Pembrolizumab, a programmed death (PD)-1 inhibitor, stands as a first-line option for patients with advanced non-small cell lung cancer (NSCLC) patients and a high programmed death-ligand 1 (PD-L1) expression [tumor proportion score (TPS) ≥50%], showing superior overall survival (OS), progression-free survival (PFS) and overall response rate (ORR) compared to chemotherapy, with better toxicity profile (1, 2). Results after 5 years of follow-up have been reported, with a median OS of 26.3 months and 31.9% of patients alive at 5 years (3). In addition, several real-world studies have confirmed these results in clinical practice (4–7). The PD-1 inhibitor cemiplimab, and the PD-L1 inhibitor atezolizumab have also shown efficacy in first-line setting with high PD-L1 expression (8, 9).
Of note, randomized clinical trials with pembrolizumab and chemotherapy have also shown OS benefit regardless of PD-L1 status, including patients with PD-L1≥50% expression (10, 11) and, more recently, results from clinical trial EMPOWER-Lung 3 also confirm better outcomes with cemiplimab and chemotherapy in patients with PD-L1≥50% (12). Other combinations of chemotherapy and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and anti PD-(L)1 have also shown similar outcomes but with higher rates of adverse events (13, 14).
However, patients with potential negative predictive factors such as poor Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (15), advanced age or receiving concomitant treatments with immune-modulating effects, such as antibiotics, corticosteroids or proton pump inhibitors (PPi) (16) are usually underrepresented in immunotherapy clinical trials. Recent research has also suggested that other factors such as a low body mass index (BMI) (17, 18) or certain metastatic sites (19) can also negatively impact on the efficacy of first-line pembrolizumab.
As there are several treatment options available for patients with advanced NSCLC and high PD-L1 expression, many efforts have been made to identify predictive factors that may help to select the best strategy in this scenario. Two meta-analyses have suggested that chemo-immunotherapy may improve OS and PFS compared to immunotherapy alone (20, 21) in some subgroups of patients, such as women or never-smokers.
The objective of our study was to evaluate the real-world outcomes of patients with advanced NSCLC and high PD-L1 expression receiving first-line pembrolizumab therapy and to assess potential predictive factors in this population.
Materials and methodsThis multicenter retrospective analysis included patients with advanced NSCLC with a PD-L1 ≥50% that had received at least one dose of first line pembrolizumab monotherapy outside of clinical trials between August 1st 2017 and January 1st 2023. Patients had to be treatment-naive or have a tumor relapse ≥6 months after curative treatment and have not received previous immunotherapy as part of their treatment. Sample size was not restricted due to the exploratory nature of the study. Data were anonymized at inclusion in the data base and collected from medical records. The data cut-off was June 30st 2023 to ensure a minimum follow-up of six months. The study was approved by a local ethics committee and confirmed by other institutions.
PD-L1 expression was determined by immunohistochemical staining in histological or cytological samples from primary tumors, lymph nodes or distant metastases, in each institution. Samples were considered valid if ≥100 viable cells were analysed.
Patient data and concomitant treatments were recorded from the medical history. Exposure to antibiotics, corticosteroids and PPi was considered during treatment and within four weeks before starting immunotherapy.
ORR and PFS were assessed by investigators using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (22) and iRECIST (23). Best response was categorized as complete response, partial response, stable disease and progressive disease (22, 23). Patients treated beyond radiographic progression were also recorded. PFS was defined as the time from the first dose of pembrolizumab to progression or death, and patients without disease progression were censored at the time of the last disease assessment. OS was calculated from the first dose of pembrolizumab until death. Patients who were still alive at the time of data analysis were censored at the time of last contact.
Descriptive statistics were used to report baseline characteristics of the population. Kaplan-Meier was used to estimate survival, and the long-rank test was used to compare median survival. Multivariate analyses were performed using Cox regression assuming proportional hazards. Two-sided p-values and 95% confidence intervals (CI) were used, with a prespecified <0.05 as significant. IBM Statistic SPSS version 25 software was used for the analyses.
Results494 patients were included from eight institutions. Patient clinicopathological characteristics are summarized in Table 1. White men, <75 years, former smoker, with a BMI ≥25, ECOG-PS of 1 and non-squamous histology were predominant in our population.
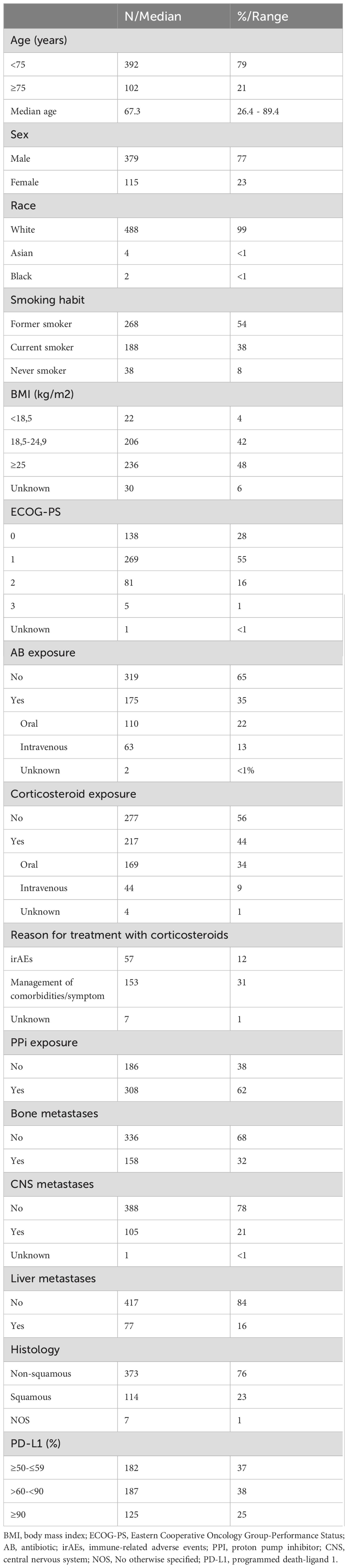
Table 1. Clinical and pathological characteristics.
PD-L1 expression was determined using 22C3 antibody (N=258, 52%), SP 263 antibody (N=164, 33%), 28-8 antibody (N=65, 13%) or Ventana SP142 antibody (N=1, <1%). Samples were obtained from primary tumor (66%), distant metastases (20%) or lymph nodes (14%). 89% were histological samples and 11% cytological samples.
454 (90%) patients discontinued treatment due to disease progression (51%), immuno-related adverse events (irAEs) (17%) or treatment completion after 35 cycles (13%). 445 patients (90%) had progressive disease at time of data cut-off, and 39 patients (8%) continued pembrolizumab beyond progression due to clinical benefit and 28 patients (6%) were rechallenged with immunotherapy in further lines. At time of data cut off, 331 patients (67%) had died, with 228 (69%) due to progression of disease.
With a median follow-up of 14.22 months (95% IC 12.5-16.0, IQR 23,13), the median OS and PFS were 15.9m (95% CI 13.1 to 18.8) and 9.9m (95% CI 7.7 to 12.1), respectively (Figures 1, 2). Of 444 patients (90%) evaluable for response, 31 patients (6%) had complete response, 184 patients (37%) partial response, 112 patients (23%) stable disease and 117 patients (24%) progressive disease. ORR for the overall population was 43%.

Figure 1. Kaplan-Meier curves for OS in the overall population and patients with similar inclusion criteria to Keynote-024 trial.
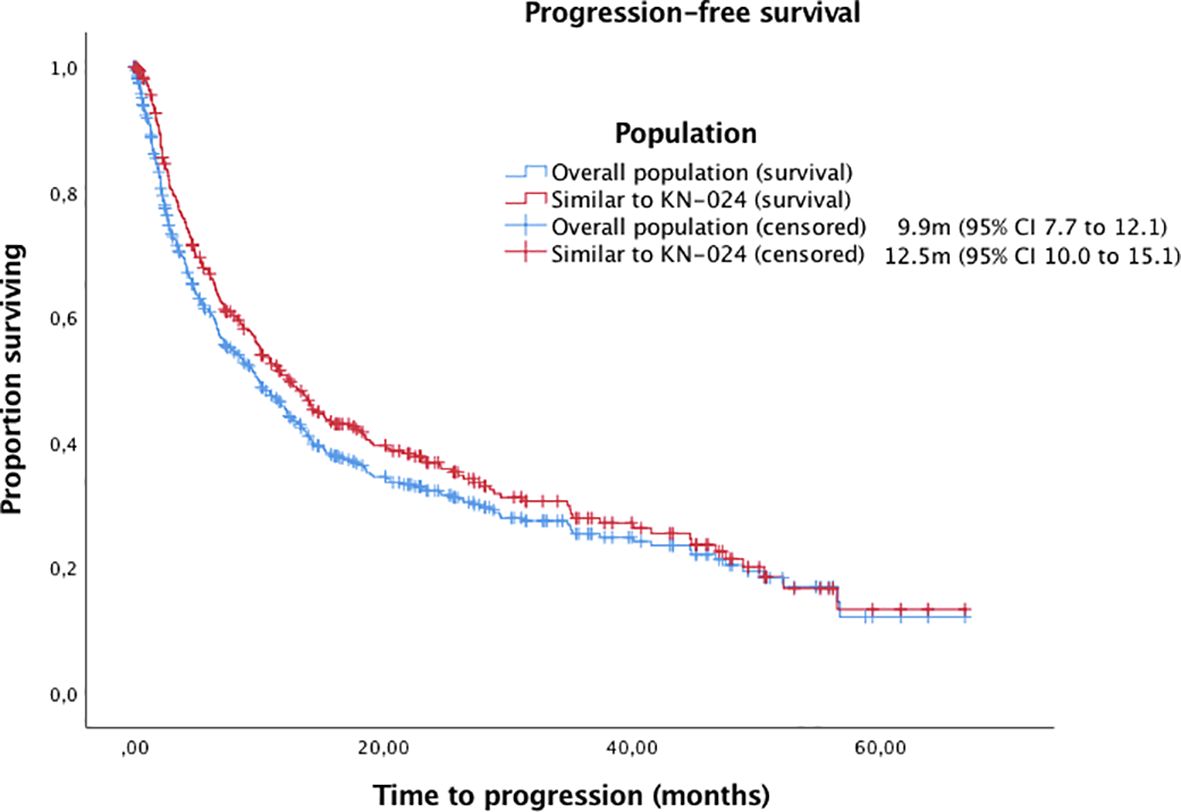
Figure 2. Kaplan-Meier curves for PFS in the overall population and patients with similar inclusion criteria to Keynote-024 trial.
Median OS and PFS according to clinicopathological characteristics are shown in Table 2. ECOG-PS, corticosteroid exposure, PPi exposure and bone metastases were associated with shorter OS and were therefore included in a multivariate model. Figures 3–6 show survival curves according to different prognostic factors. An interaction between ECOG-PS and corticosteroid exposure was also observed in the multivariate analysis for OS: corticosteroid exposure (HR 1.5, 95% CI 1.1 to 1.8) and ECOG-PS (HR 2.4, 95% CI 2.0 to 2.8) (Table 3). We did not find a statistically significant association between survival outcomes and age, sex, smoking status, AB exposure nor PD-L1 status.
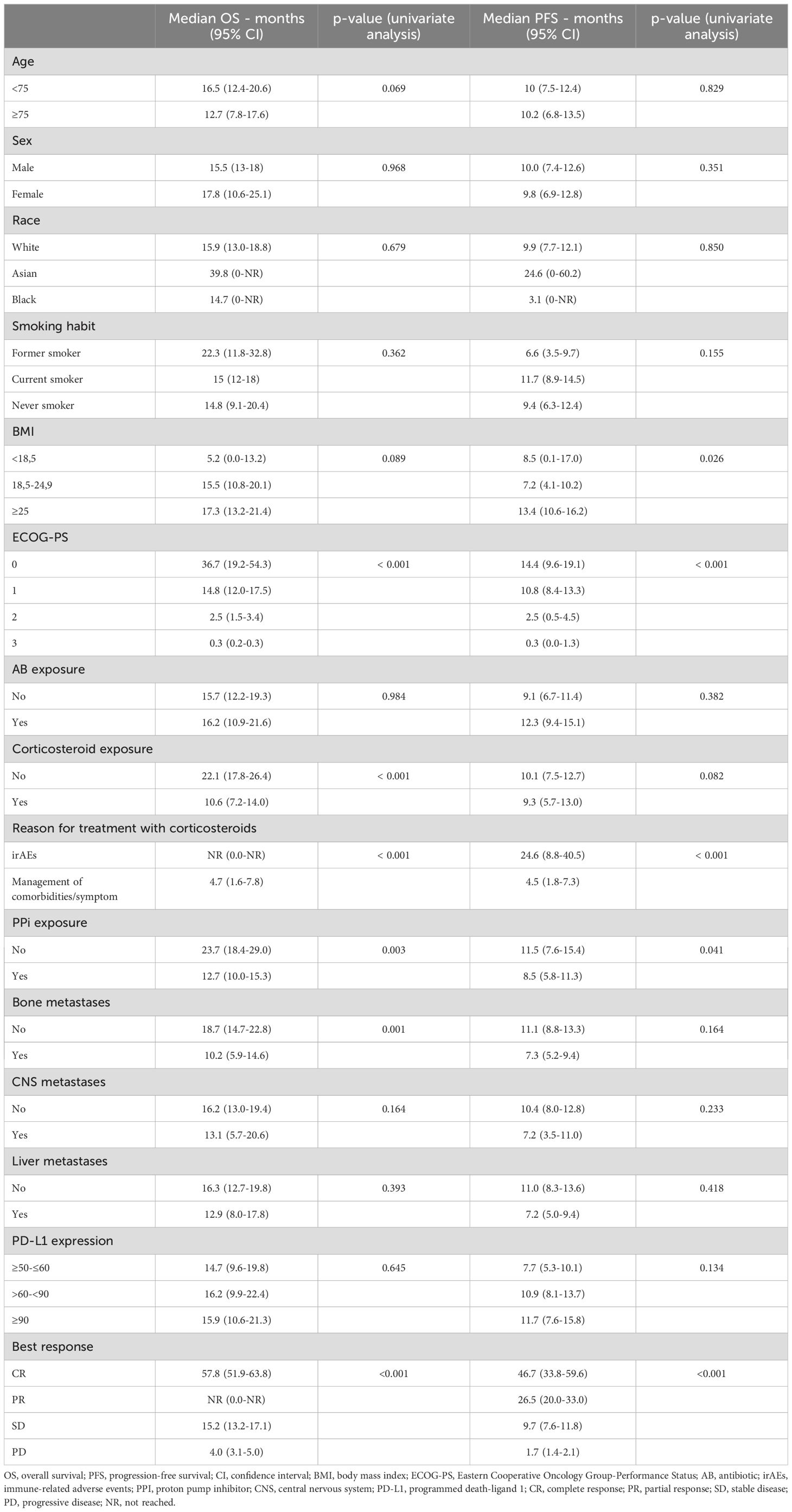
Table 2. Survival outcomes according to clinicopathological characteristics.
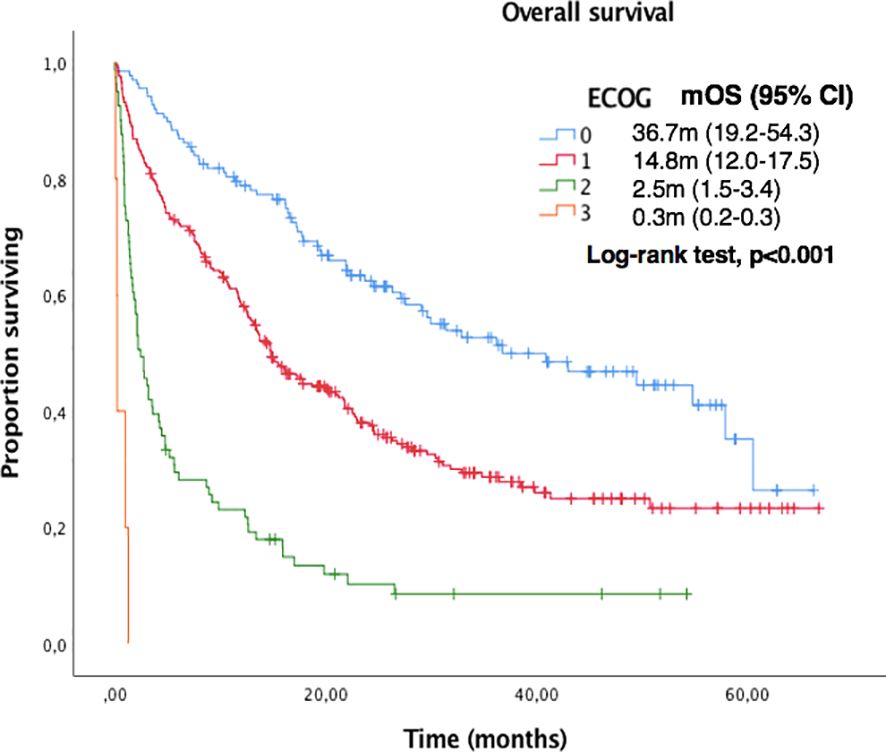
Figure 3. Kaplan-Meier curves for OS according to ECOG-PS in the overall population.
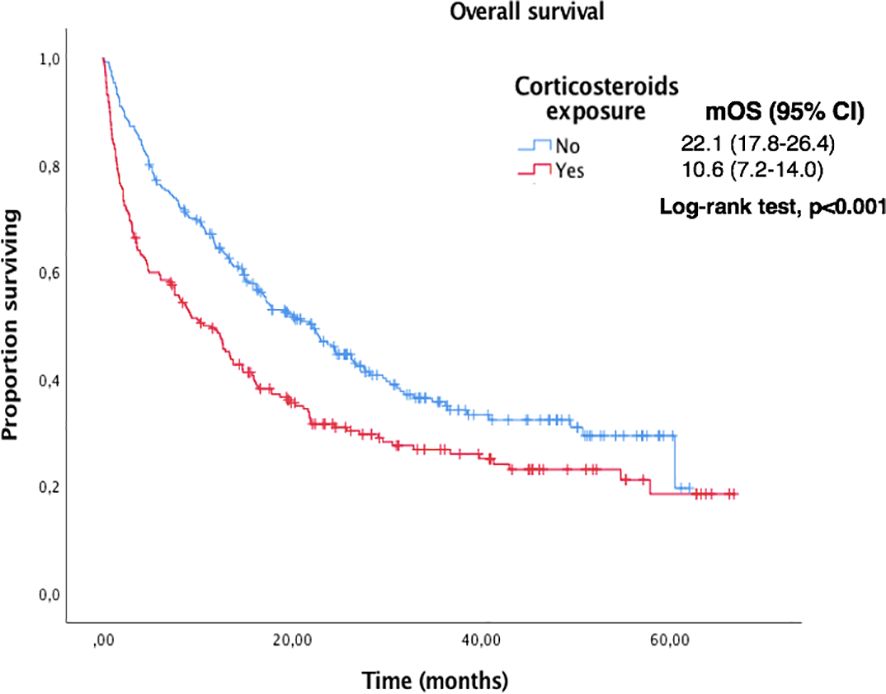
Figure 4. Kaplan-Meier curves for OS according to corticosteroids exposure in the overall population.
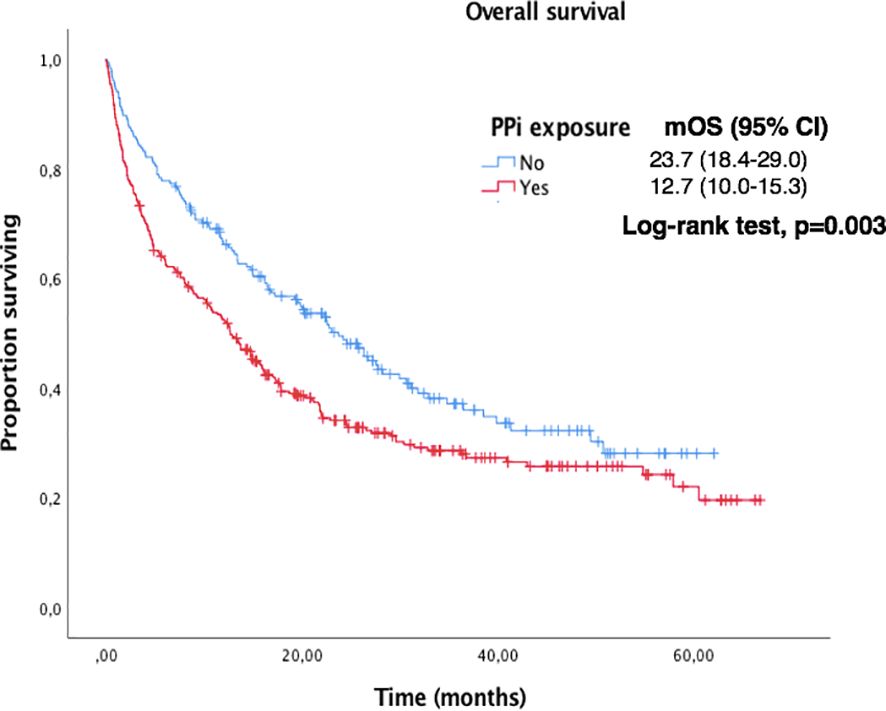
Figure 5. Kaplan-Meier curves for OS according to PPi exposure in the overall population.
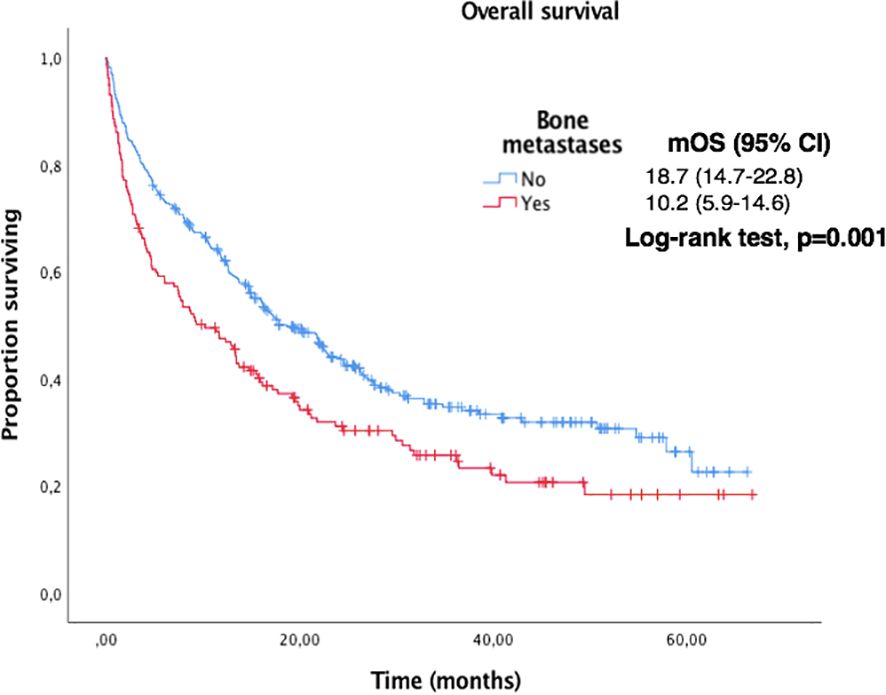
Figure 6. Kaplan-Meier curves for OS according to the presence of bone metastases in the overall population.
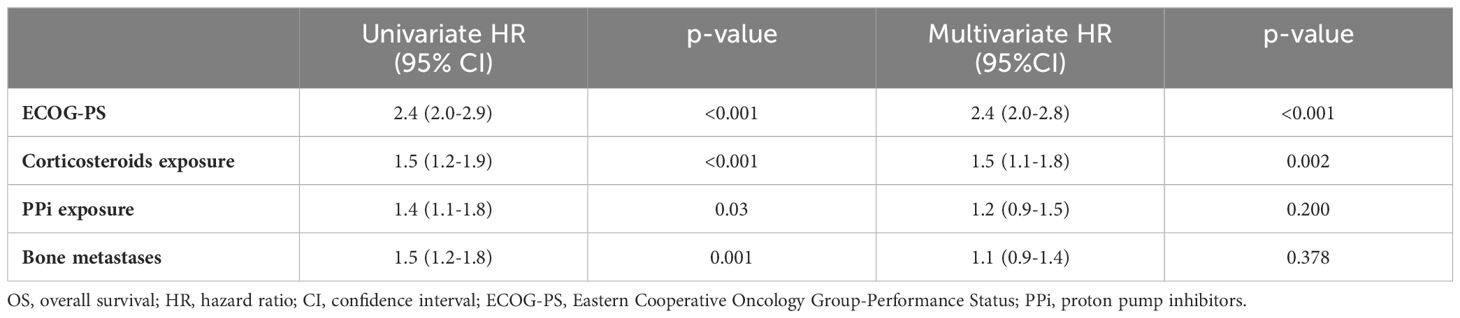
Table 3. Analysis of prognostic factors for OS.
Type of antibiotic and its indication are shown in Table 4. 175 (35%) patients received treatment with antibiotic: 64% received oral administration (64%) and 36% intravenous administration. The median OS in patients receiving oral antibiotics vs. those receiving intravenous antibiotics was 21.9m (95% CI 11.7 to 32.2) vs. 10.4m (95% CI 3.1 to 17.7), p=0.005. There was no significant difference in terms of survival between an antibiotic exposure ≤7 days (57%) or >7 days (43%).
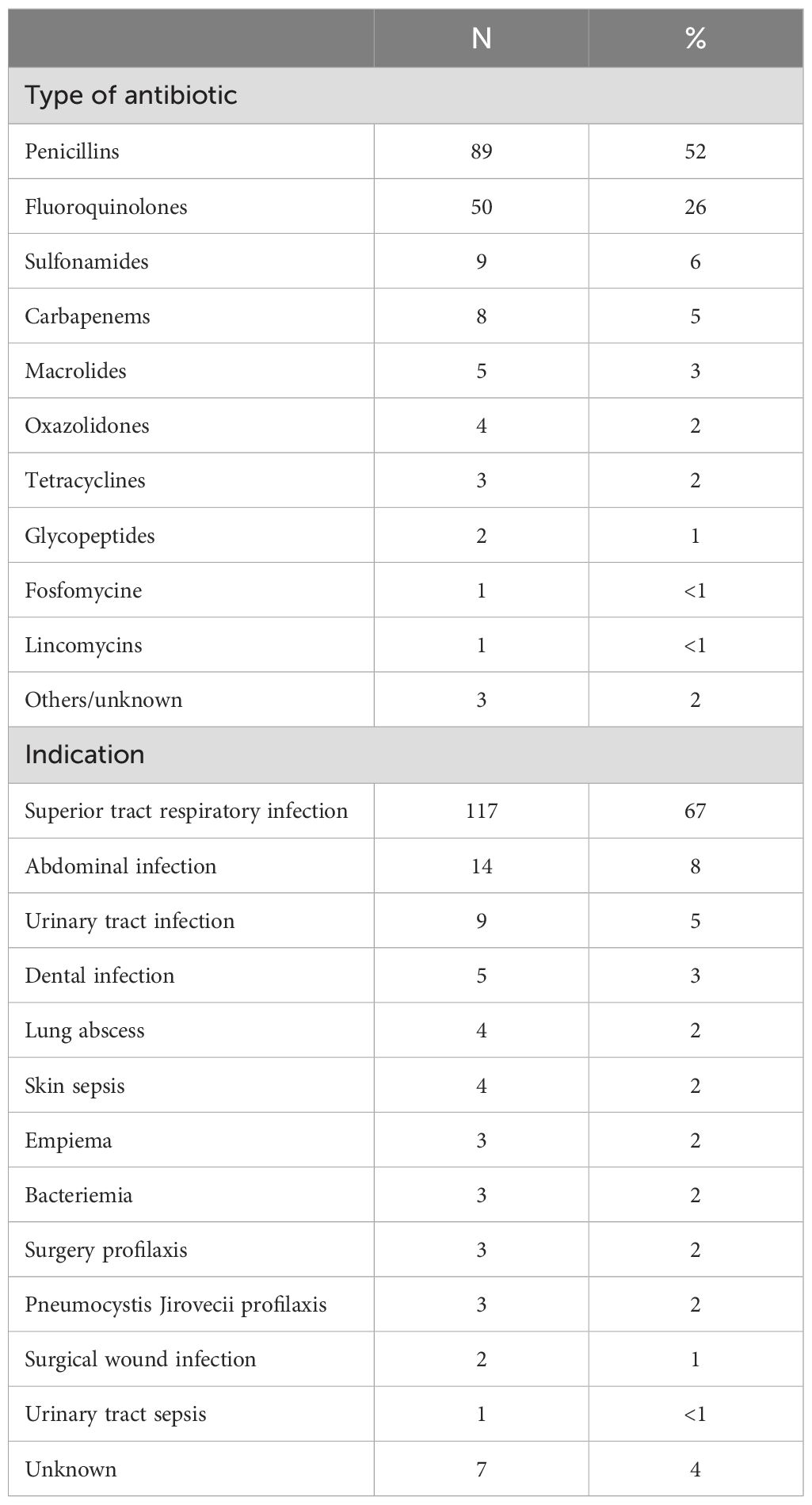
Table 4. Type of antibiotic and indication.
217 (44%) patients received oral (78%) or intravenous (20%) corticosteroids and presented shorter OS: the median OS in patients receiving corticosteroids vs. those who did not was 10.6 m (95% CI 7.2 to 14.0) vs. 22.1m (95% CI 17.8 to 26.4), p<0.001. The median OS in patients with oral vs. intravenous corticosteroids was 12.9m (95% CI 8.1 to 17.7) vs 3.52m (95% CI 0.0 to 8.4), p=0.001.
Finally, we analysed those patients with similar inclusion criteria to Keynote-024 trial (1, 2) (excluding patients with ECOG-PS≥2 or untreated brain metastases). Of the 329 patients (67%) included, the median OS and PFS were 22.3m (95% CI 17.4 to 24.1) and 12.5m (95% CI 10.0 to 15.1), respectively, with an ORR of 48%.
DiscussionOur real-world study confirms that corticosteroid treatment and poor ECOG-PS are negative predictive factors for first-line pembrolizumab monotherapy in patients with advanced high PD-L1 NSCLC.
The negative impact of corticosteroids was observed when they were administered as symptomatic treatment or for concomitant diseases, but not for the management of irAEs. Corticosteroid treatment should only be used under necessary conditions. Our findings are in line to previously reported series in advanced NSCLC patients (16, 24) and in other solid tumors (25). However, there are also conflicting results regarding the detrimental effect of corticosteroids for irAEs (26, 27), so the impact of corticosteroids for the management of irAEs in survival outcomes remains unclear.
Bone metastases and treatment with PPi were found to be negative predictive factors in the univariate analysis but were not confirmed in multivariate analysis. Other potential factors such as smoking habit, liver or CNS metastases, BMI or older age were not found to have negative impact, although there was a trend to a shorter median PFS and OS. Those findings are in line to similar previous studies with immunotherapy in advanced NSCLC (16–18).
We did not find a negative impact of antibiotic exposure, nor BMI in outcomes of patients receiving pembrolizumab. Interestingly, this finding was also observed in patients receiving first line chemotherapy and immunotherapy (28, 29). Of note, survival outcomes were significantly better in patients receiving oral than intravenous antibiotics in the univariate analysis, probably because patients receiving intravenous antibiotics presented a more serious infection.
To date, PD-L1 expression is the only validated biomarker in advanced NSCLC although it has several limitations (30). However, outcomes do not always correlate with PD-L1 expression level, as observed in our study, even in patients with high PD-L1 expression (8). There is an urgent need to define new biomarkers to better select the best treatment option. In the absence of more accurate biomarkers, the combination of chemotherapy and immunotherapy might be a good alternative in patients with advanced high PD-L1 NSCLC with negative predictive factors (10–14, 31).
One important finding of our study is that survival outcomes were similar to those observed in the Keynote-024 (1, 2) when patients with comparable characteristics were analysed, that were significantly better than in the overall population. This finding reaffirms the fact that patient with worse conditions receive treatment outside clinical trials. Additionally, our patient cohort has many similarities with the biggest real-world cohort reported in terms of age, histology, ECOG-PS, bone metastases and smoking history (16). We report a median age of 67.3 years vs. 70.1.; 76% of non-squamous histology vs. 77.9%, 83% of ECOG-PS 0-1 vs. 82.6%, 32% of patients with bone metastases vs. 33.6 and 92% of former/current smokers vs. 89.2%. A surprising fact, however, is that in our cohort we did not find a negative impact of antibiotics and PPI exposure, although a higher exposure was observed in our series. These differences may be explained by a smaller sample size, a shorter follow up in our cohort, or the possible different data frames for considering concomitant medication exposure.
Our study presents some limitations. Due to the retrospective design, some inherent selection bias may be implied, although this should be minimized by the consecutive patient selection criteria. In addition, data collection may have been heterogeneous between different institutions and data frames of some treatments may not be exact. However, the optimal time to collect concomitant antibiotic exposure is unclear, so we considered the same treatment period proposed in previous series (16).
Finally, as already indicated, the sample size and the follow-up may have meant that some numerical trends and significance of univariate analyses on predictive factors could not be confirmed after multivariate analysis, as well as the survival data may be immature. Despite these limitations, our real-world study supports the available evidence provided by randomized trials and other real-world cohorts of the efficacy of first line pembrolizumab in patients with advanced high PD-L1 NSCLC.
In conclusion, our study reaffirms the efficacy of first line pembrolizumab monotherapy in patients with advanced NSCLC and high PD-L1 expression with similar outcomes to those previously reported. Patients receiving corticosteroid treatment and with ECOG-PS 2 present worse outcomes after multivariate analysis. Alternative treatment options may be explored for patients receiving detrimental concomitant medication or unfit patients.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Hospital de la Santa Creu i Sant Pau Ethics Committe. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsAP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. SM-R: Conceptualization, Data curation, Formal Analysis, Investigation, Resources, Validation, Writing – original draft, Writing – review & editing. AH: Data curation, Investigation, Resources, Writing – original draft, Writing – review & editing. TM: Data curation, Investigation, Resources, Writing – original draft. EA: Data curation, Investigation, Resources, Writing – original draft. JR-B: Data curation, Investigation, Resources, Writing – original draft. MC: Data curation, Investigation, Resources, Writing – original draft. PC: Data curation, Investigation, Resources, Writing – original draft. JM: Data curation, Investigation, Resources, Writing – original draft. MF: Data curation, Investigation, Resources, Writing – original draft. RG-C: Data curation, Investigation, Resources, Writing – original draft. AC (12th author): Data curation, Investigation, Resources, Writing – original draft. RÁ: Data curation, Investigation, Resources, Writing – original draft. MZ-G: Data curation, Investigation, Resources, Writing – original draft. DI: Data curation, Investigation, Resources, Writing – original draft. AC (16th author): Data curation, Investigation, Resources, Writing – original draft. PI: Data curation, Investigation, Resources, Writing – original draft. JS-L: Data curation, Investigation, Resources, Writing – original draft. AB: Data curation, Investigation, Resources, Writing – original draft. IS: Data curation, Investigation, Resources, Writing – original draft. EF: Data curation, Investigation, Resources, Writing – original draft. MM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe following authors declare potential conflicts of interest outside the submitted work:
DI: Consultation Honoraria: Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, F. Hoffmann-La Roche, Johnson & Johnson, Lilly, Merck, MSD, Pfizer, Sanofi, Takeda. Speaker Honoraria: Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, F. Hoffmann-La Roche, Johnson & Johnson, MSD, Novartis, Pfizer, Takeda. Clinical Trials: Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, F. Hoffmann-La Roche, GSK, Janssen, Lilly, Merck, Mirati Therapeutics, MSD, Novartis, Pfizer, Sanofi. Research grant: AstraZeneca, BMS, F. Hoffmann-La Roche, GSK.
AC 12th author: AstraZeneca, Advisory Board, Personal. Bayer, Other, Personal, Speaker honoraria. Boehringer Ingelheim, Advisory Board, Personal. Bristol-Myers Squibb, Advisory Board, Personal. Janssen, Advisory Board, Personal. Lilly, Advisory Board, Personal. Merck Sharp & Dohme, Advisory Board, Personal. Novartis, Advisory Board, Personal. Pfizer, Advisory Board, Personal. PharmaMar, Invited Speaker, Personal. Regeneron, Advisory Board, Personal. Roche, Advisory Board, Personal. Sanofi, Advisory Board, Personal. Takeda, Advisory Board, Personal. Merck Sharp & Dohme, Research Grant, Institutional, Financial interest, Drug-only for Investigator-initiated trial.
PI: Advisory role and/or travel compensation: Bristol‐Myers Squibb, F. Hoffmann, La Roche AG, MSD Oncology, Pfizer, Medscape, Astra Zeneca, Takeda, Amgen.
JS-L: Astra Zeneca, Invited Speaker, Personal. Astra Zeneca, Advisory Board, Personal. BMS, Invited Speaker, Personal. BMS, Advisory Board, Personal. MSD, Invited Speaker, Personal. MSD, Advisory Board, Personal. Roche, Invited Speaker, Personal. Roche, Advisory board, Personal. Eisai, Invited Speaker, Personal. La Roche Posay, invited Speaker, Personal.
AB: Astrazeneca advisory board and personal and invited speaker, BMS expert testimony and invited speaker, MSD invited speaker, Novartis invited speaker, Pfizer invited speaker, Personal, Piere Fabre invited speaker, Roche invited speaker and advisory board, Sanofy advisory board and invited speakerBMS, Principal Investigator, Clinical Trial CA224-1044. Pfizer, Principal Investigator, Clinical Trial C4221016.
EA: Consultant or Advisory Role: MSD, Bristol-Myers, Roche, Boehringer Ingelheim, Pfizer, Novartis, AstraZeneca, Lilly, Takeda. Speaking: MSD, Bristol-Myers, Roche, Boehringer Ingelheim, Pfizer, Novartis, AstraZeneca, Lilly, Takeda. Co-founder: Trialing Health S.L.
MC: Consultant or Advisory Role: Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, Lilly, MSD, Takeda, Phyzer, Kyowa, Sanofi,Jansen. Speaking: Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, Lilly, MSD, Takeda, Kyowa, Pierre-fabre, Novocure, Sanofi, Jansen.
MF: AstraZeneca, Invited Speaker, Personal. BMS, Invited Speaker, Personal. BMS, Advisory Board, Personal. Janssen, Invited Speaker, Personal. Janssen, Advisory Board, Personal. Pfizer, Invited Speaker, Personal.
RG-C: Astra Zeneca, Invited Speaker, Personal; Astra Zeneca, Advisory Board, Personal; BMS, Invited Speaker, Personal; BMS, Advisory Board, Personal; Jansen, Advisory Board, Personal; Jansen, Invited Speaker, Personal; lilly, Invited Speaker, Personal; lilly, Advisory Board, Personal; MSD, Advisory Board, Personal; novartis, Invited Speaker, Personal; novartis, Advisory Board, Personal; pfizer, Invited Speaker, Personal; pfizer, Advisory Board, Personal; roche, Invited Speaker, Personal; roche, Advisory Board, Personal; Sanofi, Advisory Board, Personal; Takeda, Advisory Board, Personal; Takeda, Invited Speaker, Personal; Astra Zeneca, Steering Committee Member, Personal, Financial interest; Jansen, Steering Committee Member, Personal, Financial interest.
EF: Personal honoraria for advisory board participation from Abbvie, Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, BMS, Eli Lilly, F. Hoffmann-La Roche, Genmab, Gilead, GSK, Janssen, Merck Serono, MSD, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point, Daiichi Sankyo; personal speaker honoraria from Amgen, AstraZeneca, BMS, Daiichi Sankyo, Eli Lilly, F. Hoffmann-La Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, MSD, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology; Board of Director role: Grifols; financial support for meeting attendance and/or travel from AstraZeneca, Janssen, Roche.
MM: Advisory Board,consulting fees or sspeakin honoraria: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Helsinn Therapeutics, Eli Lilly, Immedica, Beigene, MSD, Novartis, Pfizer, F. Hoffmann-La Roche Ltd., Takeda, Sanofi, Janssen, Amgen, Cassen. Research funding institution: Bristol-Myers Squibb, AstraZeneca, F. Hoffmann-La Roche Ltd. Travel and accommodation support: AstraZeneca, F. Hoffmann-La Roche Ltd., Pfizer, MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. (2019) 37:537–46. doi: 10.1200/JCO.18.00149
PubMed Abstract | Crossref Full Text | Google Scholar
2. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
PubMed Abstract | Crossref Full Text | Google Scholar
3. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csöszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin. (2021) 39:2339–49. doi: 10.1200/JCO.21.00174
PubMed Abstract | Crossref Full Text | Google Scholar
4. Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M, Cappuzzo F, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer. (2020) 21:498–508.e2. doi: 10.1016/j.cllc.2020.06.010
PubMed Abstract | Crossref Full Text | Google Scholar
5. Cortellini A, Tiseo M, Banna GL, Cappuzzo F, Aerts JGJV, Barbieri F, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother. (2020) 69:2209–21. doi: 10.1007/s00262-020-02613-9
PubMed Abstract | Crossref Full Text | Google Scholar
6. Velcheti V, Chandwani S, Chen X, Catherine Pietanza M, Burke T. First-line pembrolizumab monotherapy for metastatic PD-L1-positive NSCLC: real-world analysis of time on treatment. Immunotherapy. (2019) 11:889–901. doi: 10.2217/imt-2019-0061
PubMed Abstract | Crossref Full Text | Google Scholar
7. Velcheti V, Hu X, Li Y, El-Osta H, Pietanza MC, Burke T. Real-world time on treatment with first-line pembrolizumab monotherapy for advanced NSCLC with PD-L1 expression ≥ 50%: 3-year follow-up data. Cancers (Basel). (2022) 14(4):1041. doi: 10.3390/cancers14041041
PubMed Abstract | Crossref Full Text | Google Scholar
8. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. (2021) 397:592–604. doi: 10.1016/S0140-6736(21)00228-2
PubMed Abstract | Crossref Full Text | Google Scholar
9. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. (2021) 16:1872–82. doi: 10.1016/j.jtho.2021.06.019
PubMed Abstract | Crossref Full Text | Google Scholar
10. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
PubMed Abstract | Crossref Full Text | Google Scholar
11. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov KD, et al. LBA51 - EMPOWER-Lung 3: Cemiplimab in combination with platinum doublet chemotherapy for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC). Ann Oncol. (2021) 32:S1328. doi: 10.1016/j.annonc.2021.08.2130
Crossref Full Text | Google Scholar
13. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
PubMed Abstract | Crossref Full Text | Google Scholar
14. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. (2023) 41:1213–27. doi: 10.1200/JCO.22.00975
PubMed Abstract | Crossref Full Text | Google Scholar
15. Facchinetti F, Mazzaschi G, Barbieri F, Passiglia F, Mazzoni F, Berardi R, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer. (2020) 130:155–67. doi: 10.1016/j.ejca.2020.02.023
PubMed Abstract | Crossref Full Text | Google Scholar
16. Cortellini A, Di Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JGJV, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. (2021) 9(4):e002421. doi: 10.1136/jitc-2021-002421
PubMed Abstract | Crossref Full Text | Google Scholar
17. Ichihara E, Harada D, Inoue K, Sato K, Hosokawa S, Kishino D, et al. The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer. (2020) 139:140–5. doi: 10.1016/j.lungcan.2019.11.011
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. (2020) 6:512–8. doi: 10.1001/jamaoncol.2019.5241
PubMed Abstract | Crossref Full Text | Google Scholar
19. Cortellini A, Cannita K, Tiseo M, Cortinovis DL, Aerts JGJV, Baldessari C, et al. Post-progression outcomes of NSCLC patients with PD-L1 expression ≥ 50% receiving first-line single-agent pembrolizumab in a large multicentre real-world study. Eur J Cancer [Internet]. (2021) 148:24–35. doi: 10.1016/j.ejca.2021.02.005
PubMed Abstract | Crossref Full Text | Google Scholar
20. Pathak R, De Lima Lopes G, Yu H, Aryal MR, Ji W, Frumento KS, et al. Comparative efficacy of chemoimmunotherapy versus immunotherapy for advanced non-small cell lung cancer: A network meta-analysis of randomized trials. Cancer. (2021) 127:709–19. doi: 10.1002/cncr.v127.5
Crossref Full Text | Google Scholar
21. Zhou Y, Lin Z, Zhang X, Chen C, Zhao H, Hong S, et al. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J Immunother Cancer. (2019) 7(1):120. doi: 10.1186/s40425-019-0600-6
PubMed Abstract | Crossref Full Text | Google Scholar
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
PubMed Abstract | Crossref Full Text | Google Scholar
23. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. (2017) 18:e143–52. doi: 10.1016/S1470-2045(17)30074-8
PubMed Abstract | Crossref Full Text | Google Scholar
24. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. (2019) 37:1927–34. doi: 10.1200/JCO.19.00189
留言 (0)