Dear Editor,
Janus kinase (JAK) inhibitors have the potential to replace immunosuppressants for varied autoimmune and inflammatory conditions. Although safer than traditional steroids, these molecules have a labelled concern for thromboembolic events like deep vein thrombosis and pulmonary embolism. We report a refractory case of bullous pemphigoid who developed bilateral hemiretinal vein occlusion (HRVO) on tofacitinib therapy. We discuss the possible association and elaborate the concerning ocular side effect of tofacitinib.
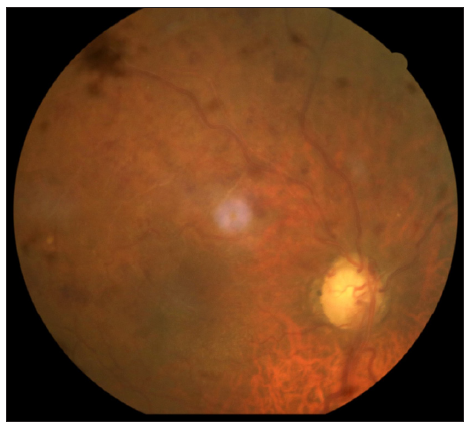
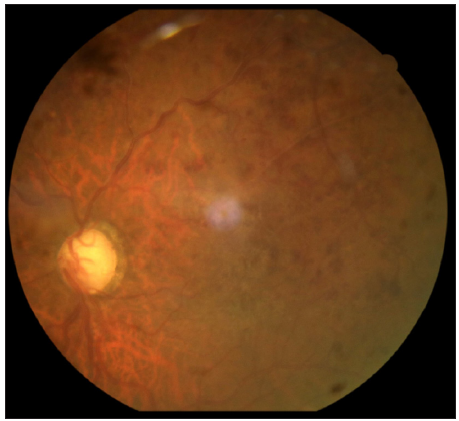
A 72-year-old male, a known case of bullous pemphigoid, in remission for four years, presented with acute flare of disease activity for two months, associated with intense itching (Bullous pemphigoid disease area index [BPDAI]. Subjective ‒24/30, Objective 30/360). The patient reported a past history of treatment with steroids and azathioprine for a year. He also complained of gradual painless progressive diminution of vision in both eyes for the last six months. Ophthalmological examination showed advanced glaucomatous changes in both eyes. In view of these ocular changes, the patient was started on doxycycline 100 mg twice a day, niacinamide 1 gm twice a day, antihistamines and high-potency topical steroids for the management of bullous pemphigoid. The daily lesional count was reduced, but the intractable pruritus persisted worsening his sleep quality. For this we administered Tofacitinib 5 mg BD, which reduced his itching markedly within six days, but he complained of acute painless worsening of vision. Repeat ophthalmological examination revealed attenuated arteriole with dilated tortuous venules and extensive superficial retinal haemorrhage in the superior half of the retina in both eyes, suggestive of HRVO [Figures 1 and 2]. Fundus fluorescein angiography confirmed the diagnosis showing a prolonged arteriovenous phase, delayed filling of venules, its staining and non-perfused areas. Intraocular pressure was 12 mmHg (right eye) and 14 mmHg (left eye), respectively, with no sudden spike on diurnal charting. There was no personal or family history of any neurological disorder, stroke, diabetes and hypertension. He was a non-alcoholic and non-smoker. An extensive haematological investigation ruled out any evidence of hyperviscosity or thromboembolism. A two-dimensional echocardiography and computed tomography angiography of the brain was normal, ruling out atherosclerotic thrombi as a cause.
Export to PPT
Export to PPT
For the management, tofacitinib was withdrawn, based on the temporal association between the drug and symptoms and the patient remained under close follow-up. His intraretinal haemorrhage resolved with few collaterals in the ischemic area without neovascularisation. Retinal photocoagulation of non-perfused areas was done. The intraocular pressure was normalised. His inferior altitudinal field loss persists with visual acuity stable at 6/18 in either eye.
bullous pemphigoid is a chronic, relapsing autoimmune disease frequently affecting the elderly patients and the treatment is aimed at arresting the development of new lesions along with cutaneous healing and control of pruritus. Topical/systemic steroids remain the first-line treatment, but in view of glaucomatous changes in the eye, this was not an option. The patient had persistent pruritus despite the reduction in BPDAI with second-line medications and hence tofacitinib was administered based on a previous report.1 The isolated event of bilateral HRVO without any evidence of a systemic venous thromboembolic event and normal intraocular pressure, with no sudden spike on diurnal charting and no prior veno-occlusive changes ruled out glaucoma, suggesting that tofacitinib could be a cause of HRVO. Non-specific Jakinibs like tofacitinib that target IL-10R associated JAKs and IFNβ and IFN λ associated JAK may result in the imbalance of prothrombotic and antithrombotic mechanisms resulting in thrombus formation.2 We hypothesise that endothelial dysfunction and damage secondary to glaucoma, coupled with advanced age, bullous pemphigoid as a comorbid disease, may have led to acute HRVO, developing within a week of tofacitinib initiation. There have been similar reports of retinal vein occlusion reported with tofacitinib and with other JAK inhibitors.3–5 The previous case of RVO was reported after a period of two months in a 47-year-old female with hypertension, rheumatoid arthritis treated with tofacitinib 5 mg BD for scleritis, resulting in drug withdrawal.3 Another report of tofacitinib induced RVO was seen in a 45-year-old, non-smoking woman with no risk of thromboembolic disease necessiating drug withdrawal.5 We further searched the US Food and Drug Administration (FDA) adverse events reporting system (FAERS) public dashboard, which revealed 17 similar events, even though these adverse events reported do not implicate a drug causation [Table 1].6
Table 1: Ocular reactions reported with tofacitinib as per FDA adverse events reporting system (FAERS) database (2013–2023)*.
Reaction Number of cases Percentage of total cases (%) Number of Cases 5033 Visual Impairment 1064 21.14047 Cataract 834 16.57063 Dry Eye 640 12.71607 Vision Blurred 478 9.497318 Eye Disorder 448 8.901252 Blindness 365 7.252136 Glaucoma 277 5.503676 Eye Pain 218 4.331413 Ocular Hyperaemia 160 3.179018 Eye Swelling 140 2.781641 Eye Irritation 138 2.741903 Uveitis 129 2.563084 Lacrimation Increased 115 2.28492 Macular Degeneration 103 2.04649 Retinal Vein Occlusion 17 0.338While a pre-existing ocular pathology existed in our patient, ocular complaint is uncommon. This report highlights the importance of ocular evaluation before starting tofacitinib, especially in elderly patients with comorbidities.
留言 (0)