In the treatment of generalized myasthenia gravis (gMG), high-dose chronic oral steroid therapy has reduced the mortality and frequency of severe disease since the 1970s (1–3), but many gMG patients have still been living with poor health-related quality of life (QOL) due to an inability to sufficiently reduce oral steroid doses and unstable MG symptoms caused by inadequate therapeutic intervention (3–6). New treatment strategies (7–9) and targeted drugs (10–14) have recently been developed to further improve QOL for gMG patients. Given that the rate of full remission from MG remains low even today (1, 5, 7, 8), setting achievable and appropriate treatment goals to optimize QOL is important.
Treatment goals for MG often comprise components for both improvement status and burden due to treatment (7, 8, 15–19). International consensus guidance (18, 19) and Japanese clinical guidelines (7, 8, 16, 17) for MG together indicate a component of MG symptoms for the initial treatment goal as: achieving a state of minimal manifestations or better (MM-or-better) in the MG Foundation of America postintervention status (MGFA-PIS). In addition to achieving MM-or-better, the Japanese clinical guidelines for MG recommend reducing the oral prednisolone (PSL) dose to ≤5 mg/day (termed “MM-5 mg”) as soon as possible (8), whereas international consensus guidance for MG encourage goals of MM-or-better with no more than grade 1 Common Terminology Criteria for Adverse Events medication side effects (18, 19).
MM is defined as the status of no symptoms with functional limitations from MG, but some weakness on examination of some muscles (20). Ultimately, in patient-centered clinical settings it should be up to the patient to decide whether they have symptoms that interfere with a normal lifestyle, where MM do not necessarily mean a ‘MG activities of daily living scale (MG-ADL) (21) score of 0 point’. MM is a realistic and achievable treatment goal that can lead to good QOL for patients, given that complete stable remission (CSR) and pharmacologic remission (PR) are frequently difficult to achieve. However, the subjective nature of MM may lead to ambiguity in goal setting in clinical practice. To avoid such ambiguity, employing some objective reference values for MM-or-better may prove helpful. Regarding endpoints in rigorous clinical trials, criteria for MGFA-PIS status have been suggested to require definition in each study protocol based on quantitative assessments (20). Demonstrating quantitative criteria for MM could therefore be useful for determining MM in the context of rigorous clinical trials and also as supplementary information in clinical settings.
In this study, scores from several MG severity scales such as the MG-ADL (21), quantitative myasthenia gravis score (QMG) (22), and myasthenia gravis composite scale (MGC) (23) from 2784 patients with gMG were analyzed to determine significant cutoff values for MM-or-better and to provide objective criteria for evaluating the clinical severity of MM-or-better. Furthermore, using these cutoffs, we classified patients assigned subjectively as MM-or-better into strict MM-or-better (below a cutoff) or optimistic MM-or-better (above a cutoff) groups, compared clinical characteristics between the groups and reported their implications in rigorous trials and clinical settings.
2 Materials and methods2.1 PatientsOur cross-sectional multi-center surveys were conducted in 2010, 2012, 2015, and 2021 at 20 neurological centers in total [Japan MG registry (JAMG-R) study]. In each of these surveys, consecutive and established MG patients with various stages of illness over a short duration (4 months) were all enrolled to avoid potential bias. Our study was based on accurate reports by motivated neurologists (not on reports from patients), with many detailed data all quantified and entered on special case cards created in Claris FileMaker Pro® (Claris International Inc., California, The United States), which were then directly expanded and integrated into a large Excel® file (Microsoft Corporation, Washington, The United States) and used as the source of the database for the analyses. Input errors and missing values were corrected or re-surveyed through discussion between the secretariat (Y.N.) and the neurologist in charge. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The ethics committees of each participating institution approved the study protocols. Written informed consent was obtained from all patients enrolled in the study.
Clinical information obtained routinely in our surveys included: sex, current age, age at onset, duration of disease, duration from onset to start of immunotherapy administration, presence of bulbar symptoms, history of MG crisis, presence of AChR-Ab or MuSK-Ab, history of thymectomy and thymic histology, current dose and maximum dose of PSL, use of non-steroid oral immunosuppressants such as calcineurin inhibitors (CNIs), plasmapheresis, and/or intravenous immunoglobulin (IVIg), MGFA classification at worst condition of the disease, current clinical status according to MGFA-PIS, severity scores at current and worst condition, body mass index (BMI), and QOL of patients (15-item myasthenia gravis quality of life scale [MG-QOL15]). Clinical severity was determined according to the MG-ADL, QMG, and MGC. Due to the coronavirus disease 2019 (COVID-19) pandemic, use of a spirometer was avoided, and QMG was not evaluated in the 2021 survey. Regarding QMG, only data obtained in 2010 (1st survey), 2012 (2nd survey) and 2015 (3rd survey) were available.
Data from 3800 patients in total were collected from a series of four surveys in 2010, 2012, 2015, and 2021 (Figure 1). Of those, 267 were excluded due to missing data from the MGFA-PIS, MG-ADL, QMG, and/or MGC. In total, data from 3533 patients, representing a real number of 2486 patients (428 patients enrolled in two surveys, 143 patients in three surveys, and 111 patients in all four surveys), were analyzed (287 patients from the 2010 survey (3, 6), 640 patients from the 2012 survey (24), 923 patients from the 2015 survey (9), and 1683 from the 2021 survey). Of these 3533 patient data, 749 patients with ocular MG were excluded, given that levels of severity scores corresponding to MM-or-better naturally differ between patients with ocular MG and gMG. The remaining 2784 patients with gMG were divided into two groups: 1432 patients with MM-or-better status (achieving CSR, PR, or MM); and 1352 patients with I-or-worse status [not achieving MM or better status; status of improved (I), unchanged (U), worse (W), or exacerbation (E) in MGFA-PIS] (20).
Figure 1. CONSORT diagram of study participants; I, improved status; MM, minimal manifestations; MG, myasthenia gravis.
Diagnosis of MG was performed according to the diagnostic criteria set forth in the 2014 Japanese clinical guideline for MG (8, 16): In brief, the diagnosis was based on clinical findings (fluctuating muscle symptoms with easy fatigability and recovery after rest) and the presence of antibodies against skeletal muscle acetylcholine receptor (AChR-Ab) or muscle-specific tyrosine kinase (MuSK-Ab), or, when neither AChR-Ab nor MuSK-Ab was detected, on clinical findings and clinical improvement after administration of anticholinesterase, decremental muscle responses to a 3-Hz train of repetitive nerve stimuli (25) and/or an eyelid ice pack test with thorough exclusion of other diseases. A therapeutic diagnosis by response to plasma exchange was considered to confirm the diagnosis. Single-fiber electromyography was not performed systematically. AChR-Ab were measured by radioimmunoassay, using 125I-α-bungarotoxin ≥ 0.3 nmol/L considered positive (5) and MuSK-Ab were measured via radioimmunoprecipitation assay, with a value ≥ 0.02 nmol/L considered positive (26). In Japanese clinical guidelines, low-density lipoprotein receptor-related protein (LRP) 4 antibody-positivity using current assay systems are not considered diagnostic findings due to reasons including a lack of disease specificity (17), and therefore are not measured systematically but are only measured in antibody-negative cases as a reference finding.
The MG-QOL15 is a 15-item patient-administered questionnaire, with each item scored from 0 to 4 points, for a maximum total score of 60 points (27). The revised MG-QOL15 (MG-QOL15r) is a simplified version of the MG-QOL15, with very similar questions that have a 0–2 points distribution and a maximum total score of 30 points (28). As both the original and revised MG-QOL15 were taken as measures of QOL at different time points in our survey, a conversion value of total MG-QOL15 score/60 or total MG-QOL15r score/30 was used to create a corrected MG-QOL15 (cMG-QOL15) for analysis.
2.2 Strict and optimistic MM-or-betterClinically assigned MM-or-better patients (n = 1432) were classified into two groups using the individual cutoff values for three severity metrics (MG-ADL, QMG, and MGC): the strict MM-or-better group and the optimistic MM-or-better group, with severities below or above the cutoff level. Clinical characteristics were compared between strict and optimistic MM-or-better groups on each scale. Due to the COVID-19 pandemic, use of a spirometer was avoided, and QMG score was not evaluated in the 2021 survey. Therefore, regarding the strict/optimistic MM-or-better group on QMG score, data for a total of 745 patients from the 2010, 2012, and 2015 surveys were analyzed.
2.3 Statistical analysisComparisons between two groups were performed using the Mann–Whitney U test for continuous variables. Categorical variables were analyzed using Fisher’s exact test to compare the two groups, and the chi-square test to compare the three or more groups. Multiple comparisons for analyses of outcome measures were corrected using Bonferroni correction, in which the level of statistical significance is divided by the trial number of univariate analyses between the two groups, resulting in p-values < .002 on Table 1 as statistical significance, <.004 on Table 2, and <.003 on Table 3. Receiver operating characteristic (ROC) curves were used to determine the ideal cutoff values for the MG-ADL, QMG, and MGC. The cutoff point on the ROC curve statistically corresponded to the point at which sensitivity - (1 - specificity) is maximal. This point was the maximum Youden index, which was used as the optimality criterion in cutoff point selection. Sensitivity and specificity values according to the optimal cutoff attributed to the Youden index were identified. The area under the ROC curve (AUC), which measures the ability of a binary classifier to distinguish between groups, was determined to evaluate the discrimination ability of the cutoff points. AUC with 95% confidence interval (CI) was reported. JMP Pro 15 statistical software® (SAS Institute Inc., North Carolina, The United States) was employed for the analysis, and a value of p <.05 was considered to indicate statistical significance. For CIs, the lower and upper limits were analyzed from bias-corrected confidence limits based on the bootstrap method at the level of p <.05.
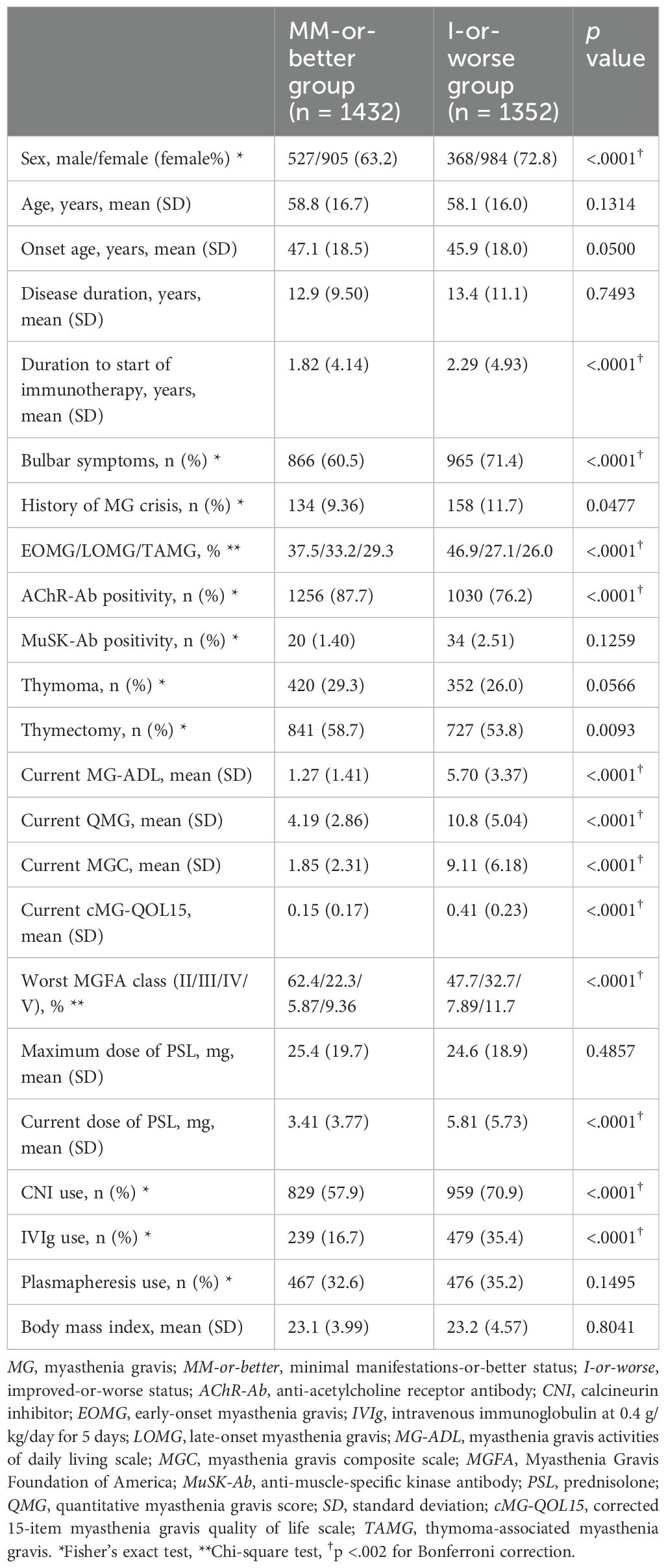
Table 1. Comparison of demographic data between MM-or-better and I-or-worse groups (Mann–Whitney U-test).
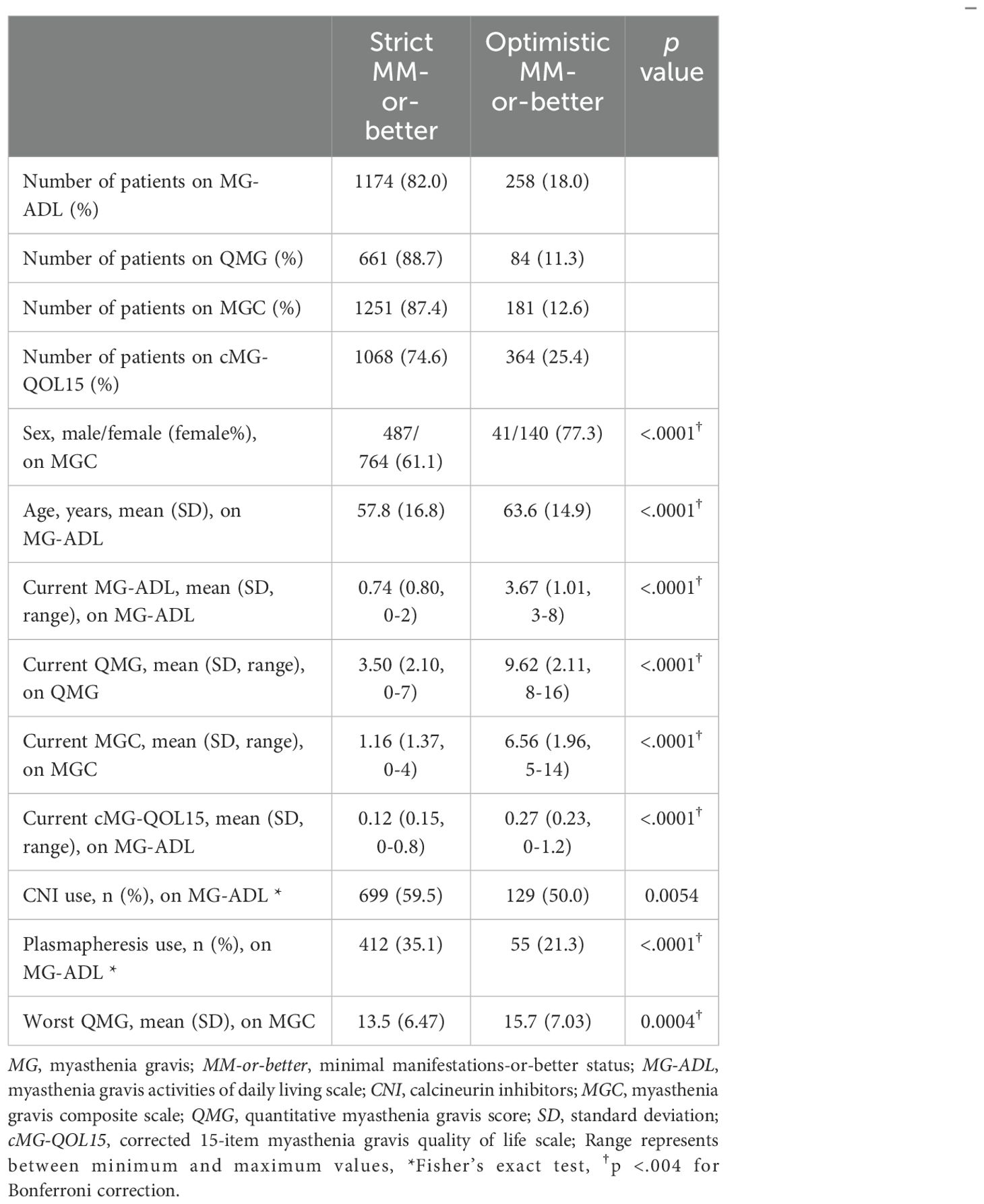
Table 2. Differences between strict MM-or-better and optimistic MM-or-better groups on MG severity scales.
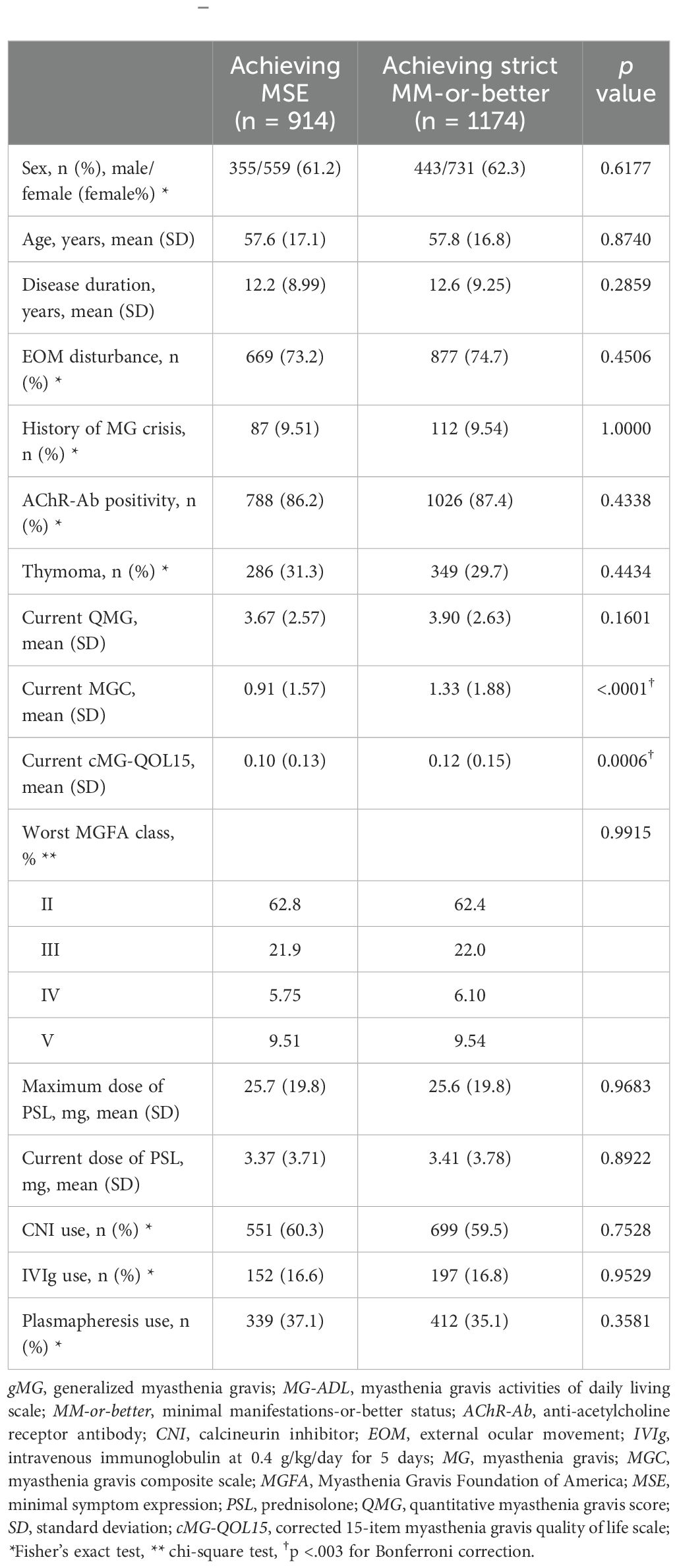
Table 3. Comparison between gMG patients achieving minimal symptom expression group and achieving strict MM-or-better on the MG-ADL scale (Mann–Whitney U-test).
3 Results3.1 MM-or-better cutoff values based on each severity rating scaleComparison of demographic data between MM-or-better and I-or-worse groups showed that the MM-or-better group had a significantly lower frequency of women, a shorter time to initiation of immunotherapy, and a higher rate of positivity for AChR-Ab (Table 1). Naturally, current MG-ADL, QMG, MGC, and cMG-QOL15 were significantly lower in the MM-or-better group than in the I-or-worse group. Regarding treatment in the MM-or-better group, current PSL dose and frequency of CNI use were lower, maximum dose of PSL and frequency of plasmapheresis did not differ significantly, and frequency of IVIg use was lower when compared to the I-or-worse group.
To analyze cutoff points between MM-or-better and I-or-worse groups on MG scores, ROC curves were drawn for MG-ADL, QMG, MGC, and cMG-QOL15, respectively. (Figures 2A–D). The AUC for MG-ADL, QMG, MGC and cMG-QOL15 were 0.912, 0.888, 0.906 and 0.832 respectively (Table 4). Based on the ROC curve, cutoff values were analyzed as follows: for MG-ADL, a cutoff of 2 points offered 82.0% sensitivity and 85.0% specificity; for QMG, a cutoff of 7 points offered 88.7% sensitivity and 70.0% specificity; for MGC, a cutoff of 4 points offered 87.4% sensitivity and 77.9% specificity; for cMG-QOL15, a cutoff of 0.2 points offered 74.6% sensitivity and 77.8% specificity (Table 4).
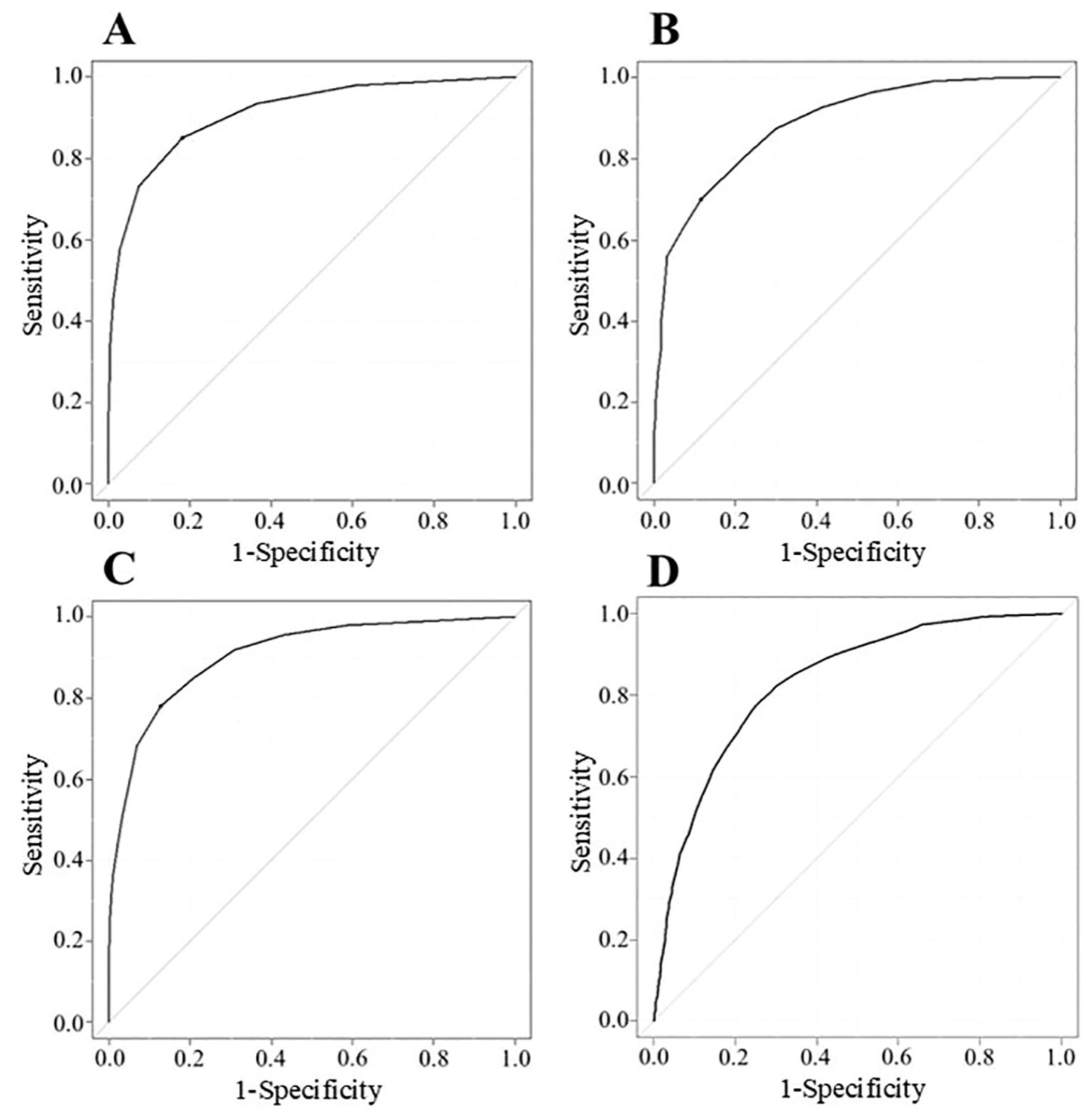
Figure 2. ROC curve described with data of MG-ADL (n = 2784) (A), QMG (n = 1473) (B), MGC (n = 2784) (C), and cMG-QOL15 (n = 2784) (D). Vertical axis shows sensitivity (true positive); horizontal axis shows 1-specificity (false positive). Cutoff points correspond to the point on the ROC curve where sensitivity - (1 - specificity) is maximal; cMG-QOL15, corrected 15-item myasthenia gravis quality of life scale; MG-ADL, myasthenia gravis activities of daily living scale; MGC, myasthenia gravis composite scale; QMG, quantitative myasthenia gravis score; ROC, receiver operating characteristic.
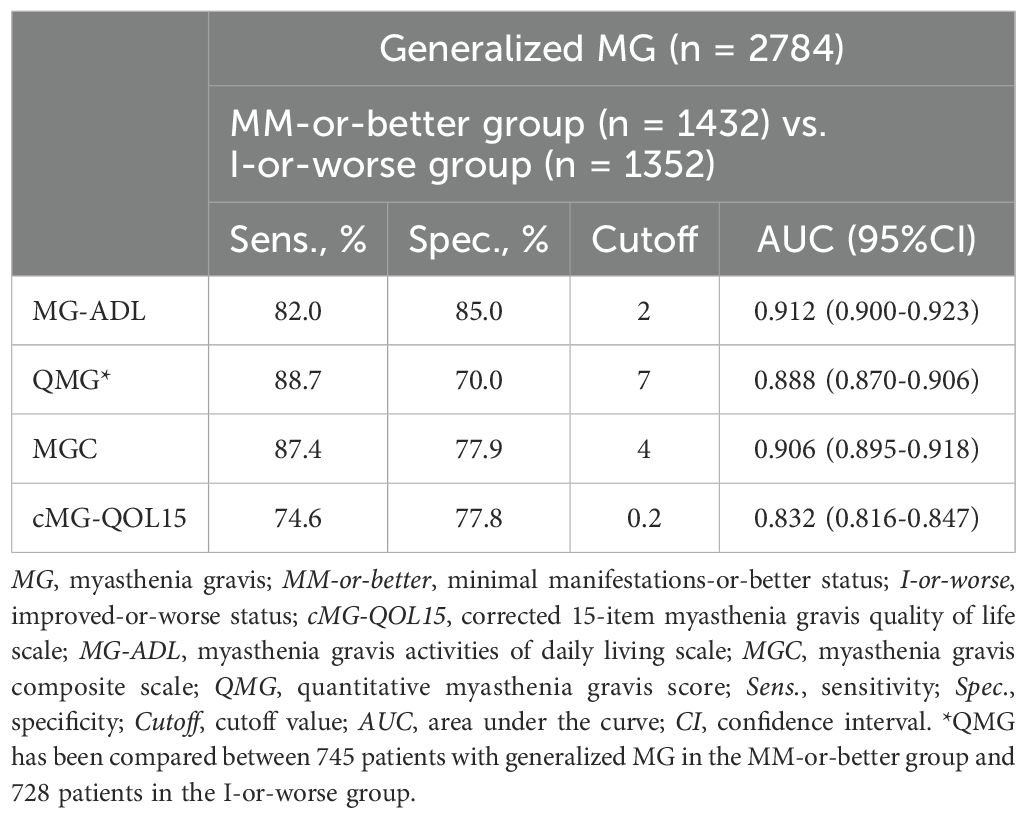
Table 4. Cutoff values, and sensitivity and specificity of MG severity metrics between MM-or-better and I-or-worse groups.
To examine the impact of overlapping data, we created another dataset (n=1936) by deleting the older data from duplicate patients (first data for double duplicates, first and second data for triple duplicates, and first, second and third data for quadruple duplicates), and analyzed it. The analyzed data corresponding to Tables 1, 4 are shown in Supplementary Tables 2, 3, which demonstrated almost the identical results between 2784 (Tables 1, 4) and 1936 (Supplementary Tables 2, 3) patients.
All three scale cutoffs were achieved in 622 (42.5%) of the 1462 patients with gMG from the 1st survey to the 3rd survey (patients within cutoffs of both MG-ADL and QMG, n = 695 [47.5%]; QMG and MGC, n = 725 [49.6%]; MG-ADL and MGC, n = 673 [46.0%]).
3.2 Difference between strict MM-or-better and optimistic MM-or-betterCommon characteristics of the optimistic MM-or-better group across all cutoffs naturally included significantly higher current MG-ADL, current QMG, and current MGC compared with the strict MM-or-better group (Table 2). Specifically, current cMG-QOL15, which reflects patient QOL, was significantly higher (worse QOL) in the optimistic MM-or-better group than in the strict MM-or-better group when differentiated with severity scores (Figure 3, p <.0001; with MG-ADL cutoff, 0.12 ± 0.15 vs. 0.27 ± 0.23; with QMG cutoff, 0.11 ± 0.13 vs. 0.18 ± 0.13; with MGC cutoff, 0.13 ± 0.16 vs. 0.28 ± 0.22). In addition, optimistic MM-or-better patients based on MG-ADL cutoff were less frequently treated with CNIs and plasmapheresis and included a higher proportion of elderly patients, compared to strict MM-or-better patients (Table 2; Supplementary Table 4). Optimistic MM-or-better patients based on the QMG cutoff showed a significantly higher frequency of female patients, a higher proportion of MGFA III and V at worst condition, and higher QMG score at worst condition (Supplementary Table 5). Optimistic MM-or-better patients based on the MGC cutoff showed a higher proportion of females, a higher proportion of elderly patients, and higher QMG at worst condition (Table 2; Supplementary Table 6).
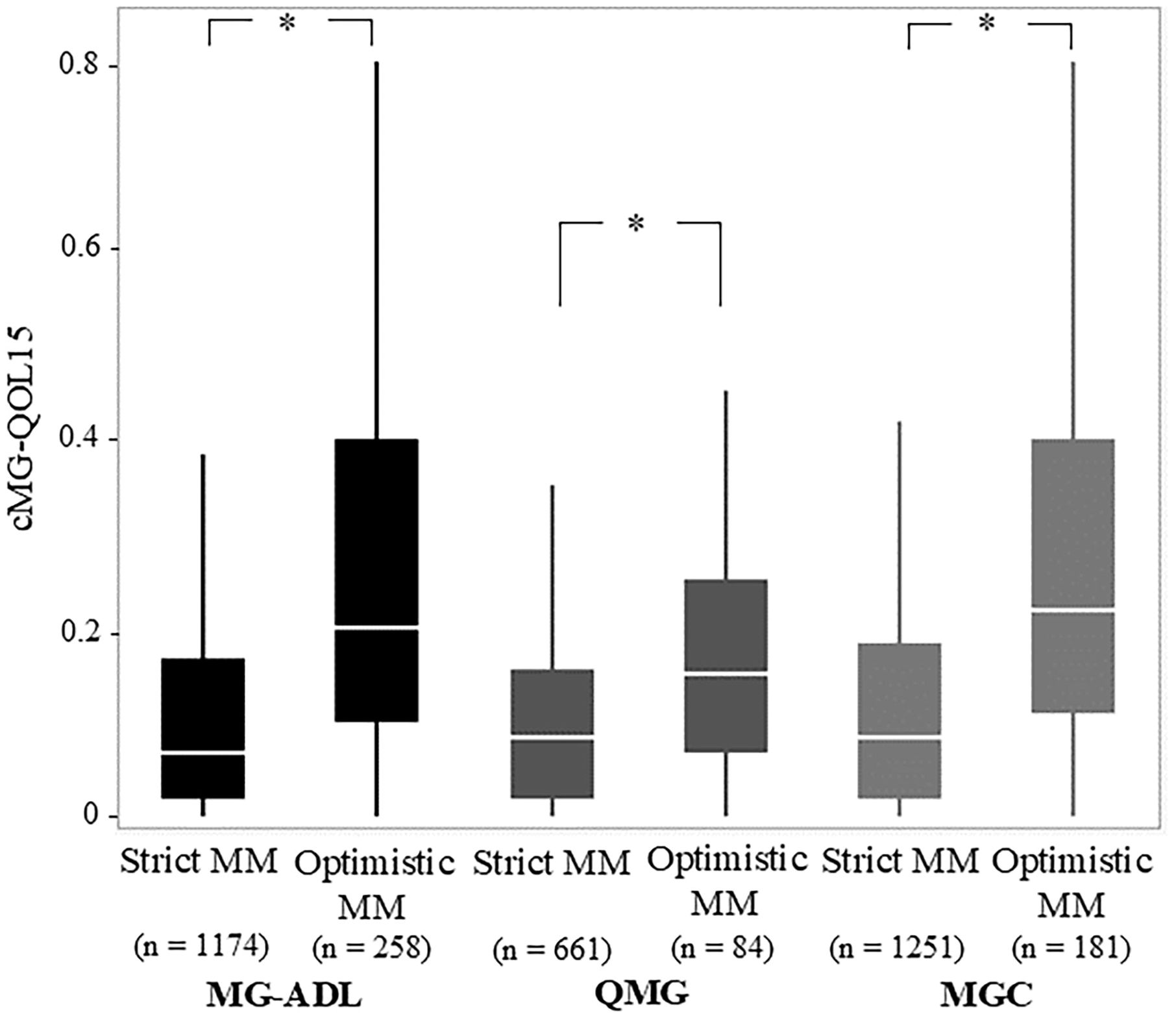
Figure 3. Comparison of Strict and Optimistic MM-or-better with cutoffs on MG-ADL, QMG, and MGC regarding corrected 15-items myasthenia gravis quality of life scale (cMG-QOL15). MG-ADL, myasthenia gravis activities of daily living scale; MGC, myasthenia gravis composite scale; MM, minimal manifestations; QMG, quantitative myasthenia gravis score; Mann–Whitney U-test; *p <.0001.
3.3 Comparisons between patients with strict MM-or-better on the MG-ADL Scale and minimal symptom expressionAmong patients with gMG clinically assigned as strict MM-or-better (n = 1174), 914 patients achieved minimal symptom expression (MSE), defined as scoring 0–1 points on the MG-ADL scale (29). Differences between the group achieving MSE (MG-ADL 0–1) and the group achieving strict MM-or-better (MG-ADL 0–2) on the MG-ADL scale are shown in Table 3. Mean MGC were significantly lower in the MSE group than in the strict MM-or-better group, whereas mean QMG did not differ significantly between groups and actual differences in cMG-QOL15 were small (0.10 ± 0.13 vs. 0.12 ± 0.15, respectively) regardless of significance, due to the large sample size.
4 DiscussionThe present study analyzed data from 2784 gMG patients in MM-or-better (n = 1432) and I-or-worse (n = 1352) groups, and demonstrated cutoff values for MM-or-better on individual severity scales, as follows: ≤ 2 points on MG-ADL; ≤ 7 points on QMG; and ≤ 4 points on MGC. On the other hand, in actuality, some patients clinically assigned as MM-or-better showed severity scores above these cutoffs (258/1432, 18.0% for the MG-ADL cutoff; 84/745, 11.3% for the QMG cutoff; and 181/1432, 12.6% for the MGC cutoff representing optimistic MM-or-better patients). Such MM-or-better patients appeared to be more frequent for the MG-ADL cutoff than for the QMG or MGC cutoffs. Considering the merits of patient-driven severity evaluation, the MG-ADL has often been employed as a primary endpoint in recent clinical trials for new therapies against gMG as well as in clinical settings (10–14). Since MG-ADL has no physician-driven evaluation item (27), use of cutoffs from QMG and MGC scores may be helpful as objective reference values in determining MM.
We proposed the idea of “strict” MM-or-better (within a severity cutoff) or “optimistic” MM-or-better (above the severity cutoff). In rigorous clinical trials, employing strict MM-or-better as a goal or endpoint may be more suitable to avoid ambiguity in determining MM. On the other hand, in clinical settings, optimistic MM-or-better should not be ignored as a means of attaching importance to the perspective of the patient. However, when assigning a patient to optimistic MM-or-better, re-evaluating the judgement of MM using the present cutoff values while taking into account the following issues may be better. In the present study, optimistic MM-or-better patients showed characteristics such as older age, higher frequency of females, and more severe disease in worst condition than strict MM-or-better patients (Table 2). Levels of MG symptoms considered as incurring ‘no limitation to a normal lifestyle’ by the individual patient may display a wide upper limit among elderly and/or female patients with experience of severe disease. Such patients may misunderstand that marked improvement in MG symptoms compared with the previous severe condition is a status of non-interference with daily life (i.e., MM-or-better), even if they still experience residual symptoms affecting good daily life. In their daily lives, the amount of activity might have already been limited in order to adapt to the symptoms. Furthermore, in this study, optimistic MM-or-better patients on the MG-ADL scale were less frequently treated with plasmapheresis and/or CNIs (Table 2), suggesting the possibility that they might have not received sufficient treatment.
Some goal statuses of MG treatment reflected in MG-specific severity metrics have been reported, such as patient-acceptable symptom state (PASS), as well as MSE (30). The PASS index represents a state in which the patient feels they are adequately well (31, 32). Cutoff values for PASS were demonstrated to be MG-ADL ≤ 2, QMG ≤ 7, MGC ≤ 3, and MG-QOL15 ≤ 8 (31). Thus, the cutoff values for strict MM-or-better shown for Japanese gMG patients (MG-ADL scale ≤ 2, QMG score ≤ 7, and MGC ≤ 4) were largely in line with those for PASS. Furthermore, it is reported that all (100%) of PASS positive patients had been simultaneously evaluated as MM-or-better (remission or MM) (31). The levels of MG symptoms at which the individual patient feels a status of non-interference with daily life may be universal on these severity metrics, regardless of ethnicity or geographic region, such as in Canada and Japan (31, 32). Strict MM-or-better and PASS can thus be considered a much practical treatment goal or endpoint as the severity component.
Recently, achieving MSE (defined as 0–1 points on the MG-ADL scale or a MG-QOL15r of 0–3) is often employed as a secondary outcome parameter in clinical trials (29). Regarding the MG-ADL scale, two items for ocular symptoms, in which ptosis and diplopia are each rated as 1 point even when not occurring every day, are difficult to discriminate between patients with and without MG symptoms interfering with daily life (21, 33, 34). These include infrequent modest ocular symptoms, which may not have an impact on QOL (33, 34). In addition, particularly among elderly patients, the effects of age- and/or comorbidity-related fatigue and frailty can affect MG-ADL scoring to some extent, even when the symptoms are unrelated to MG (35–38). The cutoff of ≤ 1 point on the MG-ADL scale for MSE may be too strict for use as a treatment goal at least in clinical settings, and in actuality, the achievement rate has not been high in clinical trials for new drugs (e.g. 14% for the zilucoplan group in RAISE study and 21.4% for the eculizumab group in REGAIN study) (10, 29). In the present study, MGC in the patient group achieving MSE (MG-ADL scale ≤ 1) were naturally lower than those in patients achieving strict MM-or-better (MG-ADL scale ≤ 2). However, QMG did not differ significantly between groups, and regarding cMG-QOL15, little numerical difference was evident.
Limitations of this study include the retrospective, cross-sectional nature, and the lack of data related to QMG score in 2021. However, we accumulated exact severity score data for a large number of cases from four cross-sectional surveys conducted from 2010 to 2021. In each of those four surveys, consecutive MG patients over a short duration were enrolled to avoid potential bias, and examined and reported by motivated neurologists. The present sample is probably one of the largest to date. Among the total of four surveys conducted, some patient overlap was seen. However, even if the present database included some data from the same patients, this had little impact on our analysis (Supplementary Tables 2, 3). The present findings do not necessarily mean that strict MM-or-better is a better category than PASS or MSE as a treatment goal, but we believe that the results are meaningful and useful for determining MM-or-better. In this study, data were collected from Japanese MG patients. However, the cutoff values for strict MM-or-better in the present study were largely in line with those for PASS as reported from Canada (31). Large differences in the levels of acceptable symptoms for patients on MG-specific severity metrics do not seem to exist among regions or ethnicity.
In conclusion, we have demonstrated that cutoff values for strict MM-or-better on MG-specific severity metrics were MG-ADL ≤ 2, QMG ≤ 7, and MGC ≤ 4. However, we do not believe that these cutoffs should be strictly used to determine MM in clinical settings. MG treatment and efficacy evaluation should be patient-centered, and the cutoffs may thus be useful as reference values. To avoid ambiguity in determining MM-or-better in rigorous settings such as clinical trials, employing the ideas of strict MM-or-better as needed may prove helpful. Multicenter studies in various regions are warranted to investigate objective MM indicators that can be applied globally.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the ethics committee of Hanamaki General Hospital (Institutional Review Board No. R3-1). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsGW: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. YT: Supervision, Writing – review & editing. YN: Data curation, Formal analysis, Investigation, Writing – review & editing. TKu: Investigation, Writing – review & editing, Data curation. MY: Data curation, Investigation, Writing – review & editing. HA: Data curation, Investigation, Writing – review & editing. YO: Data curation, Investigation, Writing – review & editing. AU: Data curation, Investigation, Writing – review & editing. NK: Data curation, Investigation, Writing – review & editing. MM: Conceptualization, Data curation, Investigation, Writing – review & editing. SK: Data curation, Investigation, Writing – review & editing. IA: Data curation, Investigation, Writing – review & editing. NM: Data curation, Investigation, Writing – review & editing. TKi: Data curation, Investigation, Writing – review & editing. MS: Data curation, Investigation, Writing – review & editing. TS: Data curation, Investigation, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing. MT: Data curation, Investigation, Writing – review & editing. SS: Data curation, Investigation, Writing – review & editing. HM: Data curation, Investigation, Writing – review & editing. MA: Writing – review & editing. KU: Conceptualization, Data curation, Investigation, Project administration, Validation, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors are grateful for the support received from the Japan Myasthenia Gravis Registry study group.
Conflict of interestGW has received honoraria for lectures from Argenx. YN has received speaker honoraria from Argenx, Alexion Pharmaceuticals, Japan Blood Products Organization, Takeda Pharmaceutical Company Limited and UCB. TK reports honoraria for lectures from Argenx, Alexion Pharmaceuticals and UCB Pharma. AU has received honoraria from Alexion Pharmaceuticals, UCB, and Argenx. MM has served as a paid consultant for UCB Pharma and Alexion Pharmaceuticals, and has received speaker honoraria from Alexion Pharmaceuticals, Argenx Japan, UCB Japan, Asahi Kasei Pharma, Takeda Pharmaceutical, Japan Blood Products Organization and Chugai Pharmaceutical Co., Ltd. NM has received honoraria from Argenx. MT reports unrestricted research grants from Japan Blood Products Organization and Astellas Pharma outside the submitted work, and has served as a paid consultant for Alexion, Argenx, Hanall BioPharma, and UCB Pharma and received honoraria for lectures from Argenx, Alexion Pharmaceuticals, and UCB Pharma. SS has received personal fees from Alexion Pharmaceuticals, Argenx, and UCB Pharma, the Japan Blood Products Organization, and Asahi Kasei Medical. HM has served as a paid consultant for Alexion, Argenx, UCB Pharma and Roche, and has received speaker honoraria from the Japan Blood Products Organization and Chugai Pharmaceutical. KU has served as a paid consultant for UCB Pharma, Argenx, Janssen Pharma, Viela Bio, Chugai Pharma, Hanall BioPharma, Merck and Mitsubishi Tanabe Pharma, and has received speaker honoraria from Argenx, Alexion Pharmaceuticals, UCB Pharma and the Japan Blood Products Organization.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1502721/full#supplementary-material
AbbreviationsAChR-Ab, anti-acetylcholine receptor antibody; AUC, area under the curve; BMI, body mass index; CI, confidence interval; CNI, calcineurin inhibitor; cMG-QOL15, corrected 15-item myasthenia gravis quality of life scale; COVID-19, coronavirus disease 2019; CSR, complete stable remission; E, exacerbation; gMG, generalized myasthenia gravis; I, improved; IVIg, intravenous immunoglobulin; JAMG-R, Japan Myasthenia Gravis Registry Group; MG, myasthenia gravis; MG-ADL, myasthenia gravis activities of daily living scale; MGC, myasthenia gravis composite scale; MGFA-PIS, Myasthenia Gravis Foundation of America - postintervention status; MGII, Myasthenia Gravis Impairment Index; MG-QOL15, 15-item myasthenia gravis quality of life scale; MG-QOL15r, revised 15-item myasthenia gravis quality of life scale; MM, minimal manifestations; MSE, minimal symptom expression; MuSK-Ab, anti-muscle-specific kinase antibody; PR, pharmacologic remission; PSL, prednisolone; QMG, quantitative myasthenia gravis score; ROC, receiver operating characteristic; U, unchanged; W, worse.
References3. Masuda M, Utsugisawa K, Suzuki S, Nagane Y, Kabasawa C, Suzuki Y, et al. The MG-QOL15 Japanese version: validation and associations with clinical factors. Muscle Nerve. (2012) 46:166–73. doi: 10.1002/mus.23398
PubMed Abstract | Crossref Full Text | Google Scholar
4. Suzuki S, Masuda M, Uzawa A, Nagane Y, Konno S, Suzuki Y, et al. Japan MG registry: Chronological surveys over 10 years. Clin Exp Neuroimmunol. (2023) 14:5–12. doi: 10.1111./cen3.12731
Crossref Full Text | Google Scholar
5. Utsugisawa K, Suzuki S, Nagane Y, Masuda M, Murai H, Imai T, et al. Health-related quality of life and treatment targets in myasthenia gravis. Muscle Nerve. (2014) 50:493–500. doi: 10.1002/mus.24213
PubMed Abstract | Crossref Full Text | Google Scholar
6. Suzuki Y, Utsugisawa K, Suzuki S, Nagane Y, Masuda M, Kabasawa C, et al. Factors associated with depressive state in patients with myasthenia gravis: a multicenter cross-sectional study. BMJ Open. (2011) 1:e000313. doi: 10.1136/bmjopen-2011-000313
PubMed Abstract | Crossref Full Text | Google Scholar
7. Murai H, Utsugisawa K, Motomura M, Imai T, Uzawa A, Suzuki S. The Japanese clinical guidelines 2022 for myasthenia gravis and Lambert–Eaton myasthenic syndrome. Clin Exp Neuroimmunol. (2023) 14:19–27. doi: 10.1111/cen3.12739
Crossref Full Text | Google Scholar
8. Murai H, Utsugisawa K, Nagane Y, Suzuki S, Imai T, Motomura M. Rationale for the clinical guidelines for myasthenia gravis in Japan. Ann N Y Acad Sci. (2018) 1413:35–40. doi: 10.1111/nyas.13544
PubMed Abstract | Crossref Full Text | Google Scholar
9. Utsugisawa K, Nagane Y, Akaishi T, Suzuki Y, Imai T, Tsuda E, et al. Early fast-acting treatment strategy against generalized myasthenia gravis. Muscle Nerve. (2017) 55:794–801. doi: 10.1002/mus.25397
PubMed Abstract | Crossref Full Text | Google Scholar
10. Howard JF, Bresch S, Genge A, Hewamadduma C, Hinton J, Hussain Y, et al. Safety and efficacy of zilucoplan in patients with generalized myasthenia gravis (RAISE): a randomized, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. (2023) 22:395–406. doi: 10.1016/S1474-4422(23)00080-7
PubMed Abstract | Crossref Full Text | Google Scholar
11. Howard JF, Utsugisawa K, Benatar M, Murai H, Barohn R, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalized myasthenia gravis (REGAIN): a phase 3, randomized, double-blind, placebo-controlled, multicenter study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1
PubMed Abstract | Crossref Full Text | Google Scholar
12. Bril V, Drużdż A, Grosskreutz J, Habib AA, Mantegazza R, Sacconi S, et al. Safety and efficacy of rozanolixizumab in patients with generalized myasthenia gravis (MycarinG): a randomized, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. (2023) 22:383–94. doi: 10.1016/S1474-4422(23)00077-7
PubMed Abstract | Crossref Full Text | Google Scholar
13. Howard JF, Bril V, Vu T, Karam C, Peric S, Margania T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalized myasthenia gravis (ADAPT): a multicenter, randomized, placebo-controlled, phase 3 trial. Lancet Neurol. (2021) 20:526–36. doi: 10.1016/S1474-4422(21)00159-9
PubMed Abstract | Crossref Full Text | Google Scholar
14. Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. (2022) 1:EVIDoa2100066. doi: 10.1056/EVIDoa2100066
PubMed Abstract | Crossref Full Text | Google Scholar
15. Nagane Y, Murai H, Imai T, Yamamoto D, Tsuda E, Minami N, et al. Social disadvantages associated with myasthenia gravis and its treatment: a multicenter cross-sectional study. BMJ Open. (2017) 7:e013278. doi: 10.1136/bmjopen-2016-013278
PubMed Abstract | Crossref Full Text | Google Scholar
16. Japanese Committee of Clinical Guidelines for Myasthenia Gravis. Japanese Clinical Guidelines for Myasthenia Gravis 2014. Tokyo, Japan: Nankodo (2014). p. 140.
17. Japanese Committee of Clinical Guidelines for Myasthenia Gravis/Lambert–Eaton Myasthenic Syndrome. Japanese Clinical Guidelines for Myasthenia Gravis/Lambert–Eaton Myasthenic Syndrome 2022. Tokyo, Japan: Nankodo (2022). p. 193.
18. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gihus NE, Illa I, et al. International consensus guidance for the management of myasthenia gravis: executive summary. Neurology. (2016) 87:419–25. doi: 10.1212/WNL.0000000000002790
PubMed Abstract | Crossref Full Text | Google Scholar
19. Narayanaswami P, Sanders DB, Wolfe GI, Benatar M, Cea G, Evoli A, et al. International consensus guidance for the management of myasthenia gravis: 2020 update. Neurology. (2021) 96:114–22. doi: 10.1212/WNL.0000000000011124
PubMed Abstract | Crossref Full Text | Google Scholar
20. Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. (2000) 55:16–23. doi: 10.1212/wnl.55.1.16
PubMed Abstract | Crossref Full Text | Google Scholar
22. Barohn RJ, Mcintire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. (1998) 841:769–72. doi: 10.1111/j.1749-6632.1998.tb11015.x
PubMed Abstract | Crossref Full Text | Google Scholar
23. Burns TM, Conaway M, Sanders DB, MG Composite and MG-QOL15 Study Group. The MG Composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. (2010) 74:1434–40. doi: 10.1212/WNL.0b013e3181dc1b1e
PubMed Abstract | Crossref Full Text | Google Scholar
24. Murai H, Masuda M, Utsugisawa K, Nagane Y, Suzuki S, Imai T, et al. Clinical features and treatment status of adult myasthenia gravis in Japan. Clin Exp Neuroimmunol. (2014) 5:84–91. doi: 10.1111/cen3.12091
Crossref Full Text | Google Scholar
25. Nagane Y, Suzuki S, Suzuki N, Utsugisawa K. Factors associated with response to calcineurin inhibitors in myasthenia gravis. Muscle Nerve. (2010) 41:212–8. doi: 10.1002/mus.21462
留言 (0)