The apolipoprotein E (APOE) gene, located on chromosome 19q13.32, encodes the 34 KDa glycoprotein known as APOE, which is essential for the transportation and metabolism of lipoproteins (1). Differences at two mutation sites (rs429358 and rs7412) in the APOE gene give rise to three distinct alleles: ϵ2 (APOE2), ϵ3 (APOE3), and ϵ4 (APOE4) allele. The APOE3 is the most common allele, with APOE 2 and APOE4 are relatively less frequent variants. The alleles account for six genotypes: APOE ϵ2/ϵ2, ϵ3/ϵ3, ϵ4/ϵ4, ϵ2/ϵ3,ϵ2/ϵ4 andϵ3/ϵ4) (2).The APOE gene polymorphism is closely associated with Alzheimer’s disease (AD), the APOE ϵ2 allele is considered the most potent protective factor against AD (3, 4). APOE alleles are involved in the development and progression of various malignancies (5, 6), such as hepatocellular carcinoma (7), testicular cancer (8), pancreatic cancer (9), melanoma (10, 11), colorectal cancer (12) and breast cancers (13, 14). However, research on their role in lung adenocarcinoma (LUAD) remains limited.
The varying effects of different APOE genotypes on lipid metabolism highlight their potential impact on cardiovascular and cancer risks (15, 16).Studies show that individuals with the APOE ϵ2 allele demonstrate diminished receptor-binding activity, which results in lower total cholesterol (TC) levels and higher triglyceride (TG) concentrations. In contrast, APOE ϵ4 carriers have higher plasma TC levels compared to those with the common APOE ϵ3 carriers. According to a recent meta-analysis, those with the APOE ϵ4 allele have a 42% higher risk of developing coronary heart disease (CHD) than people with the common APOE ϵ3 allele (2). This association may be due to the altered expression levels of low-density lipoprotein cholesterol (LDL) and high-density lipoprotein (HDL), resulting from the efficient binding of the APOE ϵ2 to LDL and HDL receptor particles (17, 18). This observation underscores the potential influence of phenotypic variations in APOE on cancer risk through changes in serum lipoprotein levels (19). Nevertheless, the specific role of APOE genotypes in LUAD remains unclear.
2 Materials and methods2.1 Study populationThe Qingdao University Affiliated Hospital’s Institutional Review Boards (IRBs) approved the study. Whole blood sample were selected from our research group’s repository collected from respiratory and critical care medicine inpatients between June 2018 and June 2022 at the hospitals. Serum was extracted from anticoagulated blood samples derived from the health examination department and preserved for further study at -80°C. The study included 400 healthy control participants and 200 LUAD patients. All participants’ clinical and demographic data were recorded, along with their alcohol and tobacco use, fatty liver disease, and lipid profiles. The criteria for hypertension included systolic/diastolic blood pressure readings of 140/90 mmHg or higher or the continuous treatment with antihypertensive medications. CHD is characterized by coronary artery atherosclerosis, leading to luminal stenosis or functional changes in coronary arteries, resulting in symptoms such as myocardial ischemia and hypoxia or the management of coronary heart disease. Diabetes is diagnosed based on fasting plasma glucose levels ≥7.0 mmol/L, a random plasma glucose level ≥11.1 mmol/L, or the administration of antidiabetic therapies. Nonalcoholic fatty liver disease (NAFLD) is defined as the accumulation of liver fat in individuals who consume little to no alcohol and do not have other identifiable causes of hepatic fat accumulation (20).
2.2 Participant groupingClinical information and histological analysis, including the outcomes of biopsies or surgical resections and TNM staging in accordance with the most recent edition of the American Joint Committee on Cancer (AJCC) guidelines, were adopted to diagnose LUAD (21). The control group participants were selected to match the age range of patients with LUAD, with a deviation of ±2 years. Individuals in the control group had no relevant medical conditions pertinent to this study, no history of tumors in any organ, and no acute or chronic diseases.
2.3 DNA extraction and APOE genotypingBlood samples were treated with the Blood Genomic DNA Extraction Kit (Catalog number DP348-03, Tiangen, China) to extract deoxyribonucleic acid (DNA), a spectrophotometer was used to measure the purity and concentration of the DNA (Thermo Fisher Scientific, United States). Standard PCR reactions employed the 2×Taq PCR Master Mix (dye-free) reagent kit. The primer sequences for APOE were the forward primer (5′- GCTTGGCACGGCTGTCCAAGGA-3)and reverse primer (5′- ATTCGCCCCGGCCTGGTACAC -3’). Following PCR amplification, the PCR products were subjected to 2% agarose gel electrophoresis to verify successful amplification. The gel was stained with ethidium bromide, and the bands were visualized under UV light to confirm the presence and expected size of the amplicons. After confirming the correct PCR products by agarose gel electrophoresis. The amplification products were transferred to Sangon Biotech (Shanghai, China) for Sanger sequencing. SnapGene software (Insightful Science, United States) was used to visualize the sequencing data of two mutation sites (rs7412 and rs 429358) to identify the APOE genotype.
2.4 Statistical analysisFor statistical analysis, we reported the data as percentages and frequency distributions using SPSS version 27.0 (IBM Corp, Armonk, NY, USA).The chi-square test or Fisher’s exact test was employed for comparing the frequency distribution of APOE genotypes and alleles in the LUAD and healthy control groups, Mann-Whitney U test was adopted for continuous or ordinal data that did not satisfy the normality assumption. To assess the relationship between genotypes and the risk of developing LUAD, binary logistic regression analysis was performed. In this analysis, odds ratios (ORs) were calculated to quantify the association between APOE genotypes and the likelihood of developing LUAD. ORs provide an intuitive measure of the strength of the relationship between predictor variables (genotypes) and the outcome (LUAD risk). This is particularly useful for understanding the magnitude of risk associated with different genotypes. For normally distributed continuous data, an independent samples t-test was performed to analyze further group differences in the APOE gene subtypes. P-value<0.05 was recognized as predictive of statistical significance.
3 Results3.1 Analysis of baseline characteristics in the study groupsThere were 600 participants in the whole, divided between both the LUAD group (n = 200) and the control group (n = 400). The baseline characteristics of the two groups, including an average age over 55 years, showed no statistically significant differences in gender, alcohol use, smoking history, hypertension, or diabetes (P > 0.05) (Table 1).
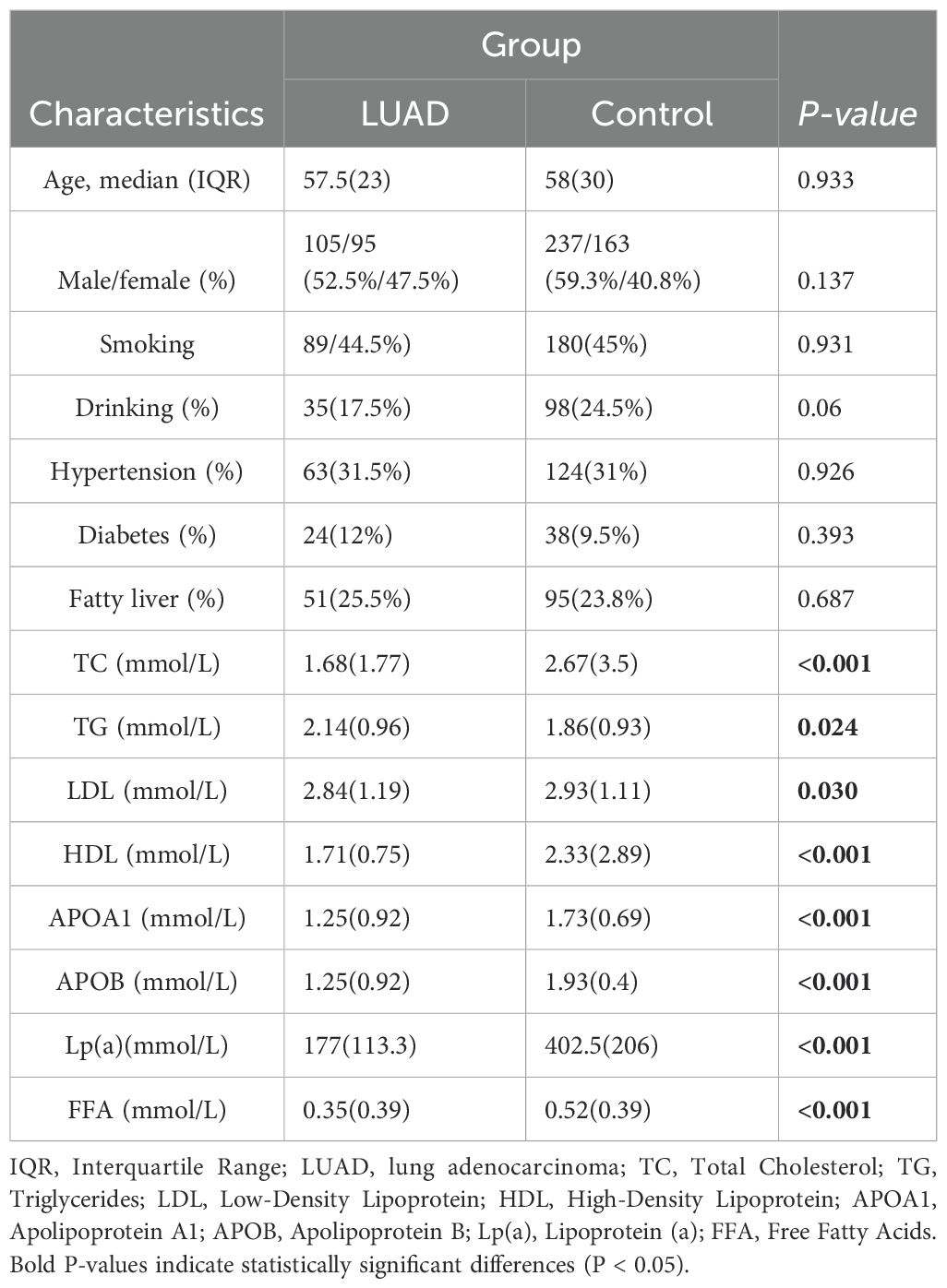
Table 1. Characteristics of the study population.
3.2 Analysis of APOE alleles and genotypes frequencies in LUAD and control groupThe statistical analysis revealed that the LUAD group had a significantly higher frequency of ϵ2 allele expression than the control group (15.5%vs7%, P< 0.001), conversely, the ϵ4 allele’s expression frequency was significantly lower than that of the control group (2.7% vs 11.1%, P < 0.001). The frequency of ϵ3 allele expression did not differ statistically significantly between the LUAD and control groups (P > 0.05) (Table 2). The binary logistic regression analysis revealed strong correlations between APOE genotypes and the incidence of LUAD. Specifically, APOE ϵ2/ϵ2 and ϵ2/ϵ3 genotypes were significantly associated with a 3.78-fold (95% CI: 1.64–8.71, P<0.001) and 3.22-fold (95% CI: 1.69–6.11, P=0.02) increase in the odds of developing LUAD, respectively. Conversely, the APOE ϵ3/ϵ4 genotype was linked to a 0.14-fold reduction in the risk of developing LUAD (95% CI: 0.05–0.4, P<0.001). The proportion of individuals of the APOE ϵ3/ϵ3 and ϵ2/ϵ4 genotypes was similar between the two groups (P>0.05) (Table 3).
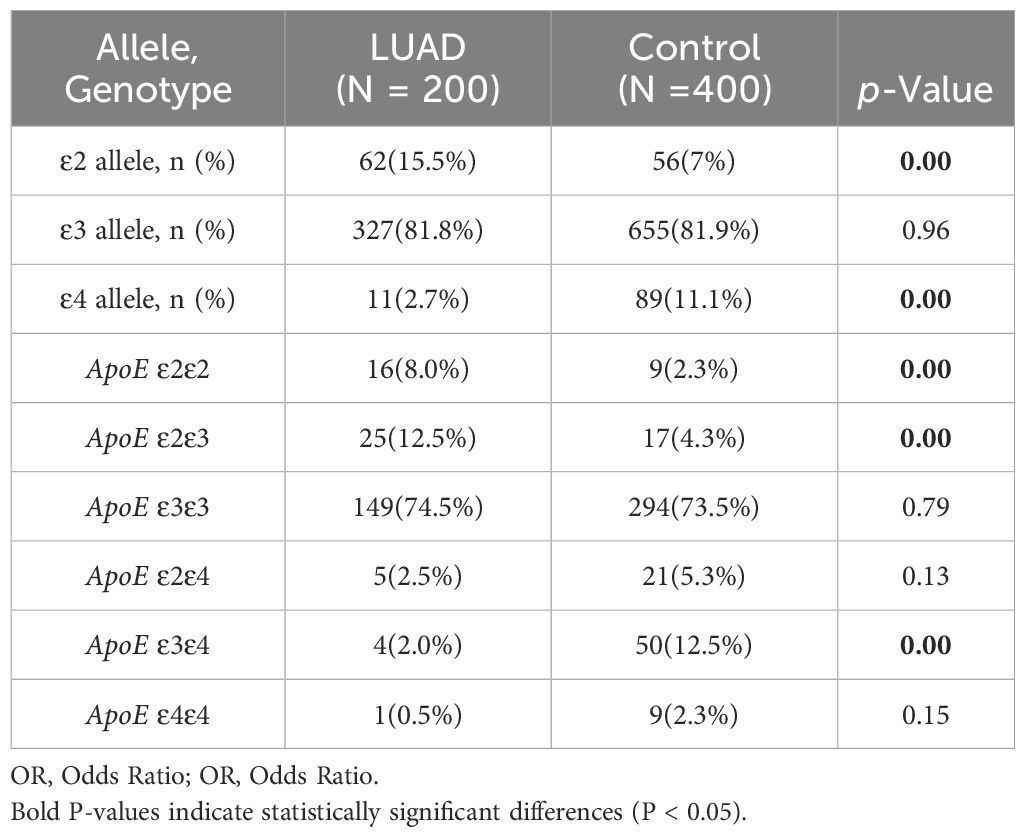
Table 2. Comparison of ApoE alleles and genotypes frequencies in LUAD and control group.
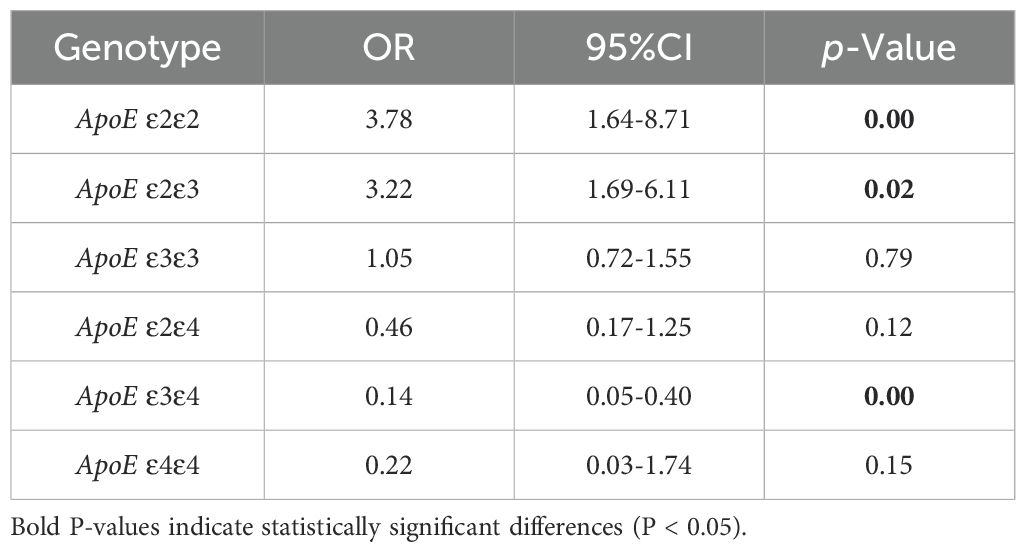
Table 3. Binary logistic regression analysis of ApoE genotypes distribution in LUAD and control group.
3.3 Analysis of APOE alleles and genotypes frequencies between the control and LUAD subgroups with different stagesLUAD patients were divided into early and late stages in order to investigate the relationship among APOE genotypes and individual LUAD stages in comparison to the control group. Both the early-stage and advanced-stage LUAD groups had a significantly higher frequency of the ϵ2 allele compared to the control group (11.6% vs. 7% and 21.2% vs. 7%, P=0.003 and P<0.01, respectively). However, the frequency of the ϵ4 allele was significantly lower in both LUAD stages compared to the control group (3.7% vs. 11.1% and 2.1% vs. 11.1%, P<0.01). The frequency of the ϵ3 allele did not differ statistically significantly between the control group and each of the other LUAD subgroups (P>0.05). Additionally, the percentage of individuals of the APOE ϵ2/ϵ3 genotype was higher in both LUAD subgroups as compared to control group, whereas the proportion of the APOE ϵ3/ϵ4 genotype was significantly lower (P<0.05) (Table 4).
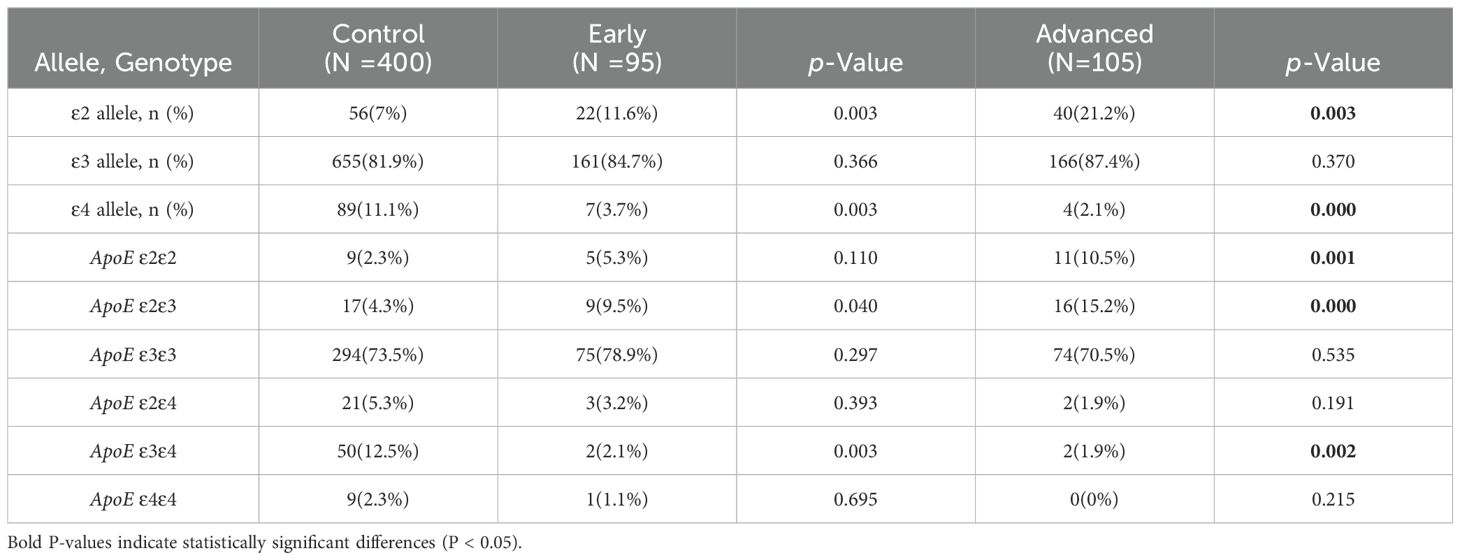
Table 4. Comparison of ApoE alleles and genotypes frequencies between the control and LUAD different stages subgroups with different stages.
Regression analysis was conducted to examine the association between specific APOE genotypes and the risk of LUAD at various stages. The APOE ϵ2/ϵ3 genotype showed a 2.36-fold increase in susceptibility to early-stage LUAD compared to the control group (P = 0.046). In patients with advanced-stage LUAD, the frequencies of the APOE ϵ2/ϵ2 and ϵ2/ϵ3 genotypes were markedly higher (OR = 5.08,95%CI,2.05-12.62, and OR = 4.05, 95%CI, 1.97-8.33, P < 0.001). Furthermore, individuals with the APOE ϵ3/ϵ4 genotype exhibited a significantly lower risk of developing both early-stage and advanced-stage LUAD (OR = 0.15,95%CI,0.04-0.63, P=0.01 and OR = 0.14, 95%CI, 0.03-0.57, P =0.006) (Table 5).
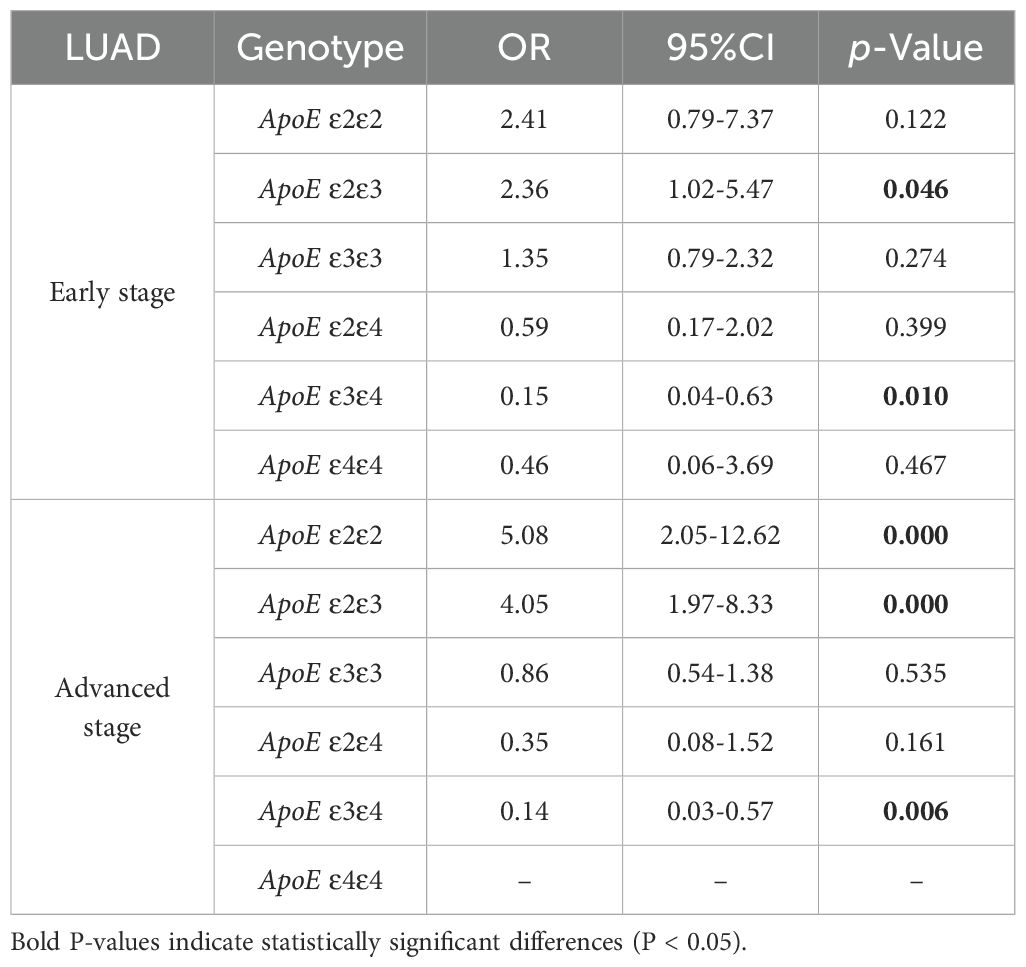
Table 5. Binary logistic regression analysis of ApoE genotypes in the control group and early and advanced LUAD stage subgroup.
3.4 Analysis of the prevalence of APOE alleles and genotypes frequencies among the control group and LUAD subgroups by differentiation levelFollowing the degree of pathological differentiation, patients with LUAD were categorized into three subgroups: well, moderately, and poorly differentiated. Based on the degree of tumor differentiation, each LUAD subgroup was compared with the control group, revealing that the frequency of ϵ2 and ϵ4 allele carriers was reduced in well-differentiated LUAD patients (0.8% vs. 7% and 4.2% vs. 11.1%, P=0.01 and P=0.02), while the frequency of ϵ3 allele carriers was significantly higher (94.9% vs. 81.9%, P<0.01). The LUAD subgroup and control group a were analyzed, revealing a much higher frequency of individuals carrying the ϵ2 allele in the moderately differentiated LUAD group (79.9% vs. 81.9%, P<0.01). In the poorly differentiated LUAD group, with respect to the control group, the frequency of the ϵ4 allele was lower (2.8% vs. 11.1%, P < 0.01), whereas the frequency of the ϵ2 allele was higher (26.1% vs. 7%, P < 0.01) (Table 6).
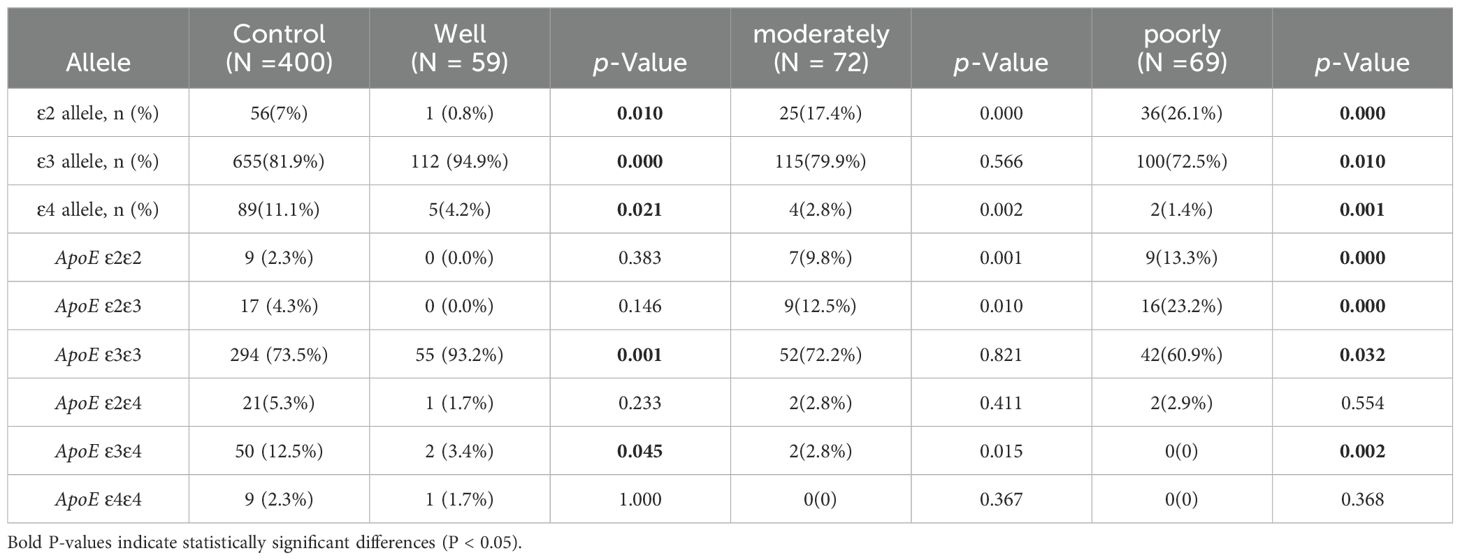
Table 6. Comparison of ApoE alleles and genotypes frequencies among the control group and LUAD group subgroups by differentiation level.
Investigating the association between APOE genotypes and poorly differentiated LUAD, we discovered that those with the ϵ2/ϵ2 and ϵ2/ϵ3 genotypes had 6.8-fold and 6.52-fold increased chances of getting this condition. Conversely, individuals with the APOE ϵ3/ϵ3 genotype had 0.56 times the odds of developing poorly differentiated LUAD (95% CI = 0.33–0.96, P = 0.03). APOE ϵ3/ϵ4 and APOE ϵ4/ϵ4 hadn’t been found in patients with poorly differentiated LUAD. The APOE ϵ2/ϵ3 and ϵ3/ϵ3genotypes raised the likelihood of developing moderately differentiated LUAD by 4.68-fold and 3.22-fold, respectively, in comparison with the healthy cohort (95% CI = 1.68–13.0 and 1.38–7.54, P < 0.05). However, the probability of developing moderately differentiated LUAD were 0.2-fold lower for carriers of the APOE ϵ3/ϵ4 genotype (95% CI = 0.05–0.84, P = 0.03). Furthermore, APOE ϵ3/ϵ3 genotype carrier showed a substantial 4.96-fold increase in the odds of developing well-differentiated LUAD (95% CI = 1.75-14.01, P = 0.003). Neither the APOE ϵ2/ϵ2 nor the ϵ2/ϵ3 genotype was present in any patient within the well-differentiated LUAD group. The APOE ϵ2/ϵ4 genotype showed no significant relationship with the probability of developing LUAD with varying degrees of differentiation (Table 7).

Table 7. Binary logistic regression analysis of ApoE genotypes frequencies in the control group and LUAD subgroups by differentiation level.
3.5 The lipid profile between individuals carrying ϵ2, ϵ3, and ϵ4 alleles in the control and LUAD subgroupsIn LUAD patients, individuals carrying the ϵ2 and ϵ3 allele exhibited significant alterations in lipid metabolism compared to the healthy control group, with LDL, HDL, total cholesterol (TC), APOB, and FFA levels downregulated (P<0.05). In contrast, no significant differences were observed in patients carrying the ϵ4 allele (Table 8; Supplementary Figures 1A–C).
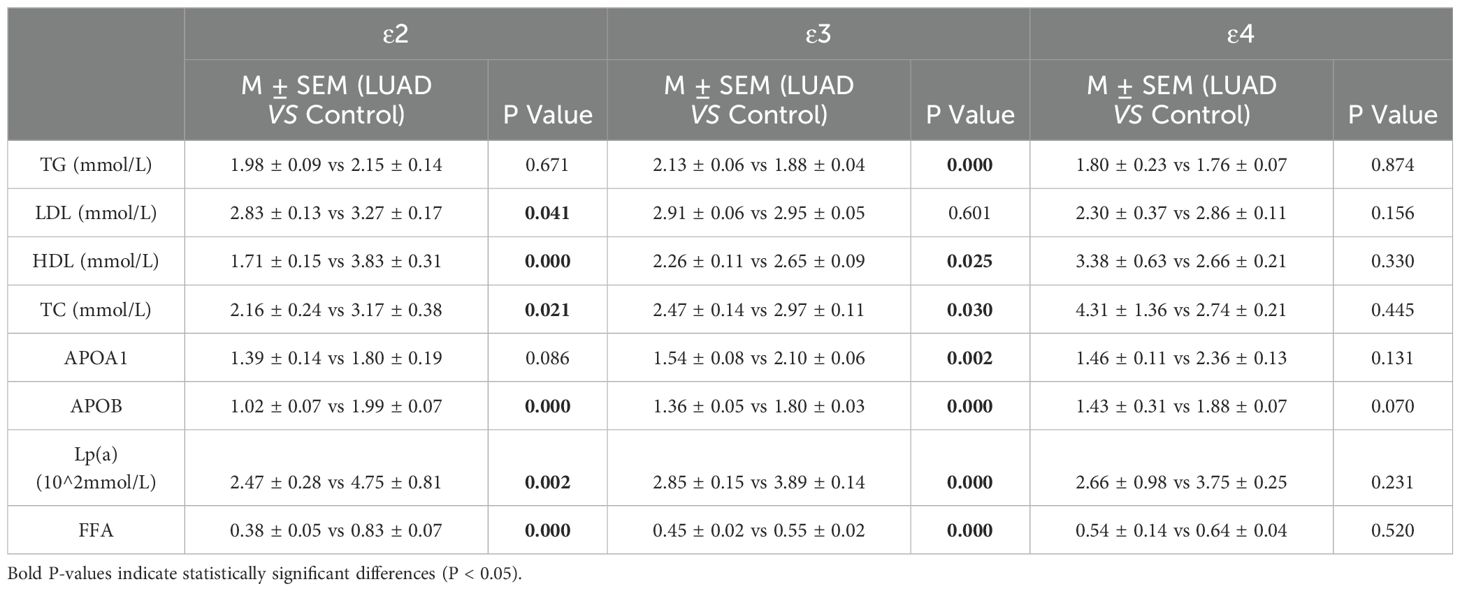
Table 8. The lipid profile between individuals carrying ϵ2, ϵ3, and ϵ4 alleles in the control and LUAD group.
To further explore the effects of different APOE alleles on lipid profiles in LUAD patients and the modulatory role of disease stage, with comparisons to a normal control group. In early-stage LUAD patients, ϵ3 and ϵ2 allele carriers were associated with the most significant alterations in lipid metabolism: ϵ3 carriers exhibited increased TG, HDL,APOA1 and ApoB levels and decreased Lp(α) and FFA levels, whereas, ϵ2 carriers had lower HDL and FFA levels and higher ApoB levels, ϵ4 carriers had significant changes only in increased ApoB levels (P < 0.05) (Table 9A; Supplementary Figure 2A–C). In late-stage LUAD patients, ϵ2 carriers primarily showed significant reductions in lipoproteins and FFA, whereas ϵ3 carriers demonstrated significant increases in TG levels along with decreased TC,APOB and FFA levels (P < 0.05) (Table 9B; Supplementary Figures 2D–F).
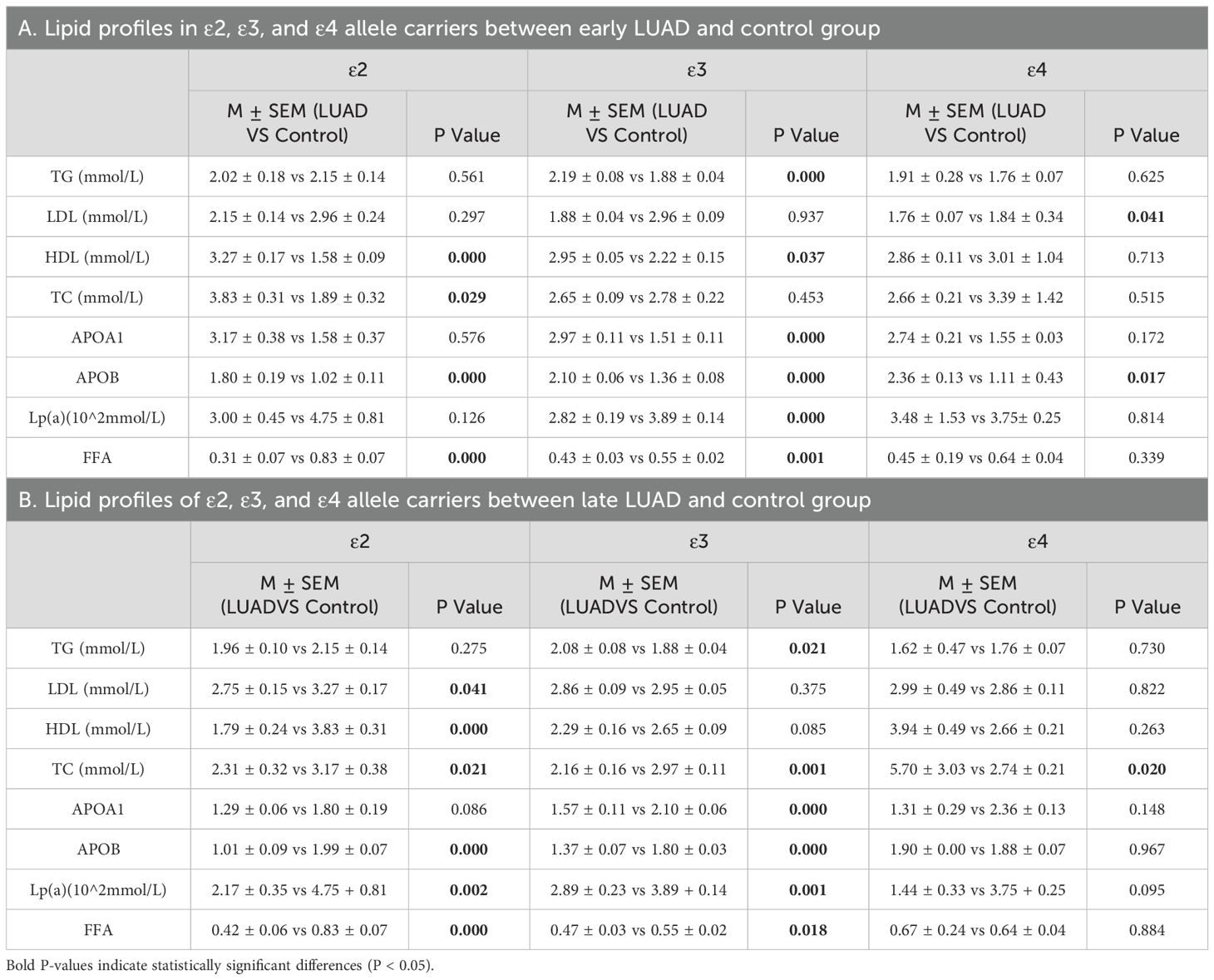
Table 9. Lipid profiles of individuals carrying ϵ2, ϵ3, and ϵ4 alleles in LUAD with different stages and control group.
To explore the relationship between APOE alleles and lipid profiles in LUAD patients with different levels of differentiation, and to compare these findings with those from a normal control group. Specifically, in well-differentiated LUAD patients, ϵ3 carriers exhibited increased TG levels, along with significantly decreased levels of HDL, Apolipoprotein A1(ApoA1), Apolipoprotein B (APOB) Lipoprotein (Lp(a)), and free fatty acids (FFA). In ϵ4 carriers, significant reductions were primarily observed in LDL, ApoA1, and ApoB levels (P < 0.05) (Table 10A; Supplementary Figures 3A, B). In moderately differentiated LUAD patients, both ε2 and ε3 carriers showed notable lipid metabolic abnormalities, including HDL, APOA1, APOB and FFA levels. In contrast, no significant changes were noted in ϵ4 carriers, suggesting a minimal or insignificant effect of the ϵ4 allele on lipid metabolism in this subgroup (P < 0.05) (Table 10B; Supplementary Figures 3C–E). In poorly differentiated LUAD patients, those carrying ϵ2 and ϵ3 allele experienced significant disruptions in lipid metabolism, particularly with reductions in ApoB and Lp(a) levels, which were consistently observed in both groups (P < 0.05) (Table 10C; Supplementary Figures 3F, G). These results suggest that APOE alleles may significantly affect lipid metabolism, especially in individuals with poorly differentiated LUAD.
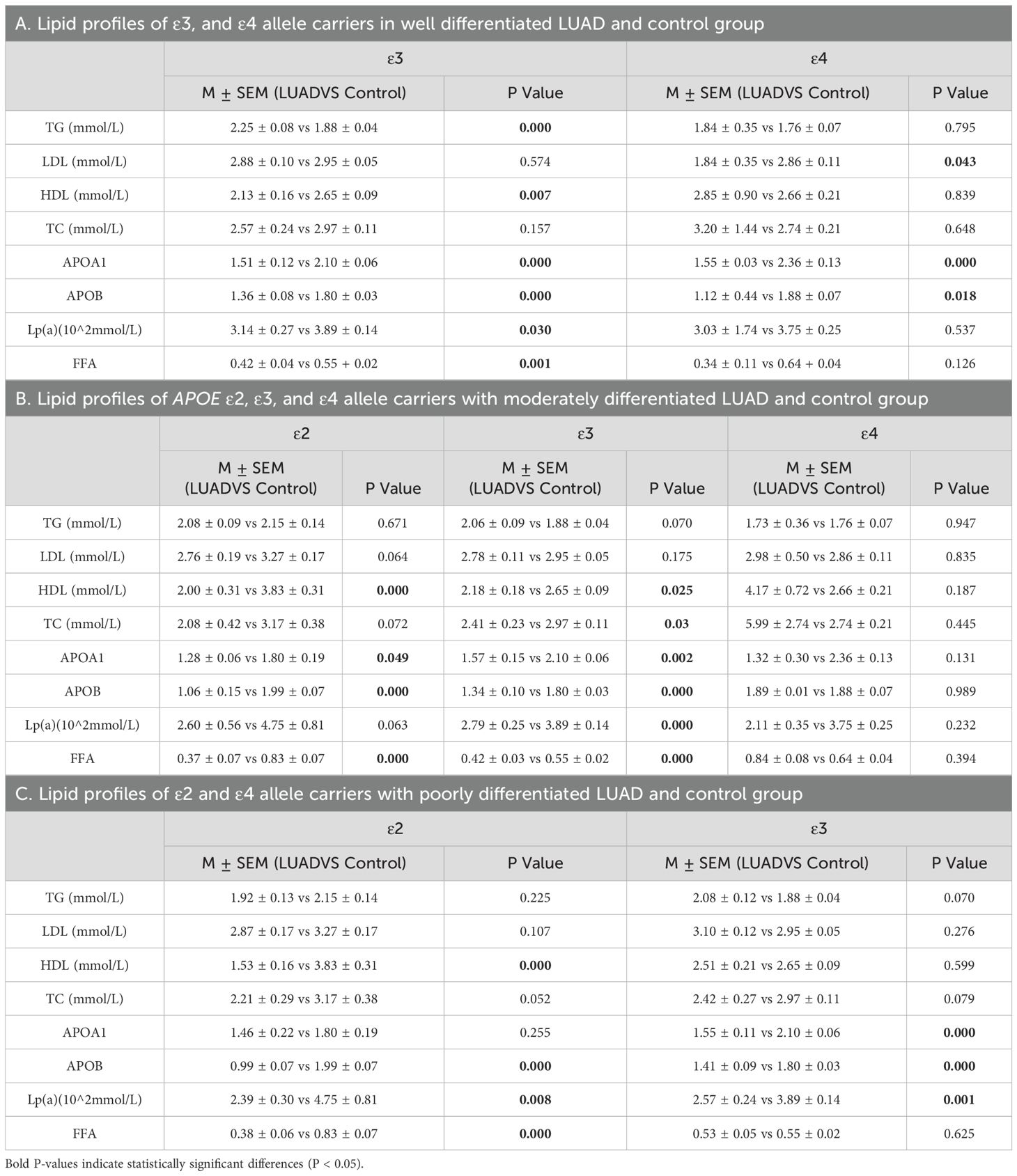
Table 10. Lipid profiles of APOE ϵ2, ϵ3, and ϵ4 allele carriers within LUAD subgroups by differentiation level and control group.
4 DiscussionOur research focused on assessing the role of APOE gene polymorphisms in LUAD susceptibility and their influence on lipid profiles. Previous reports have primarily focused on APOE protein expression levels in patients with lung cancer. This study demonstrates revealed that the presence of the ϵ2 allele increases the risk of LUAD, while the ϵ4 allele provide a protective effect. However, the APOE ϵ3/ϵ3 genotype did not exhibit a significant association with LUAD risk. The APOE ϵ2/ϵ4 genotype demonstrated no statistical difference between the healthy control and LUAD groups. Furthermore, the APOE ϵ2/ϵ2 genotype displayed a higher risk of cancer development compared to APOE ϵ2/ϵ3, particularly in patients with advanced stages of lung cancer, suggesting a potential association with cancer progression stages. Similar studies have identified the APOE ϵ4 allele as a lung cancer risk factor. However, they overlook important differences in pathological subtypes and fail to address the unique metabolic characteristics of LUAD (19). The role of the APOE alleles and their genotypes varies across different tumor types. For instance, APOE ϵ2 reduces the risk of developing colorectal cancer, while the APOE ϵ4 allele does not significantly impact colorectal cancer risk (22), In contrast, the APOE ϵ3 allele is linked to a higher risk of laryngeal squamous cell carcinoma (LSCC) (23). Different cancer susceptibility results are caused by the interaction between APOE allele carriers and their genotypes, which has a substantial impact on cancer development and progression.
The frequencies of different APOE genotype within the population are affected by gender, age and genetic variations (24). This study was conducted with baseline data such as gender, age, and medical history being consistent across the groups, which means that the potential confounding effects of these factors were eliminated in this study. Individuals carrying the APOE ϵ2/ϵ4 genotype do not have a significantly increased risk of LUAD, we hypothesize an allelic dosage effect for APOE ϵ2 and ϵ4, suggesting that the number of alleles correlates with the extent of their impact on phenotype and function. Specifically, ϵ2 appears to have a detrimental effect, while ϵ4 seems to exhibit a protective effect. The adverse impact of homozygous ϵ2/ϵ2 is more pronounced than that of ϵ2/ϵ3, whereas the protective effect of homozygous ϵ4/ϵ4 is stronger than that of ϵ3/ϵ4. In colorectal cancer, patients with the APOE ϵ2/ϵ3 genotype are more likely to progress to advanced stages once diagnosed (12). Individuals with the APOE ϵ2/ϵ4 and APOE ϵ3/ϵ4 genotypes have a reduced risk of LSCC by 2.9 and 1.5 times. Individuals with the APOE ϵ3/ϵ3 genotype have a 1.7-fold higher likelihood of developing LSCC (23). The findings align with the allelic dosage effect in cancer risk, but larger population studies are needed for definitive conclusions.
Recent studies suggest that interactions between lipid metabolism and APOE genotypes may increase cancer risk, particularly for APOE ϵ2 carriers with low TC levels (19, 25). The PROSPER-derived studies evaluated the relationship between APOE subtypes and cancer risk in the older population. These studies indicated a negative correlation between TC levels and cancer incidence and mortality (26). The study couldn’t establish a direct causal link between APOE genotype and cancer risk due to the effect of medications on TC levels in patients with vascular disease (27). The primary outcome variable of the study was cancer development, without differentiating between specific types of malignancies, however, the analysis of APOE gene polymorphism and tumor risk did not include the Chinese population (28). APOE ϵ4 female patients display elevated TG levels and an increased risk of breast cancer (29, 30). Interactions between APOE genotypes and lipid metabolism may impact cancer risk, though direct causality and population-specific effects remain unclear. The precise mechanisms through which APOE genotypes influence malignant tumor progression remain unclear. Some studies suggest that the ϵ2 allele may protect against head and neck cancer, potentially because of its enhanced antioxidant abilities (31), however, overexpression of APOE2 can induce epithelial-mesenchymal transition (EMT), thereby promoting cancer progression in pancreatic cancer (32). Mice carrying the human APOE ϵ2 allele demonstrate prolonged progression-free survival (PFS) compared to APOE ϵ4 carriers, with the mechanism linked to diminished T-cell cytotoxicity against tumor cells (11).Building on mechanisms observed in other cancers, these results underscore the importance of investigating how APOE ϵ2 may drive tumor progression in LUAD.
APOE gene can influence the uptake and breakdown of TC and TG in lung cancer patients (33, 34). Patients with LUAD carrying the ϵ2 allele showed decreased TC levels. TC levels increase in early-stage LUAD patients and are reduced in late-stage patients compared to those with the ϵ4 allele. A condition called the cancer preclinical phenomenon when cancer causes a drop in plasma TC levels prior to a formal cancer diagnosis, this effect arises as cancer cells increase their uptake of TC from the bloodstream for growth and proliferation (35, 36). Earlier studies have indicated a heightened gastric cancer incidence in individuals carrying the ϵ2 allele with lower TC levels (37), a study conducted across seven countries found that lung cancer mortality risk rises when TC levels drop below 170 mg/dL (25). The APOE ϵ2 allele affects different lipid metabolism markers across various tumors, ApoA1 levels are significantly increased in patients with early-stage gastric cancer, whereas they remain relatively stable in those with advanced-stage gastric cancer (38, 39). Increased APOA1 levels can reduce the risk of lung cancer (40), but after lung cancer patients with brain metastases, APOA1 levels are up-regulated (41). Patients carrying the APOE ϵ2 allele tend to have lower VLDL-C levels, while those with the ϵ4 allele exhibit increased VLDL-C levels (18). This association suggests that the phenotypic variation of lipoproteins among individuals can alter the cancer risk by modulating serum lipoprotein levels. Overall, APOE gene polymorphisms influence lipid metabolism in LUAD patients, characterized by reduced HDL levels and elevated TG (42), which provide essential energy and biosynthetic precursors for tumor cell proliferation. Moreover, dysregulated lipid metabolism impacts immune cell function, impairing tumor immune surveillance, such as cholesterol modulating T cell receptor signaling and immune checkpoint expression (43). Thus, targeting lipid metabolism could represent a novel strategy to enhance immune response and treat tumors. FFA and cholesterol can influence multiple stages of cancer immunity by modifying the state and function of immune cells in the tumor microenvironment (TME) (44, 45). These changes interfere with the production of tumor-associated antigens (TAAs) (43), enabling tumor cells to escape immune surveillance and impairing the normal antitumor response (46). The study indicates that macrophages with high APOE expression are enriched in metastatic gastric tumors, contributing to immune evasion by reducing CD8+ T cell infiltration (47). Targeted interventions that modulate these pathways could potentially reduce tumor growth and improve treatment outcomes for APOE ϵ2 carriers. Additionally, since APOE ϵ2 is implicated in the regulation of epithelial–mesenchymal transition (EMT), therapies aimed at inhibiting EMT may offer significant benefits for these patients. Identifying APOE ϵ2 carriers would allow clinicians to personalize treatment strategies, potentially improving prognosis and enhancing the effectiveness of therapies for advanced LUAD.
This study has certain limitations. We explored the association between APOE genotypes and TNM staging in patients with LUAD. However, subgroup analyses for the T stage, N stage, and M stage for each patient were not conducted. This limitation stems from the fact that not all patients could undergo accurate T, N, and M staging, resulting in significant data loss and reduced statistical power. Moreover, this study did not measure APOE serum concentrations. It would have been beneficial to analyze the correlation between APOE levels and lung cancer staging if such data had been available. This study focused on a specific population in the Qingdao area of Shandong Province, China. Future research should include data from other regions or populations with different genetic backgrounds to validate the generalizability of the association between APOE polymorphisms and LUAD.
5 ConclusionIndividuals carrying the ϵ2 allele have an increased susceptibility to developing LUAD, accompanied by disrupted lipid metabolism. The APOE ϵ2/ϵ2 and ϵ2/ϵ3 genotypes have been attributed to an increased probability of advanced and poorly differentiated LUAD.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statementThe studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsHB: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. DR: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing. YW: Funding acquisition, Methodology, Resources, Writing – original draft, Writing – review & editing. HW: Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. CZ: Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81670822) and Wu Jieping Medical Foundation (320.6750.19094.29).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RZ declared a shared affiliation with the authors DR, HW to the handling editor at the time of review.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1522761/full#supplementary-material
References1. Dongés B, Haupt LM, Lea RA, Chan RC, Shum DH, Griffiths LR. Role of the apolipoprotein E and catechol-O-methyltransferase genes in prospective and retrospective memory traits. Gene. (2012) 506:135–40. doi: 10.1016/j.gene.2012.06.067
PubMed Abstract | Crossref Full Text | Google Scholar
2. Xu M, Zhao J, Zhang Y, Ma X, Dai Q, Zhi H, et al. Apolipoprotein E gene variants and risk of coronary heart disease: A meta-analysis. BioMed Res Int. (2016) 2016:3912175. doi: 10.1155/2016/3912175
PubMed Abstract | Crossref Full Text | Google Scholar
3. Serrano-Pozo A, Das S, Hyman BT. APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. (2021) 20:68–80. doi: 10.1016/S1474-4422(20)30412-9
PubMed Abstract | Crossref Full Text | Google Scholar
4. Jackson RJ, Keiser MS, Meltzer JC, Fykstra DP, Dierksmeier SE, Hajizadeh S, et al. APOE2 gene therapy reduces amyloid deposition and improves markers of neuroinflammation and neurodegeneration in a mouse model of Alzheimer disease. Mol Ther. (2024) 32:1373–86. doi: 10.1016/j.ymthe.2024.03.024
PubMed Abstract | Crossref Full Text | Google Scholar
5. Lumsden AL, Mulugeta A, Zhou A, Hyppönen E. Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine. (2020) 59:102954. doi: 10.1016/j.ebiom.2020.102954
PubMed Abstract | Crossref Full Text | Google Scholar
6. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of apolipoprotein E, APOE genotype, and all-cause and cause-specific mortality in 105 949 individuals from a white general population cohort. Eur Heart J. (2019) 40:2813–24. doi: 10.1093/eurheartj/ehz402
PubMed Abstract | Crossref Full Text | Google Scholar
7. Innes H, Nischalke HD, Guha IN, Weiss KH, Irving W, Gotthardt D, et al. The rs429358 locus in apolipoprotein E is associated with hepatocellular carcinoma in patients with cirrhosis. Hepatol Commun. (2022) 6:1213–26. doi: 10.1002/hep4.1886
PubMed Abstract | Crossref Full Text | Google Scholar
8. Amidi A, Agerbæk M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. (2017) 11:769–83. doi: 10.1007/s11682-016-9552-3
PubMed Abstract | Crossref Full Text | Google Scholar
9. Wang H, Zhou HC, Ren RL, Du SX, Guo ZK, Shen XH. Apolipoprotein E2 inhibits mitochondrial apoptosis in pancreatic cancer cells through ERK1/2/CREB/BCL-2 signaling. Hepatobiliary Pancreat Dis Int. (2023) 22:179–89. doi: 10.1016/j.hbpd.2022.09.010
PubMed Abstract | Crossref Full Text | Google Scholar
10. Adaku N, Ostendorf BN, Mei W, Tavazoie SF. Apolipoprotein E2 stimulates protein synthesis and promotes melanoma progression and metastasis. Cancer Res. (2023) 83:3013–25. doi: 10.1158/0008-5472.CAN-23-1252
PubMed Abstract | Crossref Full Text | Google Scholar
11. Ostendorf BN, Bilanovic J, Adaku N, Tafreshian KN, Tavora B, Vaughan RD, et al. Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat Med. (2020) 26:1048–53. doi: 10.1038/s41591-020-0879-3
PubMed Abstract | Crossref Full Text | Google Scholar
12. Torres GG, Dose J, Hasenbein TP, Nygaard M, Krause-Kyora B, Mengel-From J, et al. Long-lived individuals show a lower burden of variants predisposing to age-related diseases and a higher polygenic longevity score. Int J Mol Sci. (2022) 23:10949. doi: 10.3390/ijms231810949
PubMed Abstract | Crossref Full Text | Google Scholar
13. Hsiao CP, Goto T, Von Ah D, Saligan LN. Cancer-Related Cognitive Impairment Associated with APOE rs7412 and BDNF rs6265 in Breast Cancer Survivors. Semin Oncol Nurs. (2024) 40:151721. doi: 10.1016/j.soncn.2024.151721
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ahles TA, Orlow I, Schofield E, Li Y, Ryan E, Root JC, et al. The impact of APOE and smoking history on cognitive function in older, long-term breast cancer survivors. J Cancer Surviv. (2024) 18:575–85. doi: 10.1007/s11764-022-01267-z
PubMed Abstract | Crossref Full Text | Google Scholar
16. Martínez-Martínez AB, Torres-Perez E, Devanney N, Del Moral R, Johnson LA, Arbones-Mainar JM. Beyond the CNS: The many peripheral roles of APOE. Neurobiol Dis. (2020) 138:104809. doi: 10.1016/j.nbd.2020.104809
PubMed Abstract | Crossref Full Text | Google Scholar
17. Zhang ZG, Li Y, Ng CT, Song YQ. Inflammation in alzheimer's disease and molecular genetics: recent update. Arch Immunol Ther Exp (Warsz). (2015) 63:333–44. doi: 10.1007/s00005-015-0351-0
PubMed Abstract | Crossref Full Text | Google Scholar
18. Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. Jama. (2007) 298:1300–11. doi: 10.1001/jama.298.11.1300
PubMed Abstract | Crossref Full Text | Google Scholar
19. Gan C, Zhang Y, Liang F, Guo X, Zhong Z. Effects of APOE gene ϵ4 allele on serum lipid profiles and risk of cardiovascular disease and tumorigenesis in southern Chinese population. World J Surg Oncol. (2022) 20:280. doi: 10.1186/s12957-022-02748-2
PubMed Abstract | Crossref Full Text | Google Scholar
21. Huang J, Osarogiagbon RU, Giroux DJ, Nishimura KK, Bille A, Cardillo G, et al. The international association for the study of lung cancer staging project for lung cancer: proposals for the revision of the N descriptors in the forthcoming ninth edition of the TNM classification for lung cancer. J Thorac Oncol. (2024) 19:766–85. doi: 10.1016/j.jtho.2023.10.012
留言 (0)