Idiopathic granulomatous mastitis (IGM), also known as granulomatous lobular mastitis, is a rare but benign chronic inflammatory disease of the breast that continues to challenge clinicians with its elusive etiology, unpredictable presentations, and diagnostic complexities. While little is known of the etiology of granulomatous mastitis (GM), an autoimmune, infectious, or hypersensitivity reaction are among the most commonly proposed theories.[1] Cases associated with trauma to the epithelium of the mammary ducts are also gaining attention.
GM typically presents in women of childbearing age, 20–40 years old, typically within the first few years postpartum.[2,3] Clinical presentation varies widely, encompassing painless localized masses that grow slowly over time, painful erythematous masses, inflammation, induration, fistula formation, skin ulceration, nipple discharge, and nipple retraction.[3] The diagnosis of GM poses a formidable challenge due to its rarity and varied clinical presentation along with non-specific radiographic findings which vary based on the timing of radiographic evaluation, extent of inflammation, and possibility of prior intervention. It is essential to rule out carcinoma of the breast due to its similar presentation. Definitive diagnosis is based on histological evidence of lobulocentric granulomatous formations within the biopsied breast tissue.[4] Treatment options are not clearly established but include observation, corticosteroids, and immunosuppressants. Often, surgical management is the last resort, although lesions may recur and result in poor esthetic outcomes.[1]
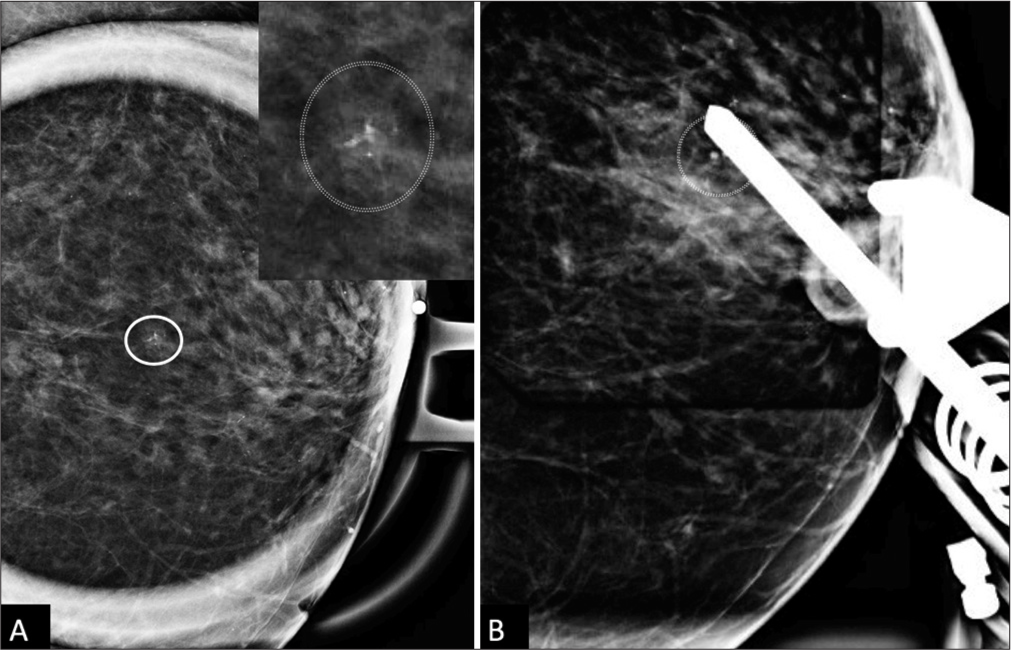
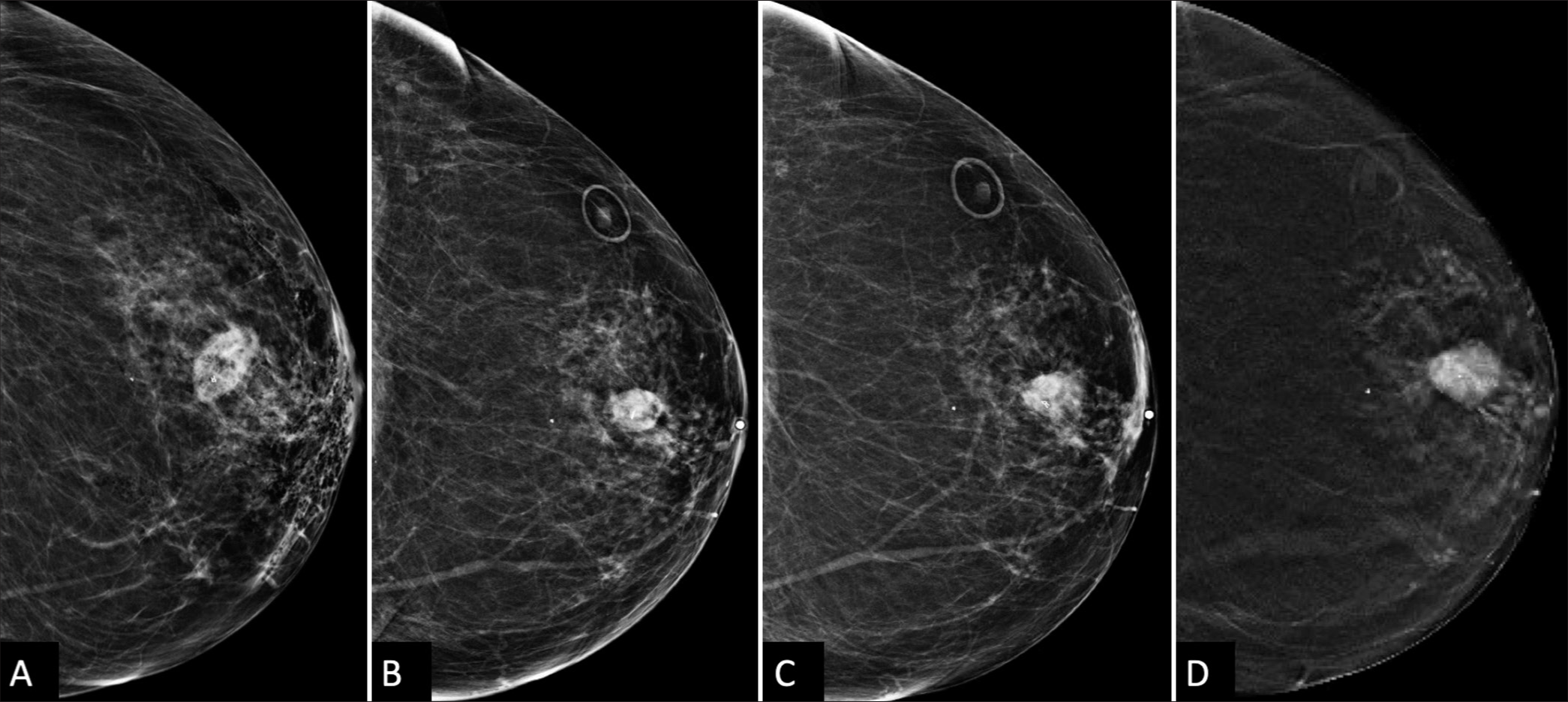
CASE REPORTA 66-year-old woman, without personal history of breast cancer, presented for her annual screening mammogram which detected a 3 mm group of calcifications in the left breast, at the 12 o’clock position, 3 cm from the nipple, which were characterized as amorphous on diagnostic mammography [Figure 1] and recommended for a stereotactic core-needle biopsy. Post-biopsy, there was a 2 cm hematoma [Figure 2A]. Pathology resulted in benign fibrosis and associated microcalcifications, concordant with imaging findings. On the initial 6-month post-biopsy follow-up, there was a slight expected decrease in size of the hematoma [Figure 2B], which remained unchanged at 1 year follow-up [Figure 2C].
Export to PPT
Export to PPT
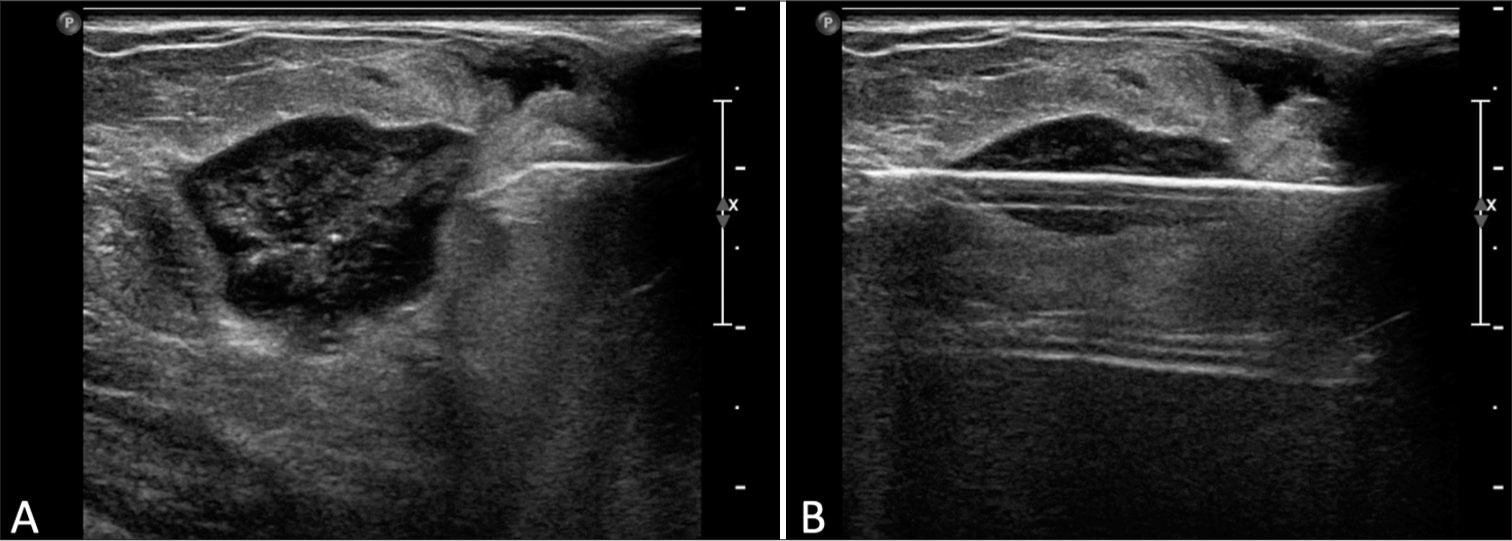
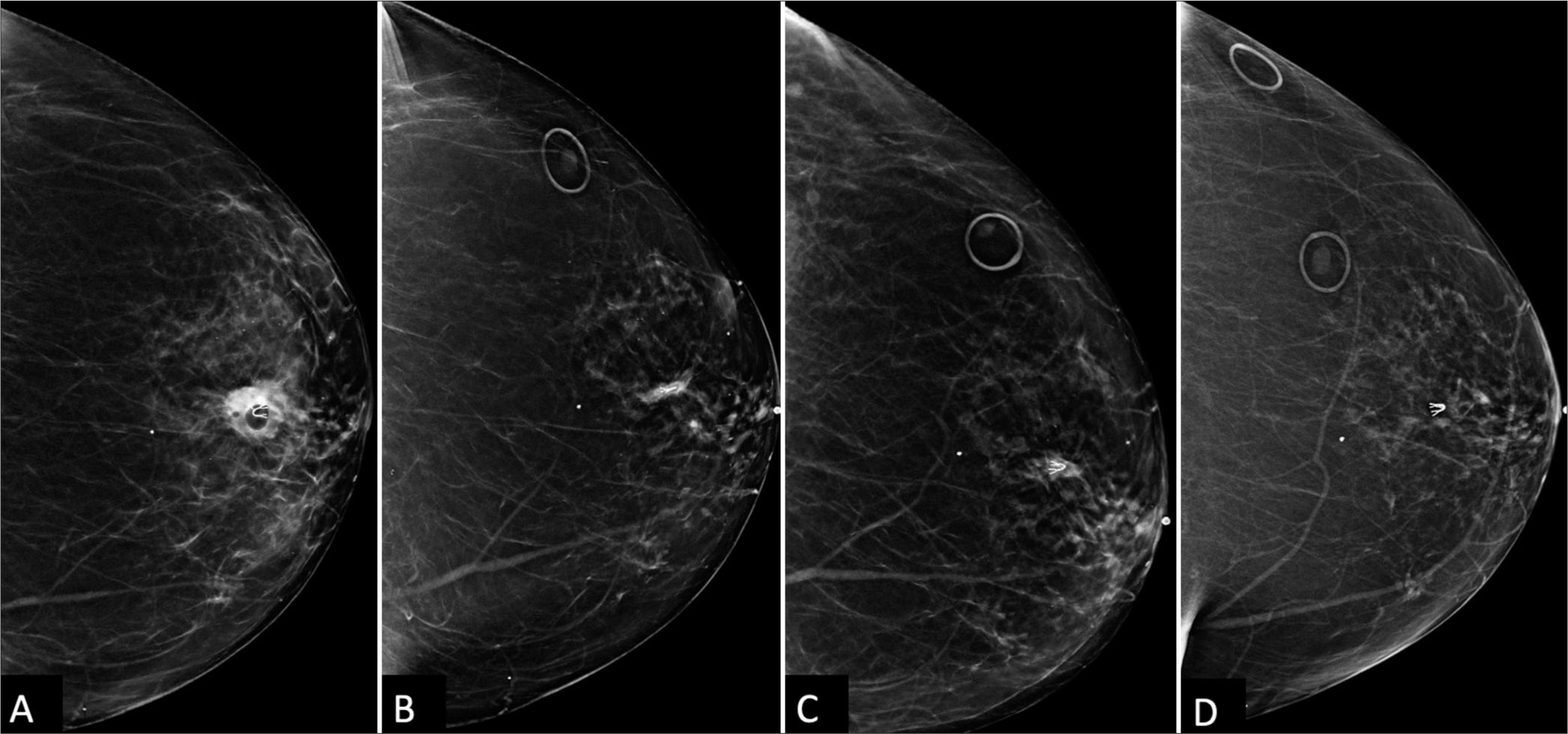
At 21 months post-biopsy, there was interval increased size, density, and subtle new distortion of the mammographic mass known to represent a post-procedural hematoma [Figure 2D]. Targeted left breast ultrasound at the 12 o’clock position, 3 cm confirmed increased size of the hematoma now 20 mm in size [Figure 3A and B]. Given interval enlargement, a subsequent ultrasound-guided core biopsy was performed with partial dissipation of the complex cystic and solid mass and resultant pathology of GM [Figure 4A]. The case was reviewed in multidisciplinary conference, and given patient was not symptomatic, a conservative watchful approach was taken.
Export to PPT
Export to PPT
At her next clinical visit, the patient reported waxing and waning of skin erythema and pain at the ultrasound biopsy entrance site [Figure 4B]. On physical examination, the left breast revealed 2 cm skin erythema with overlying cystic fluctuance. Thick yellowish drainage was expressed and the cystic fluctuance fully decompressed, with complete resolution of erythema and pain following a 10-day course of antibiotics (Bactrim DS). During the two successive screening mammograms, the mammographic asymmetry continued to shrink to full resolution [Figure 4C and D].
DISCUSSIONIGM is a rare and challenging condition characterized by chronic inflammation of the breast with an uncertain etiology. It mainly affects young women of childbearing age, but it has also been reported in men and elderly women. Differential diagnosis and common mimickers of GM include invasive ductal carcinoma, periductal mastitis, fibrocystic changes, diabetic mastoplasty, tuberculosis mastitis, non-tuberculosis infectious mastitis, and eosinophilic mastitis.[1] Diagnostic work-up of GM should include mammography, ultrasound, and biopsy of suspicious lesions to rule out other causes.
This case report describes a unique presentation of GM in a 66-year-old woman following a stereotactic core-needle biopsy of her left breast, with unexpected enlargement of a post-procedural hematoma at 1½ year interval post-biopsy, and subsequent ultrasound-guided biopsy leading to the diagnosis of GM [Figure 5A and 5B]. The location and the temporal relationship in the development of GM lead us to the hypothesis that the causative agent in this case is the direct trauma from the stereotactic biopsy and likely the chemical mastitis resulting from the formation of the chronic post-procedural hematoma.
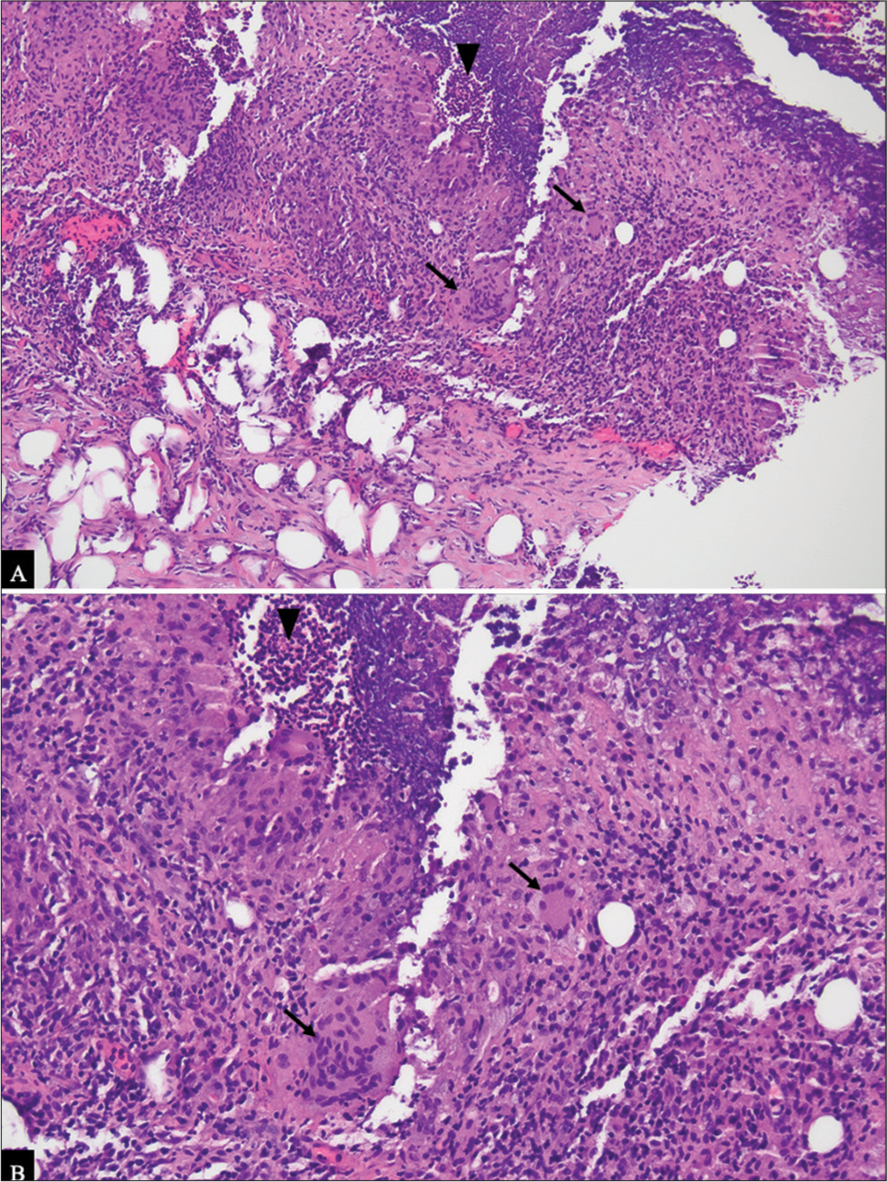
Export to PPT
Interestingly, this patient’s skin infection following the ultrasound-guided percutaneous biopsy may have been a result of an infrequent but known complication. However, it has also been reported that GM often presents with multiple tender lumps forming micro abscesses at needle biopsy sites and may express sterile pus and resemble a skin infection,[5] similarly to this case in which the patient was treated for a suspected skin infection with bactrim. If antibiotic therapy fails and an infectious cause are ruled out, corticosteroids may be considered for the treatment. Corticosteroids, while effective in reducing inflammation, carry a large side effect risk, including immunosuppression and the potential for relapse once treatment is discontinued. Methotrexate has emerged as an effective alternative treatment for IGM, particularly in cases where corticosteroids are contraindicated or poorly tolerated. It has been used successfully as a steroid sparing agent, either alone or in combination with low-dose corticosteroids, in patients with recurrent or refractory IGM.[6] However, methotrexate therapy requires careful monitoring due to its potential side effects including hepatotoxicity, myelosuppression, and pulmonary toxicity. Surgery is reserved for refractive and aggressive cases. Recurrence may occur regardless of the treatment approach. Given this patient’s stability and mild symptoms, a conservative approach was deemed appropriate with a plan for close follow-up to monitor disease progression.
To the best of our knowledge, there has been only one other case published regarding IGM occurring secondary to breast biopsy, however associated with percutaneous ultrasound guided core biopsy in a 42-year-old patient with prolonged breast feeding,[5] but there have been other cases noting the development of IGM after other forms of trauma to the breast, such as post-ductoscopy.[7]
A more comprehensive review of the literature discusses how diagnosis of IGM remains a challenge, particularly in post-biopsy scenarios. It has been suggested that trauma, including from biopsy, may trigger an exaggerated immune response leading to granulomatous inflammation,[8] as seen in this case. While there are limited reports of IGM following percutaneous biopsy, this case adds to the growing body of evidence suggesting that such trauma can play a significant role in the development of IGM.
CONCLUSIONThis case adds to the evolving understanding of IGM, particularly in the context of post-biopsy complications. Clinicians should remain vigilant in monitoring patients with a history of breast procedures, as trauma-induced GM may manifest years later. Continued research is warranted to elucidate the complex etiology of IGM and to optimize diagnostic and therapeutic strategies for this challenging condition.
留言 (0)