Cerebrovascular lesions are a major cause of mortality and morbidity and affect a large number of patients seeking neurological care. For instance, in 2020, every 40 s someone in the United States had a stroke, and every 3.5 min someone died of a stroke; resulting in more than 795,000 annual cases of stroke in the United States. Neuroradiologists commonly encounter cerebrovascular abnormalities on magnetic resonance imaging (MRI), including the incidental discovery of various intracranial vascular lesions on imaging performed for myriad indications, with conventional unenhanced T2-weighted images acquired as a routine sequence.
In spin-echo imaging, the protons in flowing fluid move out of the plane of radiofrequency pulses between the initial excitation pulse and the refocusing pulse. This movement, known as the time-of-flight effect, causes the protons to miss the refocusing pulse and dephase, resulting in no signal contribution to that specific voxel. The extent of signal loss depends on the velocity of the protons moving out of the imaging plane, slice thickness, and time to echo (TE). In addition, signal loss can occur due to spin-phase effects caused by motion within the same imaging plane. The velocity of blood varies at different points within a vessel, leading to protons acquiring distinct phases as they traverse the applied gradient used for spatial encoding. The phase shift is determined by the proton’s velocity, gradient strength, and TE. These differences cause signal loss within a voxel due to spin dephasing at a microscopic level.
Vascular structures typically demonstrate a low signal flow-void on T2-weighted images. T2 and proton-density sequences with a long TE demonstrate the most prominent flow voids. In our experience, large cerebrovascular abnormalities are easily evident. Thus, neuroradiologists should evaluate all T2-weighted images for incidental cerebrovascular lesions, irrespective of the primary indication of the study, and may recommend further evaluation with follow-up computed tomography, magnetic resonance (MR), or conventional angiogram as appropriate. In this article, we present the characteristic imaging appearance of various cerebrovascular lesions of the brain on routine non-contrast T2-weighted MRI [Table 1].
Table 1: Cerebrovascular lesion characteristics on conventional T2-weighted imaging.
Cerebrovascular lesion T2-weighted imaging characteristics Aneurysm Hypointense outpouching with presence of internal laminated thrombus in some patients Arteriovenous malformation Heterogeneous signal nidus with adjacent hypointense draining vessels Total arterial occlusion Asymmetric loss of, or abnormal flow void, with loss of normal hypointensity Capillary telangiectasia Most commonly subtle hyperintense lesion Cavernous malformation “Popcorn” or “berry” appearance with a rim of signal loss Dural arteriovenous fistula Dilated arteries and brain without intervening nidus Moyamoya disease Stenosis of the carotid terminus and proximal middle cerebral artery, with distal arterial reconstitution Proliferative angiopathy Tangle of vessels and adjacent dilated supplying arteries and draining vein, without intervening abnormal brain tissue Vein of galen malformation Flow voids are seen with a dilated median prosencephalic vein Vascular tumors Hyperintense hemorrhagic foci interspersed with flow voids Arterial atherosclerosis Narrowed lumen appearing as flow voids within hyperintense plaque components DISCUSSION AneurysmIntracranial aneurysm rupture accounts for 0.4–0.6% of total deaths, with aneurysmal subarachnoid hemorrhage affecting approximately 30,000 people every year in North America. Saccular aneurysms are the most common with about 85% located in the anterior circulation of the Circle of Willis, affecting the anterior communicating artery (35%), the internal carotid artery (30%), and the middle cerebral artery (MCA) (22%).[1] Features on T2-weighted images typically include T2 hypointense outpouching along with an internal laminated thrombus with hyperintense rim evident in some cases [Figure 1].[2]
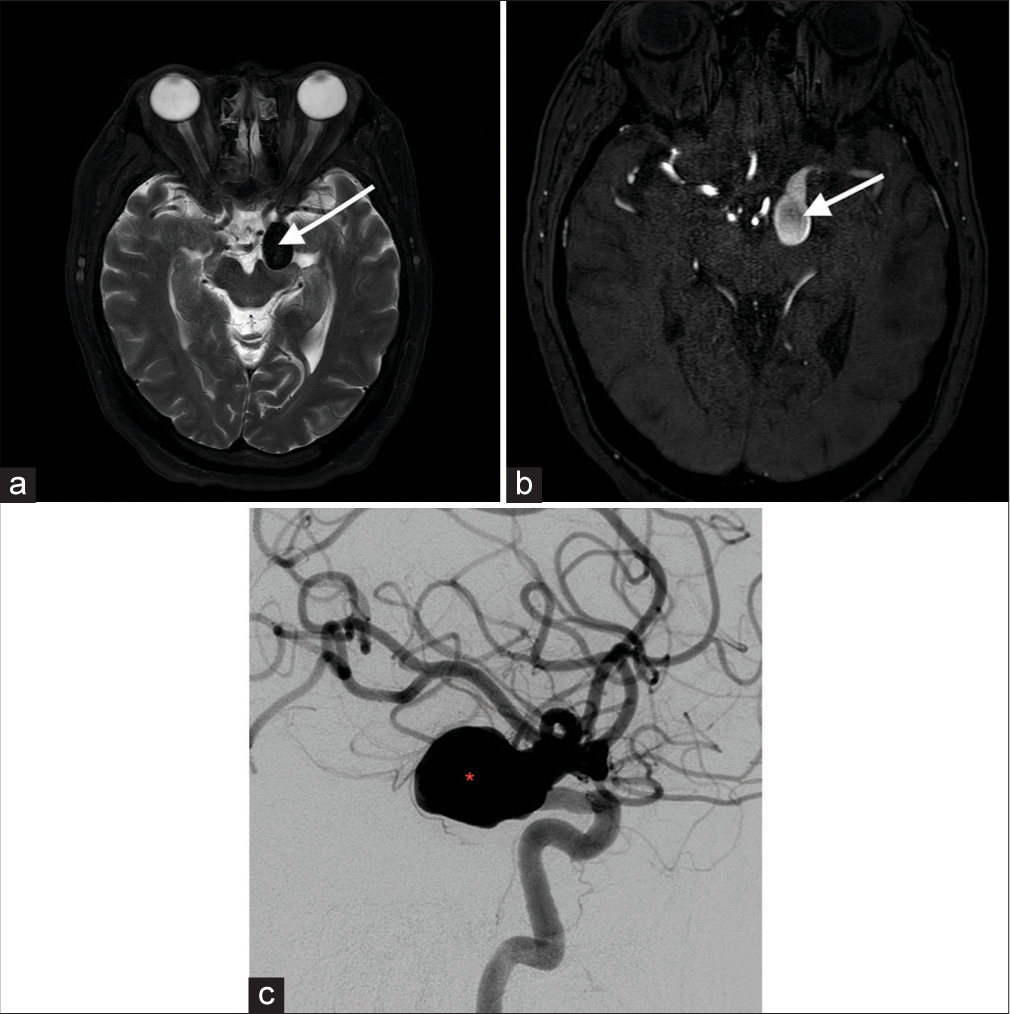
Export to PPT
Arteriovenous malformation (AVM)Diagnostic criteria for AVM include the presence of a nidus embedded within the brain parenchyma, identified on either cross-sectional imaging or conventional angiography, and early venous drainage, which is best seen in dynamic studies. On T2-weighted MR, large lesions will be evident with a nidus and adjacent dilated vessels as well as numerous serpiginous flow voids generated due to fast flow [Figure 2]. Some small AVMs may only be seen on T2-weighted sequences as flow voids.[3]
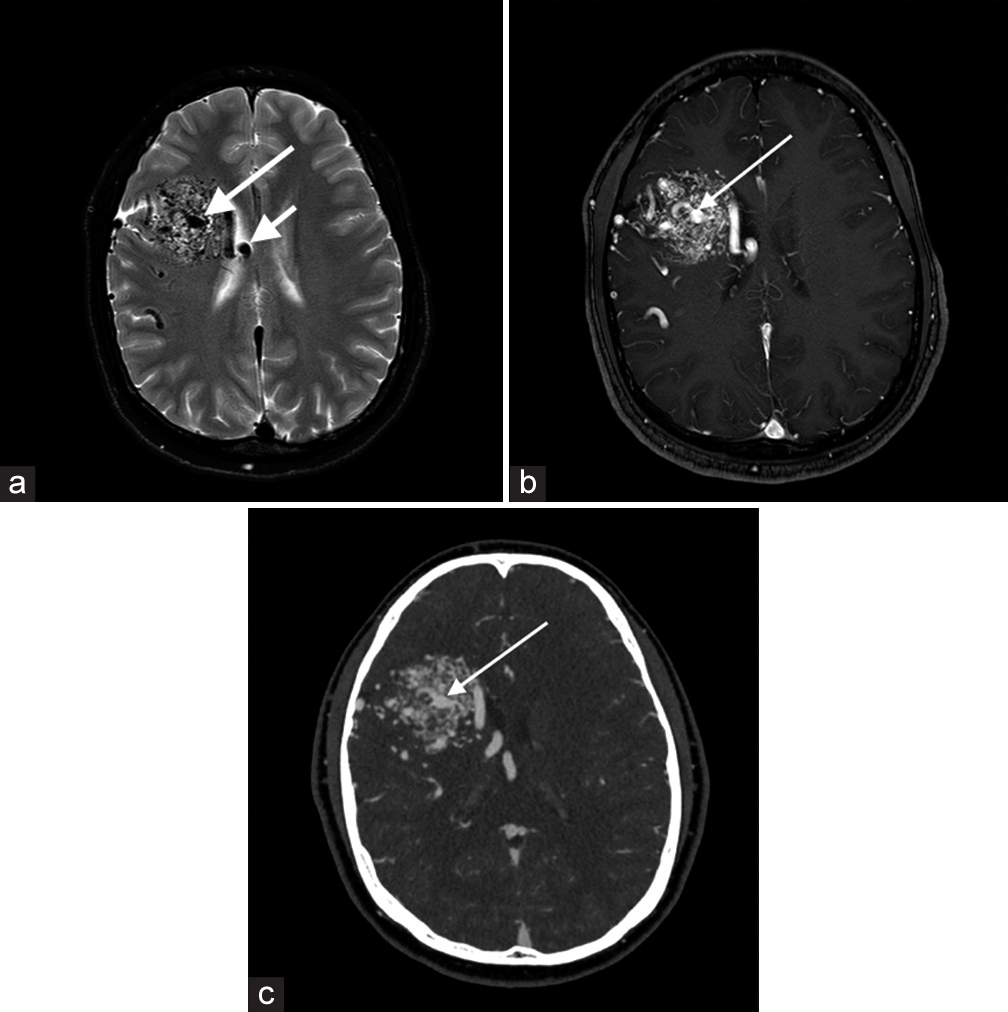
Export to PPT
Arterial occlusionTotal arterial occlusion refers to a flow signal termination on all sequences at any point along the intracranial or extracranial artery. A focal gap in flow is evident with near-occlusions. Features on T2-weighted images include asymmetric loss of flow void, with loss of normal hypointensity [Figure 3].[4] These patients can be asymptomatic or present with transient ischemic attack or stroke symptoms.
Export to PPT
Capillary telangiectasiaCapillary telangiectasia is low-flow lesions that are most commonly found in the brainstem, the majority being asymptomatic. They are usually discovered incidentally on imaging. On T2-weighted images, they are nearly always slightly hyperintense [Figure 4]. T2-weighted images may show low-signal intensity which is thought to be due to deoxyhemoglobin from sluggish flow, and not hemorrhage.[5]
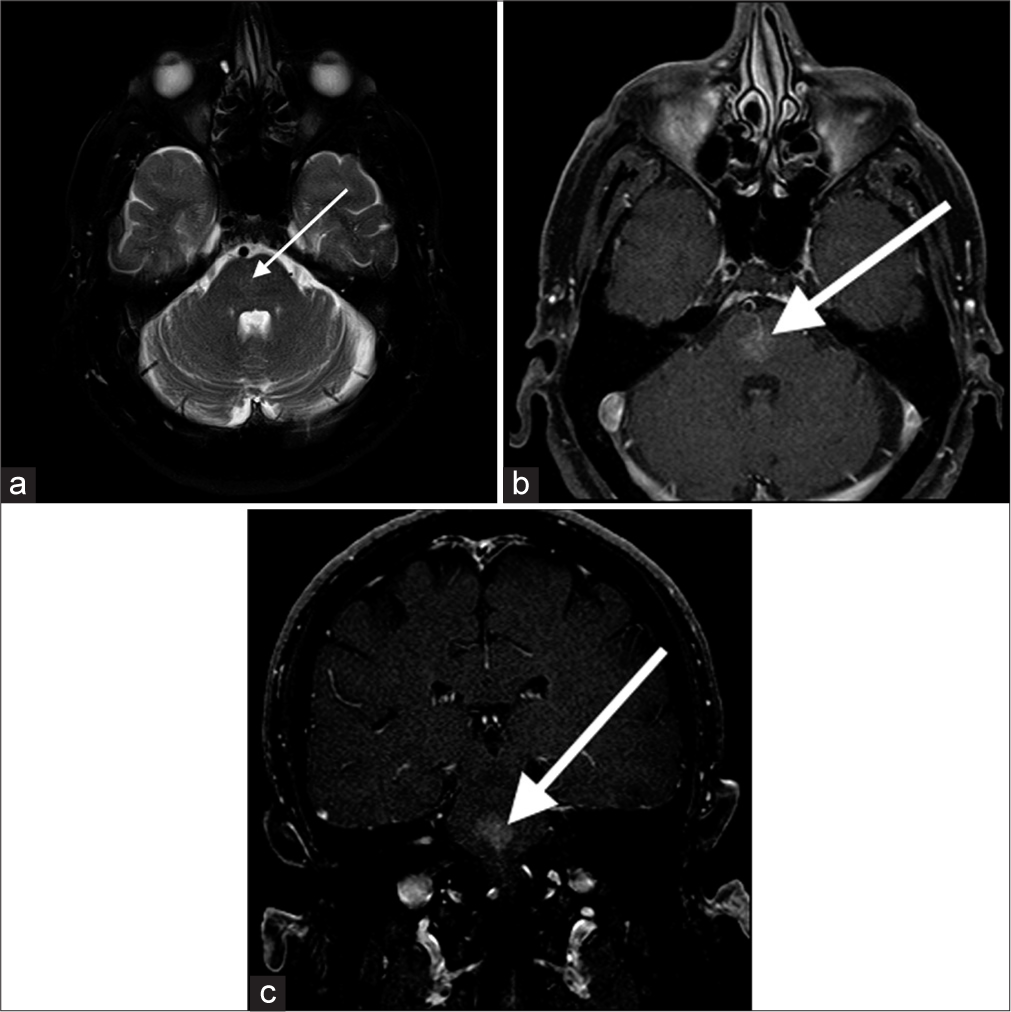
Export to PPT
Cavernous malformationsSlow-flow venous malformations, majority are asymptomatic and are found incidentally on imaging. Tend to be supratentorial but can be found anywhere including the brainstem. They are usually solitary, although up to one-third of patients with sporadic lesions have more than one lesion. T2-weighted images demonstrate a characteristic “popcorn” or “berry” appearance with a peripheral rim of signal loss due to the presence of hemosiderin [Figure 5].[6]
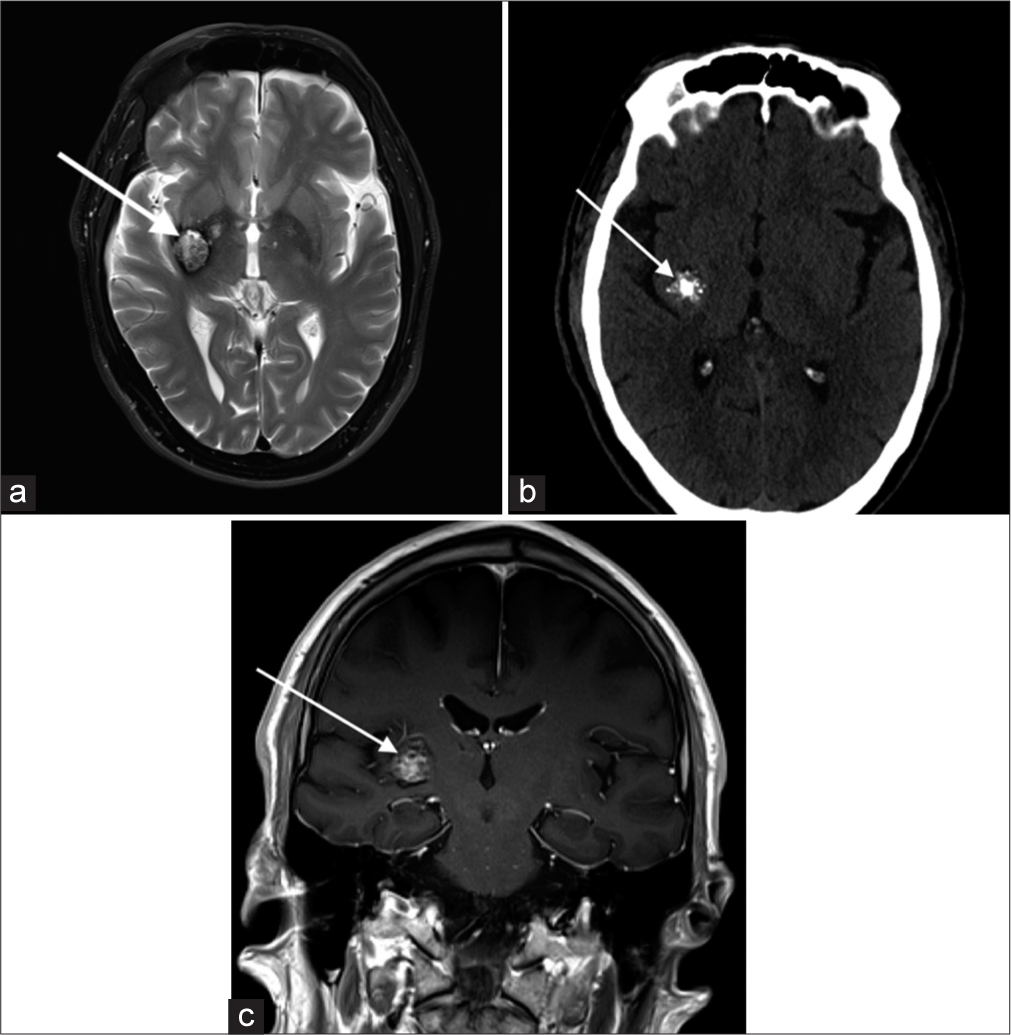
Export to PPT
Dural Arteriovenous (AV) fistulasThese are pathologic connections between dural arteries and dural veins, meningeal veins, or cortical veins, distinguished from parenchymal or pial AVMs by the presence of a dural arterial supply and the absence of a parenchymal nidus.[7] On T2-weighted MR, numerous dural or leptomeningeal flow voids, venous ectasia, or regionally increased leptomeningeal flow voids are considered key findings, with white matter hyperintensity and intracranial hemorrhage possibly representing sequelae of fistulae [Figure 6]. In cavernous sinus dural arteriovenous fistula, large flow voids within the sinus are best visualized on T2-weighted sequences.
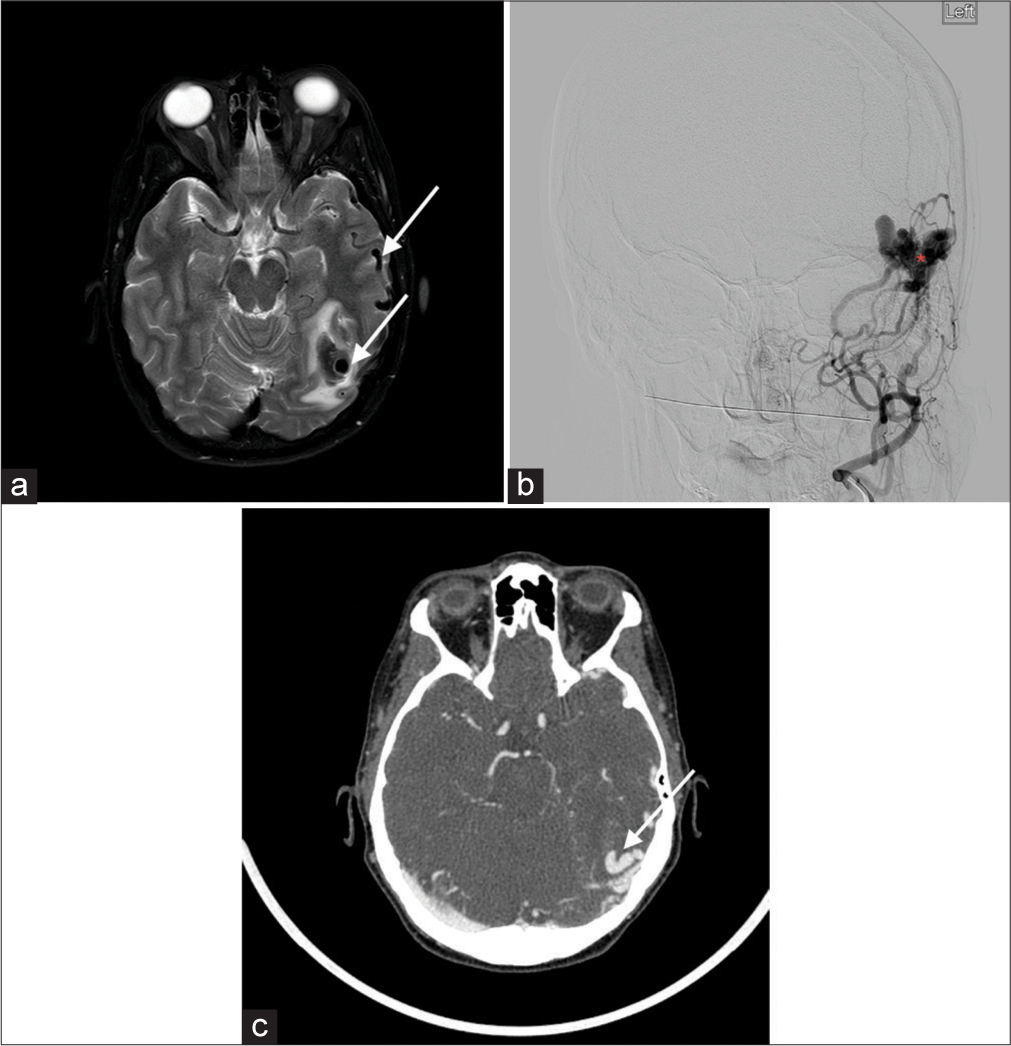
Export to PPT
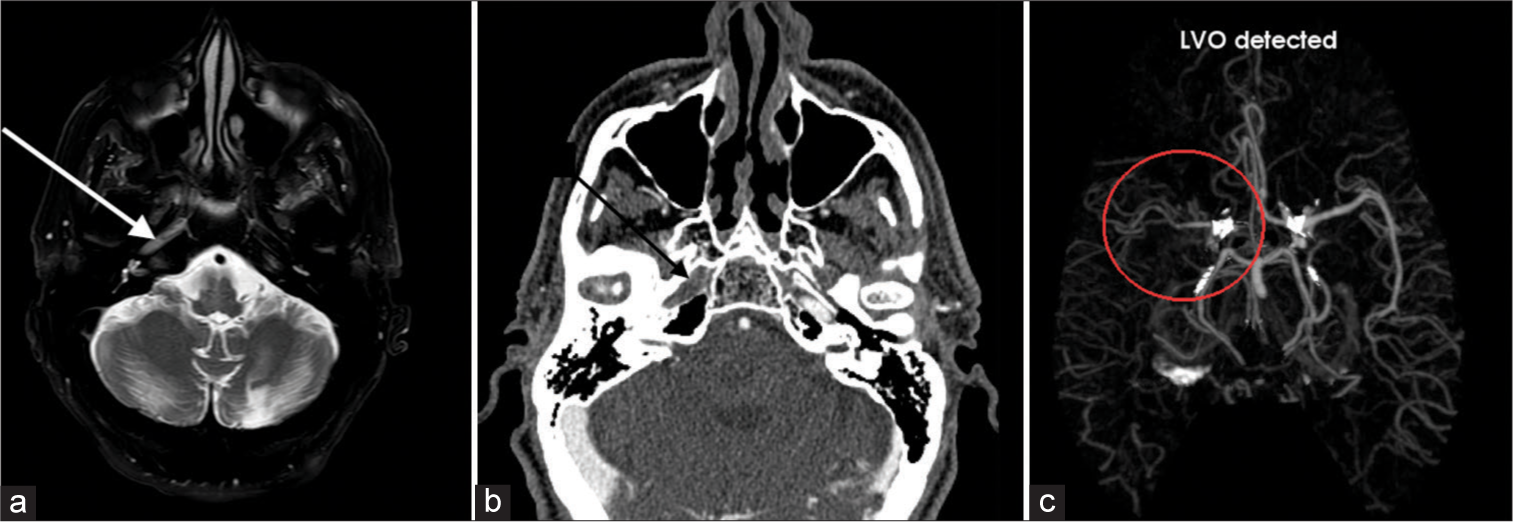
Moyamoya diseaseJapanese for “puff of smoke,” Moyamoya disease refers to the unilateral or bilateral stenosis or occlusion of carotid termini and proximal M1 segment MCAs, with further associated prominent arterial collateral formation. Leptomeningeal collaterals resulting in contrast enhancement and high signal on fluid attenuated inversion recovery (FLAIR) is known as the “Ivy sign.” Features on T2-weighted images include stenosis of the carotid terminus and proximal MCA [Figure 7].[8]
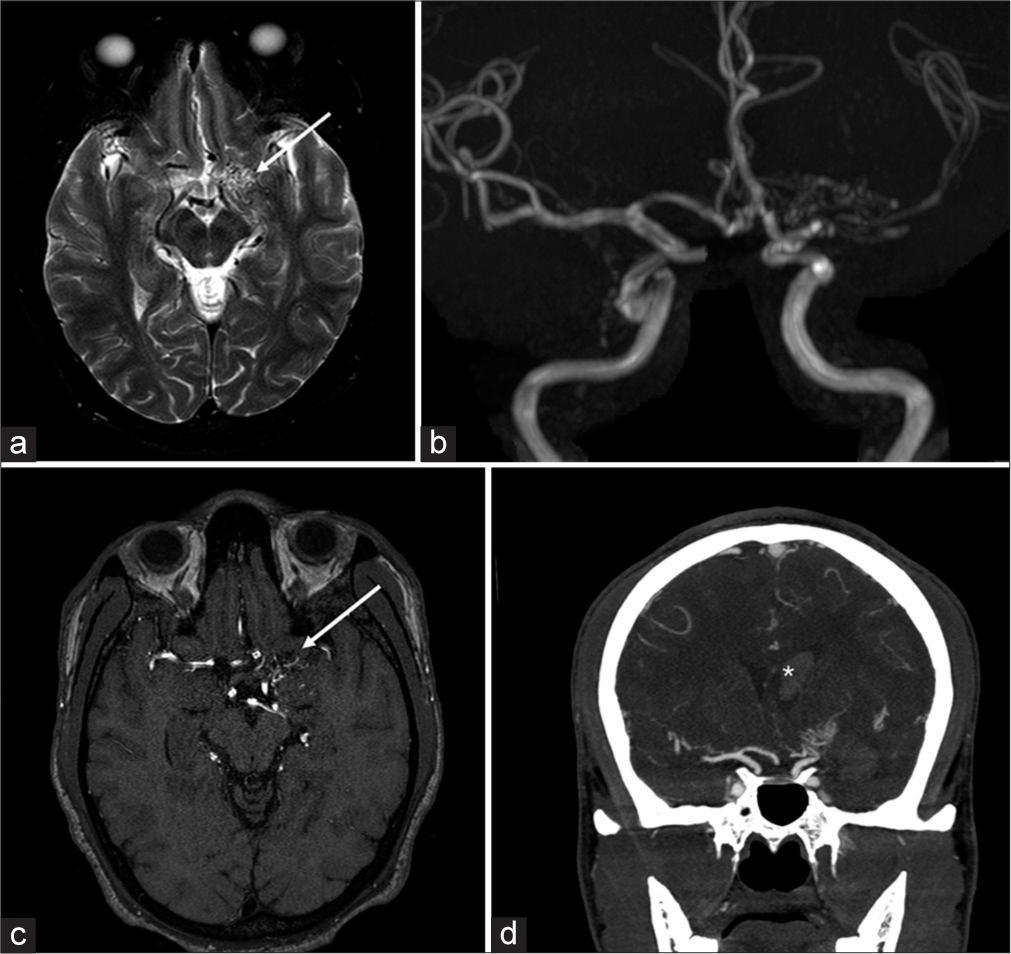
Export to PPT
Proliferative angiopathyCerebral proliferative angiopathy is a rare, relatively newly described cerebrovascular malformation that comprises the feeding arteries, nidus, and draining veins. One important feature distinguishing it from AVMs is the presence of normal brain parenchyma in-between the abnormal vascular channels. In contrast to AVMs, the natural history of the disease suggests a lower risk of hemorrhage, which makes differentiation clinically important. T2-weighted images show a tangle of vessels with adjacent dilated supplying arteries and draining veins [Figure 8].
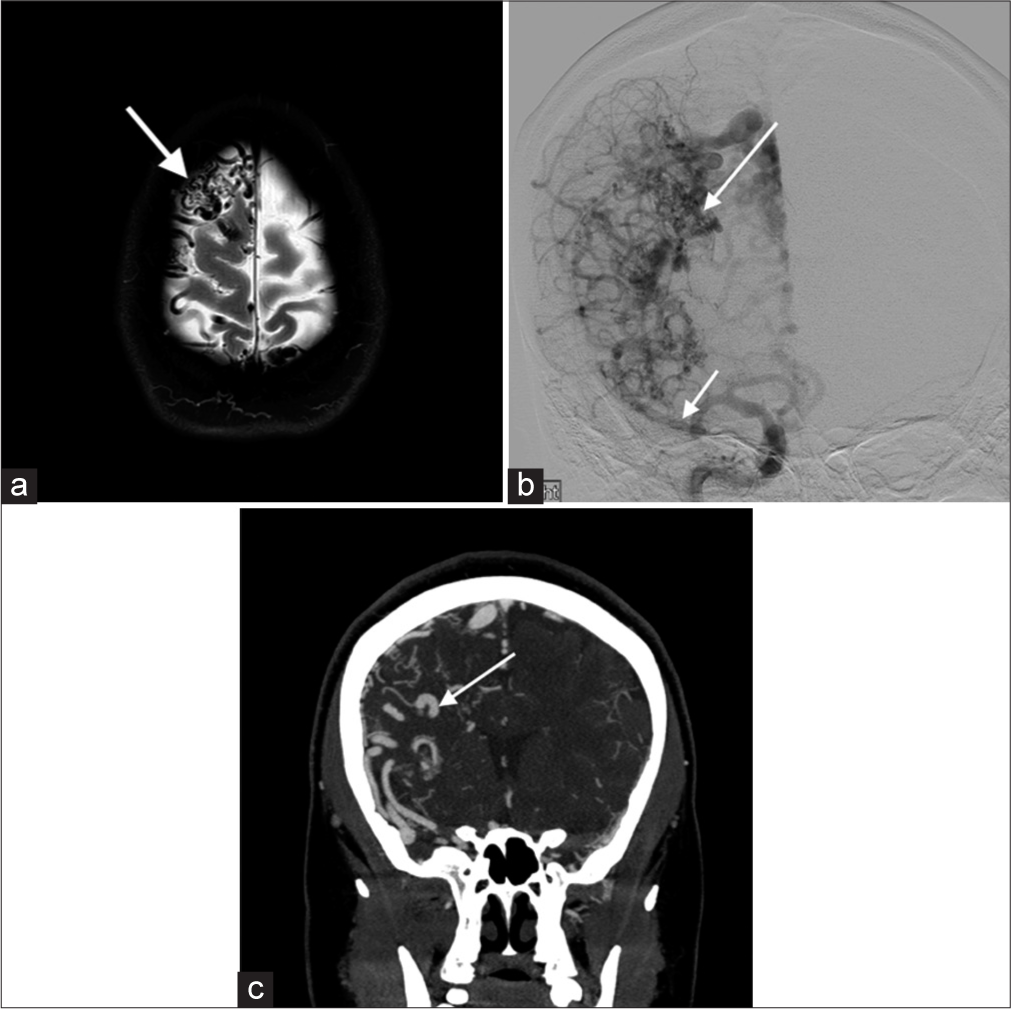
Export to PPT
Vein of Galen malformationVein of Galen malformation is a congenital AV fistula involving the median prosencephalic vein of Markowski. Lasjaunias classification further describes choroidal and mural types. Signal abnormalities including T2 prolongation and flow voids in periventricular regions are seen on T2-weighted imaging with a dilated median prosencephalic vein, the name of vein of Galen is a misnomer [Figure 9].[9]
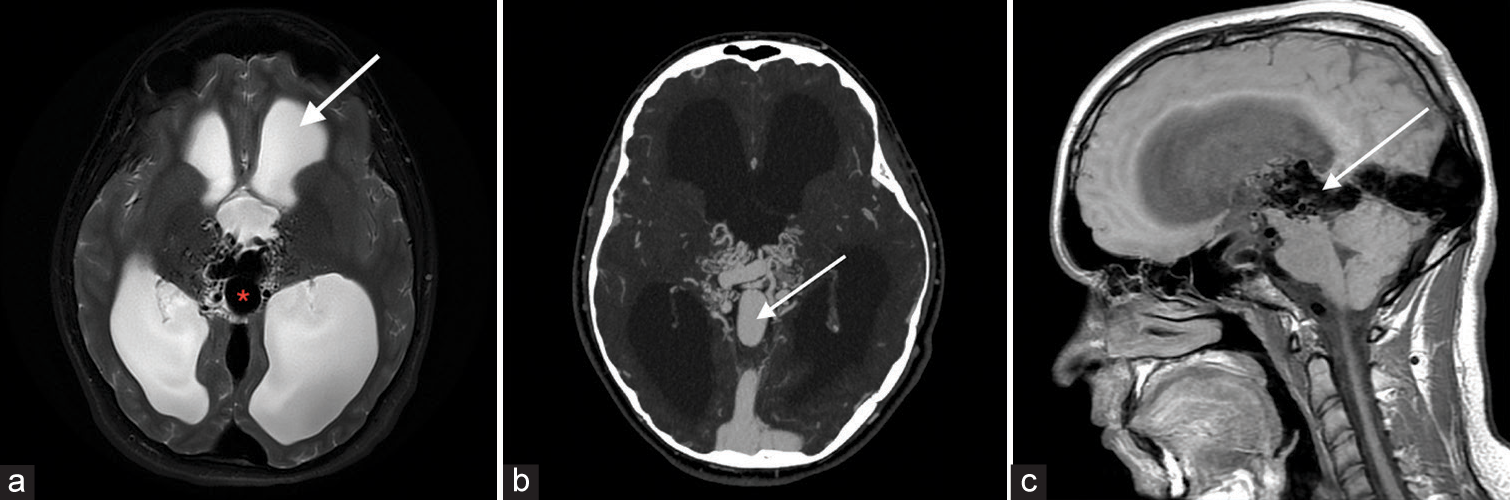
Export to PPT
Vascular tumorsAstrocytomas are hyperintense on T2-weighted images with ill-defined borders, and a T2-FLAIR mismatch sign is indicative of isocitrate dehydrogenase (IDH)-mutant subtype.[10] IDH mutations, crucial prognostic markers, confer better outcomes, distinguishing them from aggressive glioblastomas [Figure 10]. Oligodendrogliomas with intact 1p/19q show homogeneous T1/T2 signals with sharp borders, differing from “true” oligodendrogliomas with codeletion. Lesions with well-circumscribed T1 hypointensity, high T2 signal, and T2/FLAIR mismatch predict absence of 1p19q codeletion [Figure 11].[11] Calcification and hemorrhage appear as signal loss on T2* sequences; contrast enhancement is common but unreliable for grading. Diffusion weighted imaging (DWI) and MR perfusion aid in distinguishing oligodendrogliomas from astrocytomas based on vascularity and cellularity. T2-weighted imaging can help in the assessment of presence of large internal vessels while planning surgical resection.
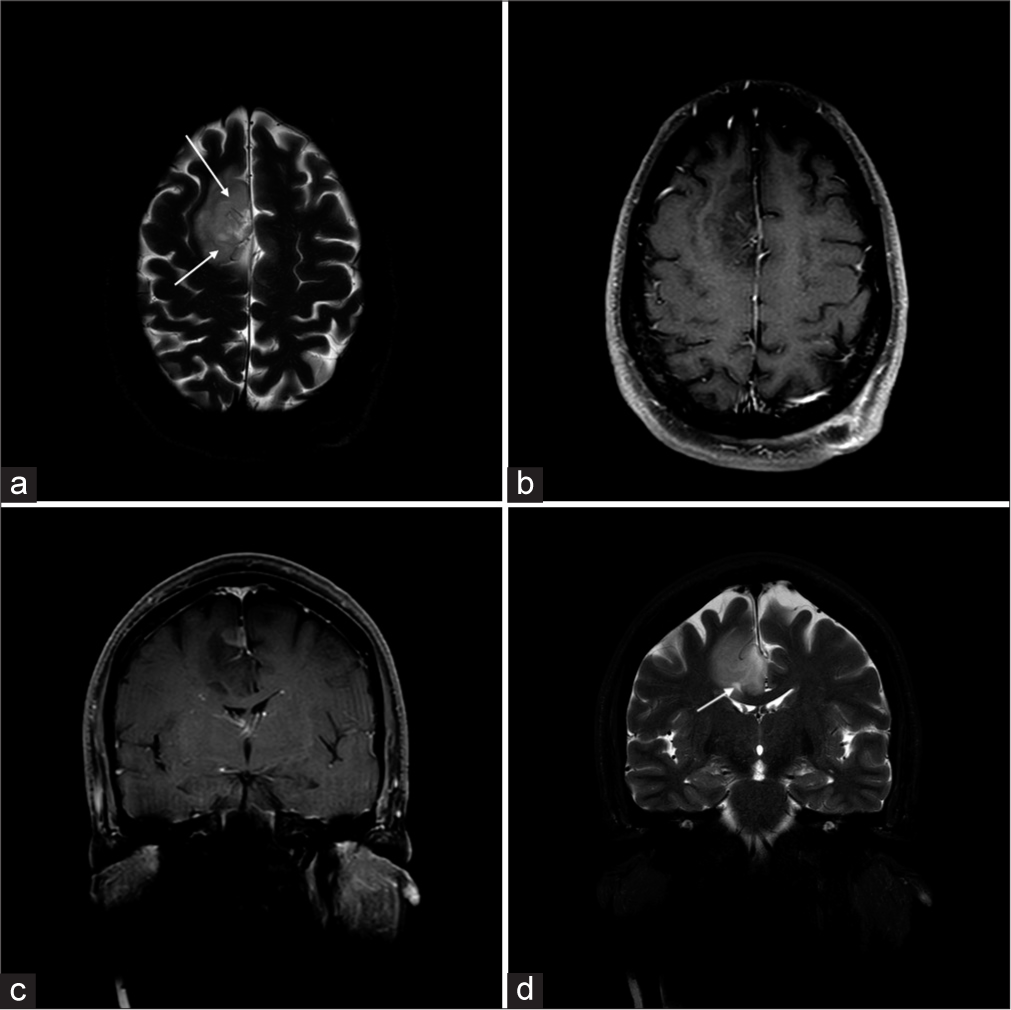
Export to PPT
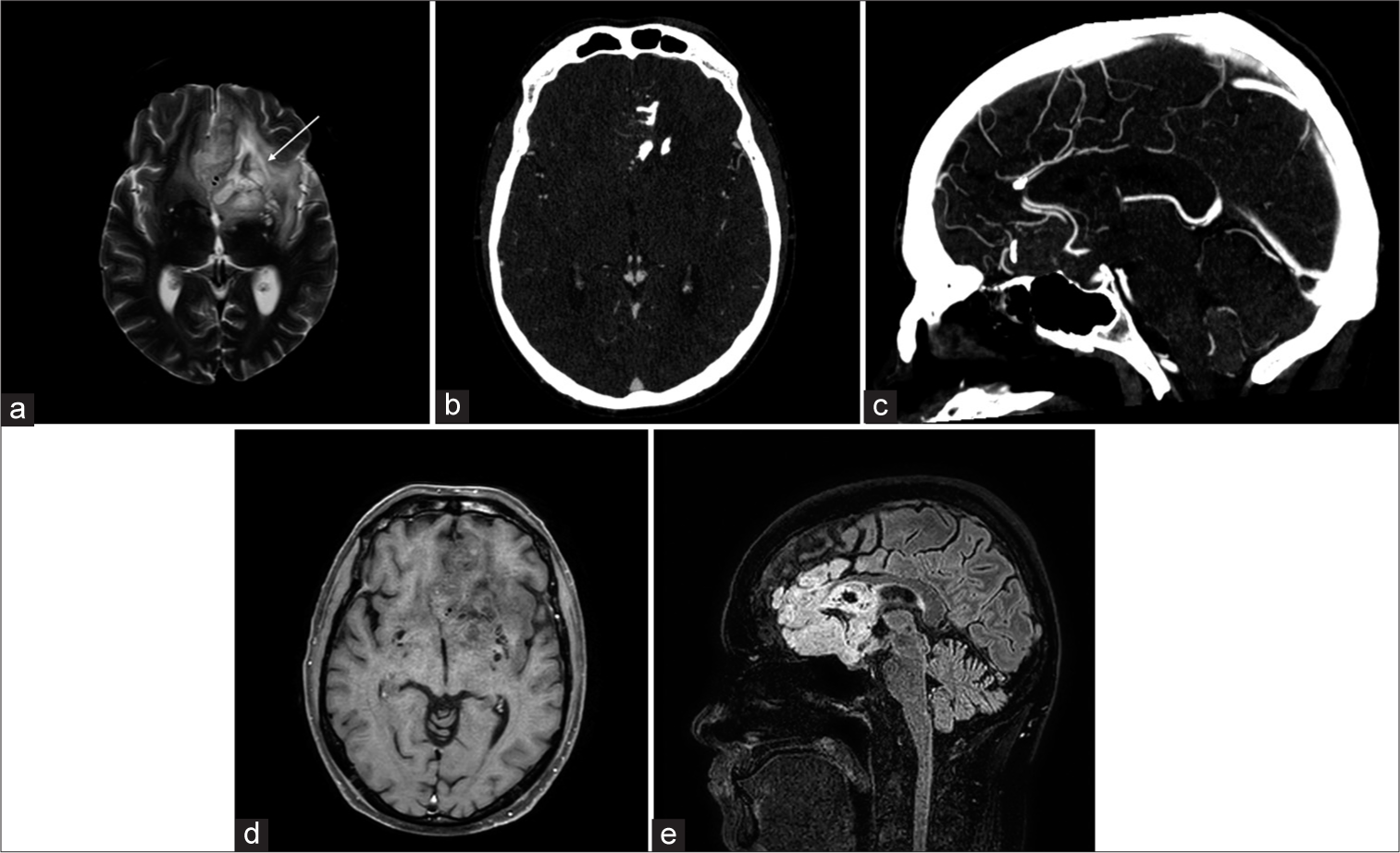
Export to PPT
Arterial atherosclerosisNarrowing of the arterial lumen can be evident as flow voids on T2-weighted sequences. The plaque components-fibrous cap, intraplaque hemorrhage, and juxtaluminal thrombus-demonstrate a hyperintense T2 signal that can be visualized adjacent to the flow void on T2-weighted sequences [Figure 12]. Calcification and hemosiderin within the plaque appear hypointense on T2. Yu et al.[12] demonstrated that MCA plaque hyperintensity on T2-weighted images (>50% stenosis) was associated with symptomatic MCA plaque, and a normalized plaque signal of 1.3–1.4 provided the highest diagnostic value for symptomatic plaque.
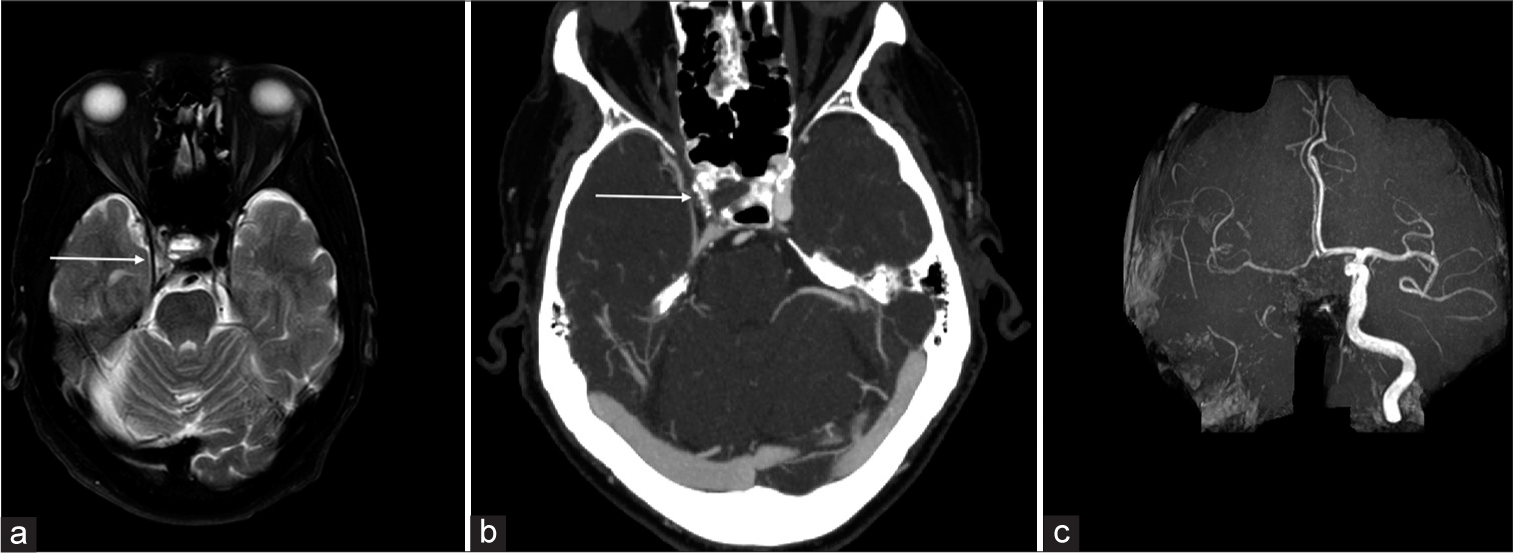
Export to PPT
CONCLUSIONIncidental discovery of intracranial cerebrovascular lesions can lead to early diagnosis and further optimal management of complex vascular lesions. Routinely acquired T2-weighted imaging can be utilized as an essential sequence to look for vascular lesions, commonly appearing as flow voids with characteristic shapes and imaging appearances. Knowledge of pathophysiology, clinical presentation, and characteristic imaging findings of cerebrovascular lesions is imperative to improve their detection rate, which can prevent future adverse events in selected patients.
留言 (0)