Normal coronary artery anatomy is characterized by the presence of two ostia rising from the left and right coronary cusps (RCCs). The left coronary system originates from the left coronary cusp, giving rise to the left main coronary artery (LMCA) that bifurcates into the left anterior descending artery (LAD) and the left circumflex coronary artery (LCX) which courses around the atrioventricular groove, and the right coronary system originates from the RCC and courses backward in the right atrioventricular groove.[1]
Congenital anomalous coronary arteries (CACAs) are rare and challenging to recognize. Coronary anomalies can occur as primary isolated forms, or as secondary forms in association with congenital heart disease (such as pulmonary atresia with intact interventricular septum or hypoplastic left heart syndrome with aortic atresia and severe mitral stenosis).[1]
The most common CACA is the separate origin of the LAD and the LCX with an incidence of 0.41% followed by LCX arising from the right coronary artery (RCA), with an incidence of 0.37%.[2]
Dual LAD is an uncommon condition characterized by different anatomical variations, all refer to two distinct arteries supplying all or a portion of the LAD territory, often a short LAD and a long LAD. The short LAD usually ends in the mid-septum but can extend high in the proximal portion.[2] The long LAD pathway frequently varies proximally and can go beyond the usual pathway, but the typical characteristic is that it reaches the distal of the anterior interventricular sulcus (AIVS).[2]
This paper aims to highlight a rare variation of the dual LAD anatomy where LAD2 originates from the RCC, separate from the ostium of the RCA.
CASE REPORTA 71-year-old male with a history of type II diabetes mellitus, hyperlipidemia, and hypertension was referred to the cardiology clinic following an abnormal ECG, which revealed sinus bradycardia and a new interventricular conduction delay with a QRS interval of 130ms. The patient denied experiencing chest pain, either at rest or during exertion, but reported dyspnea on exertion, specifically when climbing one flight of stairs.
Given these findings, the patient underwent an exercise transthoracic stress echocardiography, which was notable for moderate shortness of breath, possibly indicating an anginal equivalent. The stress electrocardiogram was non-diagnostic due to baseline ST and T wave abnormalities. Echocardiography also revealed baseline akinesia involving the basal septum and basal inferior wall, with no evidence of reversible ischemia.
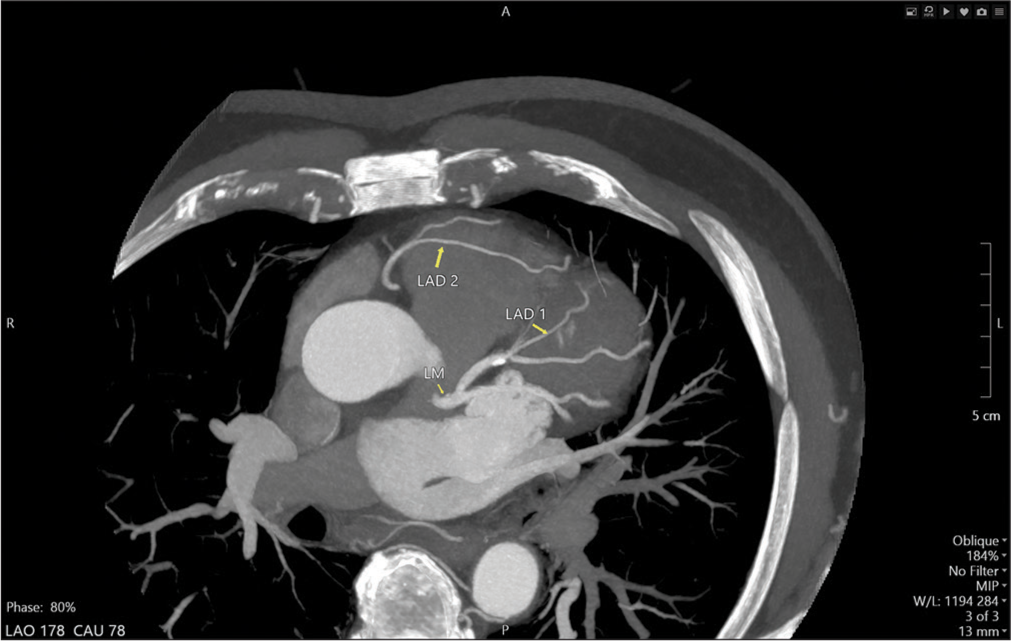
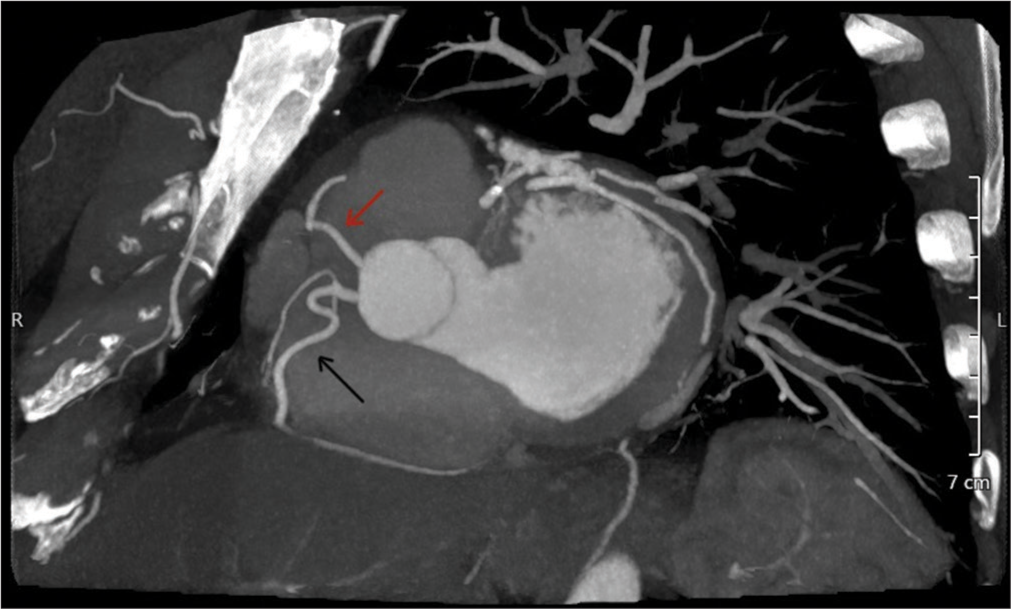
To further evaluate for coronary artery disease, a Coronary Computed Tomography Angiography (CCTA) was performed. The CCTA demonstrated dual left anterior descending (LAD) artery anatomy. The first LAD (LAD1) originated from the left main coronary artery (LMCA) and terminated in the proximal anterior interventricular sulcus (AIVS), while the second LAD (LAD2) originated separately from the right coronary cusp (RCC) via a separate ostium from the right coronary artery (RCA) and extended to the distal AIVS [Figures 1 and 2]. The CCTA also showed a calcified plaque causing 30% stenosis in the proximal LAD1. Additionally, a branching, medium-sized ramus intermedius coronary artery was observed, free of stenosis.
Export to PPT
Export to PPT
Given the findings, the patient was initiated on guideline-directed medical therapy, with no current indication for invasive coronary angiography.
DISCUSSIONSince the first recognition of dual LAD anatomy, there have been multiple suggestions for a clear classification of the anatomical variations. Beginning with Spindola-Franco et al. in 1983, followed by numerous case reports and case series with unclassified types of dual LAD, Jariwala et al. finally suggested a new classification of the various anatomical variations of dual LAD based on angiographic and autopsy findings, in an attempt to cover the unclassified types of dual LAD anatomy.[3]
Group I or the “split” dual LAD system: LAD1 and LAD2 bifurcate from the proper LAD coming from the LMCA. In this group, the whole left coronary system and its major branches arise from the left coronary sinus (LCS).
Group II or the “true” dual LAD system: The dual LAD originates partially from the LCS and the right coronary sinus (RCS). The left-sided LAD or LAD1 (coming from the LCS) terminates in the proximal AIVS and the right-sided LAD or LAD2 originates from the RCS or any segment of the RCA and enters the distal IAVS to terminate into the LV apex
Group III or the “anomalous” dual LAD system: The entire left coronary system arises from the RCS and lacks a constant morphological feature.
These three groups further divide into subgroups with a variety of characteristics, complicating the understanding of dual LAD anatomy and adding to its diversity.
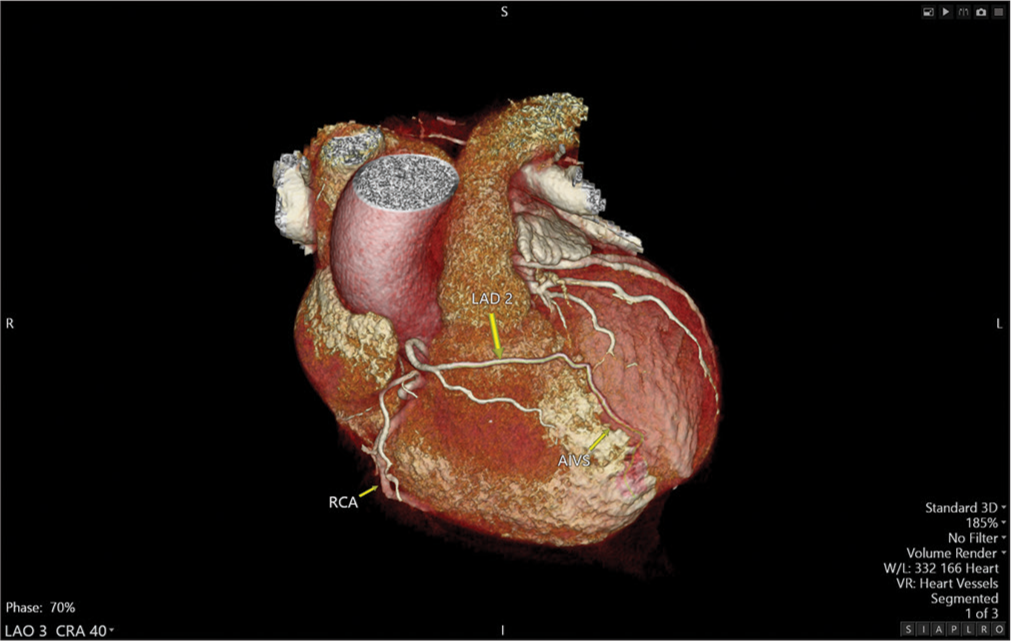
In our patient, the anterior wall of the LV was supplied by two LADs. The LAD1 emerged from the LMCA ending abruptly in the proximal IAVS. The LAD2 originated from the RCC separately from the RCA ostium, traversed toward the left side, anterior to the right ventricular outflow tract (RVOT), and entered the mid AIVS to terminate distally [Figure 3].
Export to PPT
Our case falls under class IIA based on the new classification proposed by Jariwala et al.[3] representing a rare entity within this subgroup. To the best of our knowledge, there are only two reported cases of LAD2 originating from RCC separate from the RCA: Deora et al.[4] and Prasad et al.[5] describing similar cases but with a different LAD2 course: The LAD2 after rising from the RCC, traversed toward the left, between the RVOT and aortic root, terminating in the mid to distal interventricular groove.
About only 20% of dual LAD anomalies have symptoms while the majority are asymptomatic, as in our case.[3]
Diagnosis is mainly incidental and based on the suspicion of coronary artery abnormalities on cardiac imaging. CCTA, coronary magnetic resonance angiography, and transthoracic echocardiography are valuable non-invasive imaging modalities in the diagnosis of such cases.[6] Coronary angiography, an invasive procedure, is still the gold standard for diagnosis given its ability to perform corrective intervention for coronary artery atherosclerotic disease if required, but CCTA is more 3D omniplanar in assessing the origin, course, and connection with other cardiac and non-cardiac structures.[6] These modalities can be used for planning surgical revascularization when needed, which requires a precise understanding of the coronary anatomy. The presence of a dual LAD system, with all its variations, should be clearly identified for the revascularization of the correct arterial system and the prevention of inappropriate arteriotomy.[7]
CONCLUSIONOverall, a short LAD, identified on any imaging modality, should prompt consideration of a dual LAD system, particularly if there is an absence of coronary arteries supplying the left ventricle apical region.
Recognizing and understanding the dual LAD anatomy is crucial in the decision-making process of revascularization, whether by surgical or percutaneous catheter-based approaches. Detailed anatomical knowledge of the dual LAD system can optimize procedural outcomes, ensuring comprehensive care for patients with this unique coronary artery configuration.
留言 (0)