning to baricitinib (2 mg/day) resulted in almost complete resolution of skin lesions within six weeks [Figures 3a-3b]. During a one-year follow-up, no new lesions appeared, and no adverse reactions were observed.
Export to PPT
Export to PPT
Export to PPT
Export to PPT
Export to PPT
Export to PPT
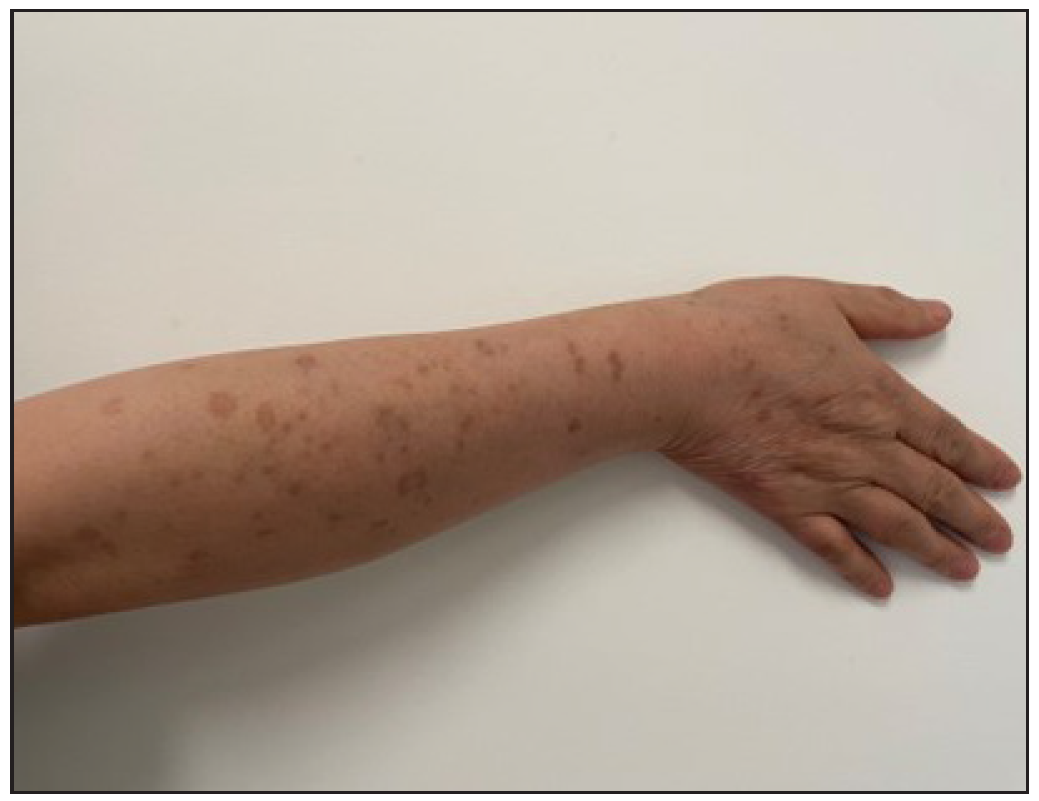
Export to PPT
Actinic Granuloma (AG) tends to preferentially affect females, with epidemiological data indicating a female-to-male incidence ratio ranging from 1.2:1 to 2:1. The pathogenesis of AG remains unclear, but studies suggest that AG is a cell-mediated autoimmune response to sun-damaged elastic fibres, primarily mediated by CD4+ cells. Interferon-γ (IFN-γ) and tumour necrosis factor (TNF-α) play critical roles in this process, inducing granulomatous inflammation. Additionally, macrophages in AG express matrix metalloproteinase-12 (MMP-12), which degrades elastic fibres and is regulated by the Th-2 cytokine IL-4 in macrophages.2 Management of AG involves traditional methods such as topical and intralesional corticosteroids, psoralen plus ultraviolet A (PUVA) therapy, antimalarials, cyclosporine, methotrexate, and cryotherapy, as well as advanced laser treatments, including Pulsed Dye Laser (PDL) and fractionated CO2 laser. Additionally, the introduction of biologic agents represents a significant advancement in the treatment of granulomatous disorders. Greb, Jacqueline et al. documented the first use of adalimumab in treating AG, achieving clinical clearance with a regimen of 40 mg administered every two weeks.3 JAK inhibitors are an emerging molecular-targeted therapy for cutaneous granulomatous diseases. They work by blocking T-cell-mediated inflammation and inhibiting the JAK/STAT pathway, thereby reducing the effects of multiple cytokines and treating inflammatory diseases. McPhie et al. observed two refractory annular granuloma (GA) patients treated with tofacitinib (5 mg BID), where one patient showed rapid lesion reduction within one hour and sustained improvement after four weeks, while the other experienced nearly complete lesion resolution after nine months.4 Xiaoyuan Hou et al. treated a case of refractory GA with oral baricitinib (2 mg/day), resulting in significant rash resolution after one month and complete clearance after three months, with no new rashes observed during a six-month follow-up period.5 Similarly, Kim et al. reported the successful treatment of two patients with refractory generalised granuloma annulare using baricitinib, achieving rapid improvement.6 Baricitinib, a small molecule oral JAK inhibitor, has been shown to treat various inflammatory diseases by inhibiting the JAK-STAT signalling pathway. Bronte Vincenzo et al. found that baricitinib also reduces serum levels of TNF-α, IL-4, and IL-13 in COVID-19 patients, thereby modulating the immune environment and preventing severe disease progression. Moreover, baricitinib blocks the secretion of multiple cytokines, such as IL-4, IL-13, IL-31, TSLP, TNF-, and IFN-γ, through the MAPK, mTOR, and PI3K-Akt signalling pathways, thereby inhibiting inflammatory responses. AG is closely related to these cytokines and inflammatory pathways.7 Based on these findings, we opted to use baricitinib as our treatment method. After six weeks of baricitinib treatment, the patient’s AG symptoms significantly improved, achieving complete resolution without any adverse events or relapse. This promising outcome highlights baricitinib’s potential in AG management and underscores the need for further clinical investigation.
留言 (0)