Childhood exposure to common microbes, pathogens, and childhood vaccines is supposed to provide appropriate immune training and surveillance capability that generates a cross-protective immunity against a number of pathogens to which children get exposed later in life (1–3). It is associated with better immune function, characterized by reduced allergies to different allergens, and reduced disease occurrence, including cancer (1, 3–9). Primary brain tumors and associated central nervous system (BCNS) cancers are the most common solid tumors in children (0–14 years old) and adolescents (15–19 years old), accounting for the highest number of solid cancer-related deaths (10, 11). The incidence of childhood BCNS cancers is highest in children less than 5 years old (i.e., 0–4Y-olds) which progressively reduces until adolescence before again starting to steadily rise with age during adulthood (10, 11). The BCNS cancers in children are inherently different from those in adults. Those found in children are predominantly malignant gliomas, embryonal tumors, and germ cell tumors, with a minority of non-malignant tumors primarily comprising pituitary tumors (11–13). A large number of environmental and genetic risk factors have been studied for their association with BCNS cancer incidences (11, 12, 14). Based on epidemiological studies, exposure to ionizing radiation (increased incidence) and inherited single-gene syndromes (~4% of childhood cases) are the only validated primary risk factors for BCNS cancer incidence (11, 12, 14). Structural birth defects (non-chromosomal) have been associated with approximately 7% of childhood BCNS cancers (11, 15, 16). Higher incidence rates have also been observed in the population’s higher socioeconomic position (SEP) subgroups (17–20). The “hygiene hypothesis” has been supposed to provide an explanation in the form of a change in SEP-associated unknown risk factor(s) that could alter the exposure of the immune system to common allergens and pathogens, resulting in aberrant immune system development or immune training and an associated risk of childhood cancer (9, 11, 12, 21). For the majority of early childhood BCNS cancers, the precise nature or identity of risk factors that could be promoting or protecting 0–4Y-old age group children against BCNS cancer incidence remains unknown, while those identified or suspected in a minority of cases lack any interventional value (11, 12).
Childhood vaccines play an important role in protecting children from major infections and diseases by augmenting the developing immune system (1). As the first year of life is important in shaping and training the developing immune system, the recommended immunization schedule (22), starting from birth to the first year of life, generally includes vaccine shots against the following common diseases: tuberculosis [Bacillus Calmette-Guérin (BCG)], polio [polio vaccine (POL)], Haemophilus influenzae B (HIB), hepatitis B (HEPB), pneumococcal illness [pneumococcal conjugate vaccine (PCV)], measles infections [measles-containing vaccine (MCV)], and diphtheria, tetanus, and pertussis (DTP). These childhood vaccines are supposed to enhance the trained innate immunity and humoral- and cell-mediated immunity in children (1–3, 23, 24). Types of these vaccines range from subunit vaccines to heat-inactivated to live-attenuated vaccines, each activating and training a specific combination of the effector arms of the developing immune system (1). The timing and number of shots vary for each, beginning soon after birth to throughout the entire first year of life, and many times boosters are given later in life based on past experiences with vaccines and perceived threats to children’s lives (22). The first doses of the BCG, HEPB, and POL vaccines are administered at birth. Two doses of MCV (MCV2), two additional doses of HEPB (HEPB3) and POL (POL3), and three doses each of PCV (PCV3), DPT (DPT3), and HIB (HIB3) are administered at different time intervals during the first year of life to generate, strengthen, and sustain immunity against target pathogens, as well as general immunity during the most susceptible period. It may be pertinent to state that the previously recommended booster doses of BCG have been discontinued worldwide due to a lowering of threat perception resulting from a general increase in hygiene and the lowered tuberculosis incidence and associated risk of exposure to the environmental Mycobacterium tuberculosis complex. In many countries of the industrialized world—which also happen to be the countries with the highest childhood cancer incidence rates—BCG vaccination has been completely discontinued, including the neonatal dose, for the perceived lack of benefit for these countries with their elimination of tuberculosis. The non-specific positive effect of the BCG vaccine on the survival of children is well recognized [References in 25–27]. It is presumed to be by providing needed immune training to enhance general immune surveillance and strengthening the “trained immunity” of the naïve developing immune system of neonates by functional reprogramming of the immune cells involved in innate immune response (23, 28–31) that ultimately seems to cross-protect them against various pathogens and diseases (1, 6, 24–27, 32, 33).
A number of epidemiological studies around 1970 found that neonatal BCG vaccination was associated with a reduced incidence of leukemia, the most frequent childhood cancer, in up to 6-year-old children (6, 34–37). The proposed mechanisms for the observed reduced cancer incidence in these children have envisaged a role for the neonatal BCG vaccinations or infections in priming and training the developing immune system to efficiently eliminate pre-existing pre-cancerous clones or cells—remnants of embryonic development (6, 36). Based on the observed need for a second hit in the genetically predisposed models of the disease and the investigation of familial cases of leukemia that are often supposed to get triggered by infections, Greaves hypothesized the need for infections that could lead to overt cancer growth following a second genetic hit (38). However, many retrospective studies failed to see the protective impact of childhood vaccination and early infections on childhood cancer incidences, with some even suggesting and arguing for an increase in cancer incidence on vaccination [References in (6, 39–42)]. For a brief account of other theories proposed for childhood leukemia incidence as a result of differential pathogen exposure and immune training, refer to the review by Hauer et al. (43). Unlike the studies that demonstrated a reduction in the incidence of childhood cancers (leukemia and BCNS cancer) with neonatal BCG vaccination, those witnessing no effect or arguing for an increase in cancer incidences had children vaccinated at times other than the neonatal period [References in (37, 39–42)]. Furthermore, their follow-up cancer incidence periods varied, ranging from beyond early childhood to old ages, when the origin and nature of cancers markedly differ from those of early childhood cancers (6, 11, 12, 36, 38, 43, 44). Some studies even seem to equate a lack of curative potential in age groups other than early childhood with a lack of preventive potential in early childhood. However, due to the lack of any clear-cut epidemiological evidence and any apparent way forward for their validation, the role of any childhood vaccination, including BCG, for childhood cancer prevention has remained highly debated and controversial (6, 32, 37, 39, 40, 42, 44, 45).
Previously, based on our observation that the incidence of BCNS cancer in up to 5-year-old children in similar Human Development Index (HDI) countries worldwide was significantly inversely correlated with tuberculosis incidence rates in these countries, we have suggested that exposure to Mycobacterium spp. could be potentially protectively linked to BCNS cancer incidence in young children (45). In this opinion article, we further develop our assertion by presenting an epidemiological analysis of early childhood BCNS cancer incidence in the WHO European region (ER), which has more similar underlying socioeconomic conditions, genetic makeup, comparable health, and medical infrastructure access, and disease reporting as compared to the rest of the world. However, there is a diversity in universal vaccination programs/policies that range from never-ever to all eligible for different vaccines, with coverage ranging from 0% to almost 100% [WHO-UNICEF Estimates of National Immunization Coverage (WUENIC): 2022 revision, updated July 2023; https://data.unicef.org/topic/child-health/immunization/] (46), potentially offering a diverse mix of supposed “trained immunity” status from different vaccines and exposure to Mycobacterium spp. in this population to identify potential protective determinants. Here, “trained immunity” refers to the existence of trained innate immune cells as a result of exposure to vaccines/immunogens/microbes, which primes the immune system as a whole to behave appropriately. With regard to BCG vaccination policy, the ER region is almost equally divisible in BCG-vaccinating (BCG) and non-vaccinating (No-BCG) countries [The BCG World Atlas, 3rd Edition, http://www.bcgatlas.org/index.php] (47). The existence of populations in the European region with 50 different levels of prevailing tuberculin immunoreactivity (TI), also referred to as “latent tuberculosis infection” (LTBI) (48–51) or Tuberculin sensitivity test (TST) positivity—a surrogate measure of existing “trained immunity” and cell-mediated immunity-boosting potential from exposure to Mycobacterium spp. (BCG or/and environmental) (51–55), offers an excellent opportunity to examine our assertions.
Early childhood vaccinations and incidence of BCNS cancerWhen the ER countries, with comparable confounders, contributing to 15% of global BCNS cancer incidences in 0–4Y-olds during 2020 (GLOBOCAN 2020; https://gco.iarc.fr/today) (56) and displaying almost two times higher age-standardized incidence rate (ASR) as compared to the rest of the world (2.4 vs. 1.3 ASR per 100,000; range: 0-5.4 ASR), were examined to identify potential impact of different early childhood vaccinations, the coverage of BCG or any other test vaccines (i.e., DTP3, MCV2, PCV3, POL3, HEPB3, and HIB3), in the same cohort of children, i.e., during 2016–2020 in 1-year-olds (WUENIC) (46), did not significantly correlate with the BCNS cancer incidence rates (Table 1) (22, 46). This apparent dissociation observed in the population-level data seems to support the contention that childhood vaccinations do not play any significant role in protecting against childhood cancers [reviewed in 39, 40], contradicting previous observations made in the 1970s on 0–6-year-old children-associated data analysis (34–36). However, among all childhood test vaccines assessed, the negative correlation, although non-significant and very weak, seemed the strongest for the BCG coverage of countries reporting non-zero BCNS cancer incidence in 0–4Y-olds [r(47): −0.1667, p-value: 0.2628] (Table 1). The countries with a small population size and reporting zero incidence for 2020 (i.e., Iceland, Malta, and Luxembourg) were excluded from the analysis. Since BCG vaccination-elicited immune responses in children are short-lived and wane away within years in the absence of boosting from rechallenge with BCG vaccine or environmental Mycobacterium spp. exposure (53, 57, 58), the observed lack of a correlation for BCG coverage would be expected in the current populations with higher hygiene standards and lower chances of boosting from re-exposure to Mycobacterium spp., even if BCG vaccination could have been protective.
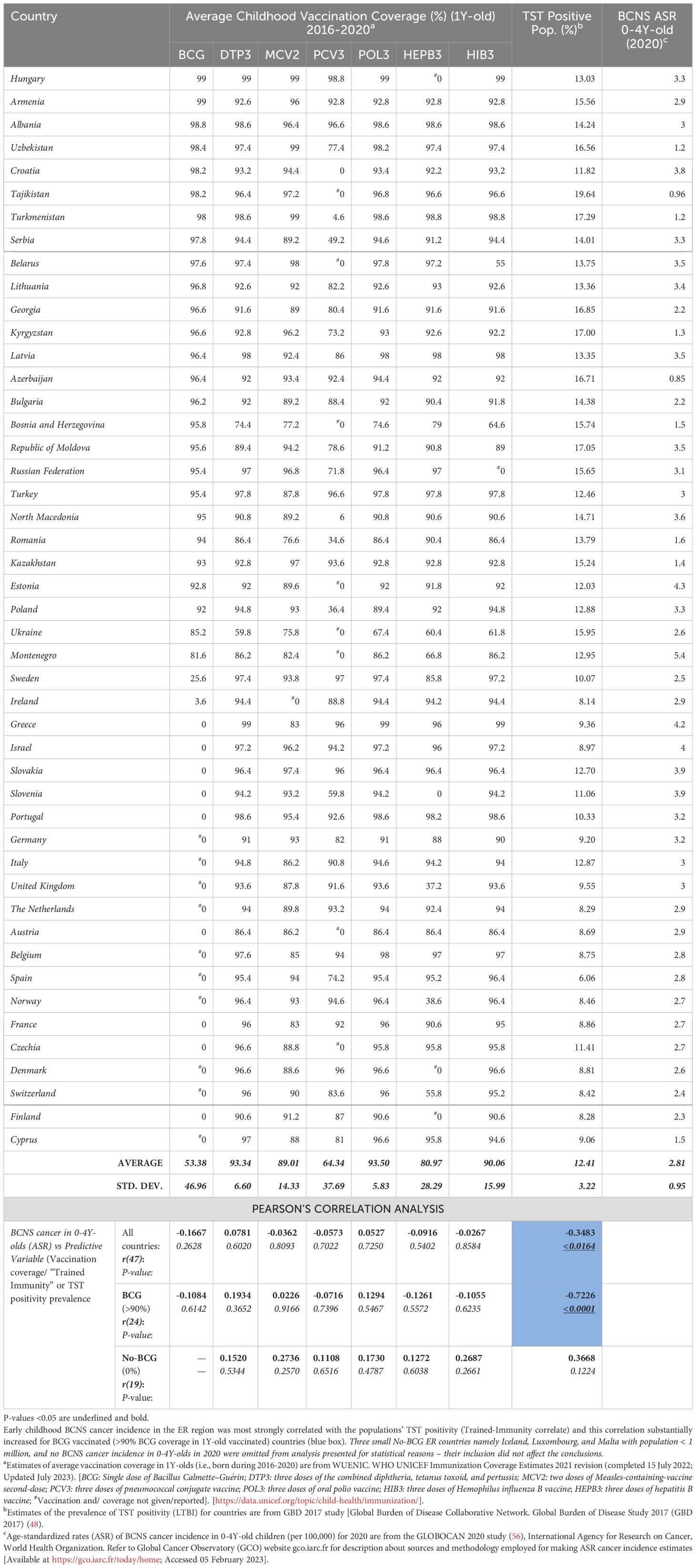
Table 1. BCNS Cancer incidence in 0-4Y-old (<5Y-old) children in European Region: Vaccination coverage in <1Y-old, ‘Trained-Immunity’ prevalence (from Mycobacterium spp. exposure) of populations (TST Positivity) and correlation analysis.
Early childhood BCNS cancer incidences in ER countries are negatively associated with the prevailing possibility of Mycobacterium spp. exposureThe TI, as measured by TST and interferon-gamma release assays (IGRAs), is employed to indirectly measure the elicited or persisting cell-mediated immune response resulting from exposure to Mycobacterium spp. (BCG or environmental) antigens, as well as to ascertain BCG vaccine efficacy (49, 50, 57). In the absence of clinically active tuberculosis disease, it is also referred to as LTBI by the WHO for tuberculosis management purposes due to these individuals supposedly “being at risk of developing TB” in their lifetime from reactivation or fresh infections (49–51, 59). Intriguingly, the prevalence of TI/LTBI in the European region countries (48), which provides a measure of the cell-mediated trained-immunity persistence in the populations resulting from their exposure to Mycobacterium spp., including that from the BCG vaccine—is found to be significantly negatively correlated with BCNS cancer incidence rates in 0–4Y-olds [Pearson’s correlation coefficient, r(47): −0.3483, p-value: <0.0164; see Table 1 and Figure 1A]. In case there is any protective role for Mycobacterium spp. exposure in reducing the incidence rate of early childhood BCNS cancer, it may be conjectured that the TI/LTBI prevalence would be more strongly but negatively correlated with early childhood BCNS cancer incidence in countries with neonatal BCG vaccination in place than in countries without BCG vaccination (No-BCG). Surprisingly, the negative correlation between BCNS cancer incidence rates and prevailing TI substantially improved for BCG-vaccinating countries (n = 24; coverage >90%) [r(24): −0.7226, p-value: 0.0001], whereas for non-BCG-vaccinating or No-BCG countries (n = 19), it became uncorrelated [r(19): 0.367, p-value: 0.122] (Table 1 and Figure 1B). A similar correlation was observed for the incidence of BCNS cancer in 2022 (data not shown). The country-wise BCG vaccination coverage in 0–4Y-olds in the European region is displayed on the map in Figure 1C. Notice that BCG vaccination is common in the Eastern region (countries colored in shades of blue) while lacking in the Western region (countries colored white). Furthermore, the inclusion of BCG countries [Ukraine, BCG coverage: 85.2%, BCNS cancer ASR: 2.6, TI/LTBI prevalence: 15.96%; Montenegro, BCG coverage: 81.6%, BCNS cancer ASR: 5.4, TI/LTBI prevalence: 12.96%] or No-BCG countries [i.e., Sweden, BCG coverage: 25.6%, BCNS cancer ASR: 2.5, TI/LTBI prevalence: 10.07%; Ireland, BCG coverage: 3.6%, BCNS cancer ASR: 2.9, TI/LTBI prevalence: 8.14%] that had BCG coverage other than >90% or 0% in respective BCG and No-BCG group countries does not change the nature of the correlation. Thus, the observed strong negative correlation between BCNS cancer incidence in 0–4Y-olds of neonatal BCG-mandating countries and their prevailing immunoreactivity to mycobacterial antigens, but not in non-BCG-mandating countries (No-BCG), indicates a potential protective association that needs further investigation. Overall, based on the existing incidences, it may be argued that the combination of neonatal BCG vaccination priming of the developing immune system of neonates and its subsequent boosting on re-exposures to Mycobacterium spp. (BCG or environmental) may be protective in early childhood BCNS cancer incidences.
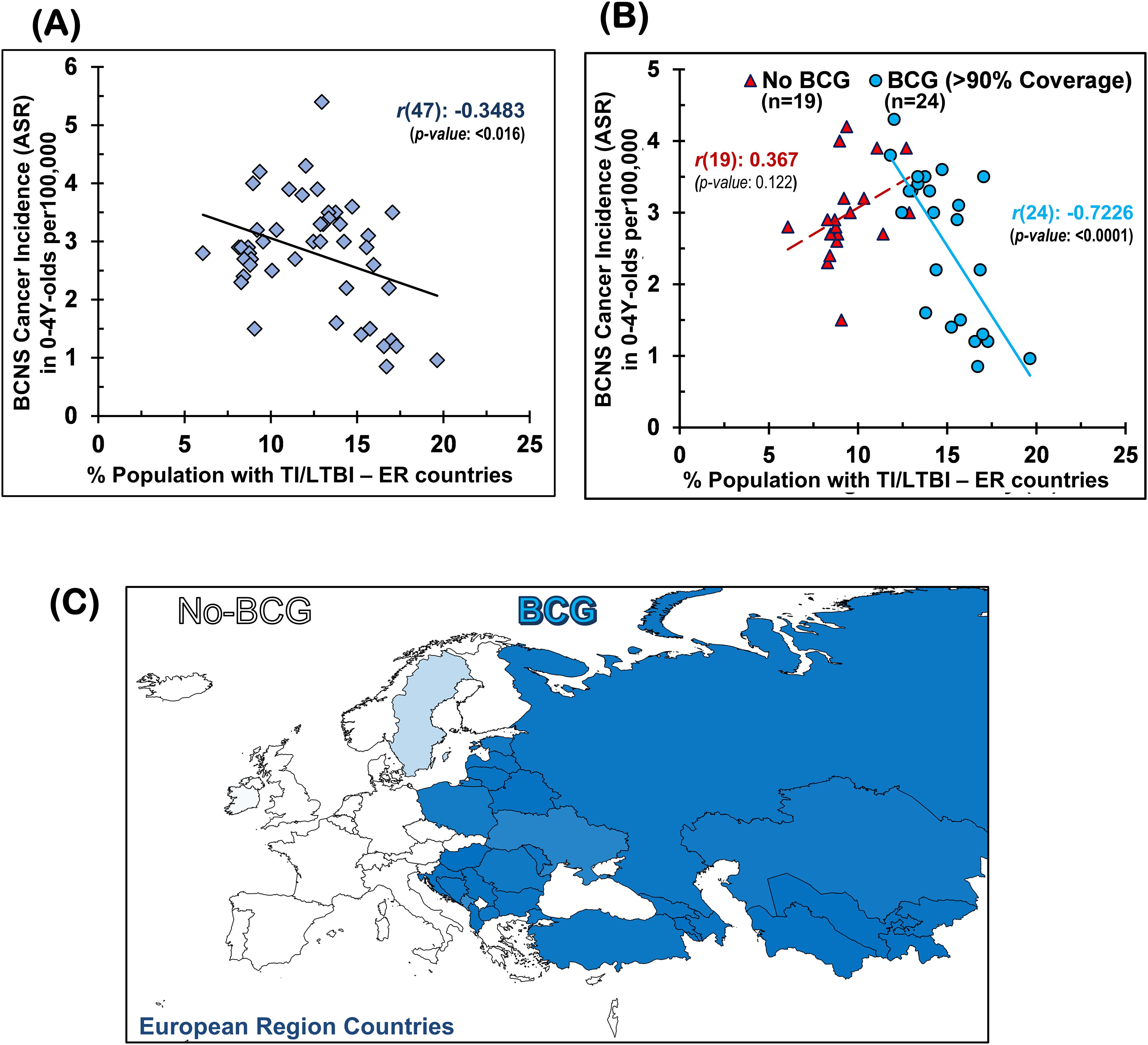
Figure 1. Early childhood BCNS cancer incidences in neonatal BCG vaccination-implementing European region (ER) countries were negatively correlated with their prevailing tuberculin immunoreactivity (TI) or LTBI estimates: (A) without regard for BCG vaccination mandate and coverage and (B) with regard to effective BCG vaccination (BCG) or lack thereof (No-BCG) [for correlation with other vaccines (e.g., BCG, DTP3, MCV2, PCV3, POL3, HIB3, HEPB or HEPB3) and their coverage, refer to Supplementary Figure 1]. (C) BCG coverage in 0–4Y-olds (<5-year-olds) in ER countries. BCG, Bacillus Calmette-Guérin; BCNS cancer, brain and central nervous system cancers; LTBI, latent tuberculosis infection.
Furthermore, this association of early childhood BCNS cancer incidence rate with the TI prevalence of countries is found to be more strongly correlated in the group of countries with effective BCG coverage (>90%) than any other early childhood vaccine coverage for country subgroups, as may be supposed from their coefficients of determination (R2 values), i.e., BCG (n = 24): R2 = 0.5221; DTP3 (n = 41): R2 = 0.1680; MCV2 (n = 26): R2 = 0.2315; PCV3 (n = 18): R2 = 0.209; POL3 (n = 41): R2 = 0.1386; HEPB3 (n = 34): R2 = 0.2052; and HIB3 (n = 39): R2 = 0.1954 (see data points shown by blue and orange circles in Supplementary Figures 1A–G). For vaccine coverage lower than 90%, most correlations are even weaker and statistically insignificant (p-value: >0.05) (see data points shown by red triangles in Supplementary Figures 1A–G). Additionally, exploratory multiple regression analysis indicates that the coverage of other vaccines (co-vaccination) or their combinations also does not seem to affect BCNS cancer incidence reduction in less than 5-year-old children, in addition to TI in the neonatal BCG-vaccinated populations. Interestingly, when all countries are considered, completely disregarding their neonatal BCG vaccination policy and coverage, the countries with medium-to-high BCNS cancer incidence rates (i.e., ASR 2–4 and >4 per 100,000) exclusively belong to low TI/TST-positive populations (<15%), whereas the low BCNS cancer incidence countries (i.e., ASR >0–2 per 100,000) are primarily BCG-vaccinating countries (8/9) with high prevailing TI/TST-positive populations (7/9 with >15% to 20%). Overall, early childhood BCNS cancer incidence in countries that use neonatal BCG vaccination is strongly but negatively linked to TI/TST positivity in the community. These findings suggest that neonatal BCG vaccination plays a protective role in countries where the immune system of any child is more likely to be boosted by exposure to environmental Mycobacterium spp. Together, these results imply that environmental Mycobacterium spp. exposure-associated boosting in children primed with neonatal BCG vaccination may play an important role in lowering the incidence of BCNS cancer in early childhood (less than 5 years of age).
DiscussionRegarding the impact of potential confounders on our observations and outcomes of any prospective study, it must be remembered that many studies in the past have positively associated the high BCNS incidence rates with SEP, non-chromosomal structural defects, a high birth rate, syndromes (e.g., neurofibromatosis types I and II, Li Fraumeni syndrome, and tuberous sclerosis), and polymorphisms in a number of genes and infections [reviewed in 11,12]. Infection of Toxoplasma gondii, a protozoan, has also been associated with an increased risk of glioma incidence (a subtype of BCNS cancers). Human leukocyte antigen (HLA) alleles, the composition of immune cells, and the genomic architecture of T, NK, and myeloid cells have been associated with glioma risk. However, the role of most viral infections studied has been inconsistent in glioma—the malignant BCNS cancer, which is responsible for the majority of deaths. Only allergies, atopic conditions, and infection with varicella zoster virus (VZV), a herpes virus that causes chickenpox and shingles, have been consistently associated with a reduced risk of glioma. The existence of a potential protective association between environmental Mycobacterium tuberculosis complex spp. exposure and BCNS cancer incidence has been suggested by us based on the observed lower incidence rates in high tuberculosis-reporting countries (45). These may very well remain underlying confounders for swaying the outcome of any exploratory study and hence the conclusions, including those of the current article. Their control, as possible, would be desired in future studies.
From a mechanistic point of view, the observed negative correlation of Mycobacterium spp. exposure with the BCNS cancer incidence in 0–4Y-olds when compared with general vaccination could be related to their ability to epigenetically reprogram innate immune cells, possibly making them better at recognizing and eliminating abnormal transformed cells (28–33, 44, 45). No matter how significant and strong any statistical correlation could appear between potential variables, it can never be considered to have a cause-and-effect relationship. Endeavors may be made to identify and validate such protective variables that may have a potential cause-and-effect relationship (51). The channelization of resources to evaluate and explore the preventive and protective potential of childhood vaccinations, especially BCG vaccination and boosting events (BCG and environmental Mycobacterium spp.), may be warranted to ascertain a potential cause-and-effect relationship, if any. It should also be remembered that there are certain inherent lineage-specific and preparation-specific differences with regard to content and immunogenicity in BCG sub-strains (60, 61) that are historically differentially employed in the Eastern and Western European region countries for children’s vaccination (47), along with differences in circulating Mycobacterium spp. (62–64) and their inherent immune activation potential (65–67) on exposure. The co-vaccination during this period may also have an impact (27, 68). While designing and performing any such exploratory study, more emphasis should be placed on identifying variables potentially causatively associated with direct reduction in early childhood BCNS cancer incidence (having cause-and-effect relationships) with some interventional value rather than just being an explanatory variable (e.g., income, Gross Domestic Product (GDP), socioeconomic position in society, and gene polymorphism, number of children (11, 12)). Additional study design considerations may be needed to control the effect of possible confounders that have been found associated previously with BCNS cancer incidences and could be supposedly overrepresented in any specific study populations (e.g., syndromes, gene polymorphisms, and mutations in genes associated with immune system functioning; see the previous paragraph). The possible modulation of the developing immune systems of children resulting from their interactions with their mother and other closely interacting individuals or social contacts, the potential reduction of the transfer of unknown causatively associated etiological agents to young children in a high TI population, or the potential of endocrine or in utero reprogramming by mothers exposed to mycobacterial antigens or other pathogens may also be considered as possible variables for any clinical trial attempting to evaluate the protective potential of the BCG vaccine, as previously suggested for leukemia (37, 38, 43).
ConclusionExposure to Mycobacterium spp. (both BCG and environmental) may potentially contribute to a decrease in BCNS cancer incidence during early childhood in countries that follow the neonatal BCG vaccination. Dedicated epidemiological studies exploring links between BCNS cancer incidence during early childhood (less than 5 years old) and childhood vaccinations, pathogen exposures, and immune training are warranted, utilizing associated children’s health records. It should be backed by follow-up randomized controlled clinical trials, preferably performed in populations with low prevalence of tuberculin immunoreactivity (TI/LTBI), explicitly exploring the impact of BCG vaccination and its boosters on early childhood BCNS cancer incidence while suitably controlling for the underlying heterologous cell-mediated immunity and “trained immunity” correlates and other possible confounders as indicated in the preceding paragraphs to conclusively determine the biological significance of the observed association and its potential practical/interventional application, if any.
Author contributionsSS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RS: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the current study. The general funding support from Banaras Hindu University (IoE-BHU and IMS) to the laboratory of SS is acknowledged. However, they had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AcknowledgmentsThe authors acknowledge general funding support from Banaras Hindu University (IoE-BHU and IMS) to the laboratory of SS. However, they had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1497436/full#supplementary-material
Supplementary Figure 1 | The negative correlation of BCNS cancer incidence in 0-4Y-olds with Tuberculin Immunoreactivity (TI/LTBI/TST-positivity) of the population (likelihood of Mycobacterium spp. exposure) was most strongly associated with the priming of the majority of children (>90%) by neonatal BCG vaccination (A), but not with the coverage (%) of any other commonly employed childhood vaccine, i.e., (B) DTP3, (C) POL3, (D) MCV, (E)PCV3, (F) HEPB (HEPB3), (G) HIB3 [n= number of countries; R2: coefficient of determination; solid and broken trend line are for p-value <0.05 and >0.05, respectively].
References2. Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe. (2019) 25:13–26. doi: 10.1016/j.chom.2018.12.006
PubMed Abstract | Crossref Full Text | Google Scholar
3. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
PubMed Abstract | Crossref Full Text | Google Scholar
8. Marron M, Brackmann LK, Kuhse P, Christianson L, Langner I, Haug U, et al. Vaccination and the risk of childhood cancer-A systematic review and meta-analysis. Front Oncol. (2021) 10:610843. doi: 10.3389/fonc.2020.610843
PubMed Abstract | Crossref Full Text | Google Scholar
9. Garn H, Potaczek DP, Pfefferle PI. The hygiene hypothesis and new perspectives—Current challenges meeting an old postulate. Front Immunol. (2021) 12:637087. doi: 10.3389/fimmu.2021.637087
PubMed Abstract | Crossref Full Text | Google Scholar
12. Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. (2019) 21:1357–75. doi: 10.1093/neuonc/noz123
PubMed Abstract | Crossref Full Text | Google Scholar
13. Gittleman HR, Ostrom QT, Rouse CD, Dowling JA, de Blank PM, Kruchko CA, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. (2015) 121:102–12. doi: 10.1002/cncr.v121.1
PubMed Abstract | Crossref Full Text | Google Scholar
14. Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer epidemiology Biomarkers prevention: Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol. (2014) 23:2716–36. doi: 10.1158/1055-9965.epi-14-0207
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lupo PJ, Schraw JM, Desrosiers TA, Nembhard WN, Langlois PH, Canfield MA, et al. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol. (2019) 5:1150–8. doi: 10.1001/jamaoncol.2019.1215
PubMed Abstract | Crossref Full Text | Google Scholar
16. Johnson KJ, Lee JM, Ahsan K, Padda H, Feng Q, Partap S, et al. Pediatric cancer risk in association with birth defects: a systematic review. PloS One. (2017) 12:e0181246. doi: 10.1371/journal.pone.0181246
PubMed Abstract | Crossref Full Text | Google Scholar
17. Francis SS, Wang R, Enders C, Prado I, Wiemels JL, Ma X, et al. Socioeconomic status and childhood central nervous system tumors in California. Cancer causes control: CCC. (2021) 32:27–39. doi: 10.1007/s10552-020-01348-3
PubMed Abstract | Crossref Full Text | Google Scholar
18. Erdmann F, Hvidtfeldt UA, Sørensen M, Raaschou-Nielsen O. Socioeconomic differences in the risk of childhood central nervous system tumors in Denmark: a nationwide register-based case-control study. Cancer causes control: CCC. (2020) 31:915–29. doi: 10.1007/s10552-020-01332-x
PubMed Abstract | Crossref Full Text | Google Scholar
19. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. (2015) 26:179–85. doi: 10.1007/s10552-014-0496-x
PubMed Abstract | Crossref Full Text | Google Scholar
20. Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PloS One. (2013) 8:e60910. doi: 10.1371/journal.pone.0060910
PubMed Abstract | Crossref Full Text | Google Scholar
24. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. (2016) 352:aaf1098. doi: 10.1126/science.aaf1098
PubMed Abstract | Crossref Full Text | Google Scholar
26. Trunk G, Davidović M, Bohlius J. Non-specific effects of bacillus calmette-guérin: a systematic review and meta-analysis of randomized controlled trials. Vaccines (Basel). (2023) 11:121. doi: 10.3390/vaccines11010121
PubMed Abstract | Crossref Full Text | Google Scholar
27. Higgins JPT, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. (2016) 355:i5170. doi: 10.1136/bmj.i5170
PubMed Abstract | Crossref Full Text | Google Scholar
28. Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. (2014) 345:1251086. doi: 10.1126/science.1251086
PubMed Abstract | Crossref Full Text | Google Scholar
29. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U.S.A. (2012) 109:17537–42. doi: 10.1073/pnas.1202870109
PubMed Abstract | Crossref Full Text | Google Scholar
30. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. (2014) 345:1250684. doi: 10.1126/science.1250684
PubMed Abstract | Crossref Full Text | Google Scholar
32. Singh AK, Netea MG, Bishai WR. BCG Turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J Clin Invest. (2021) 131:e148291. doi: 10.1172/JCI148291
PubMed Abstract | Crossref Full Text | Google Scholar
33. Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, et al. BCG-Induced cross-protection and development of trained immunity: Implication for vaccine design. Front Immunol. (2019) 10:2806. doi: 10.3389/fimmu.2019.02806
PubMed Abstract | Crossref Full Text | Google Scholar
34. Rosenthal SR, Crispen RG, Thorne MG, Piekarski N, Raisys N, Rettig PG. BCG Vaccination and leukemia mortality. JAMA. (1972) 222:1543–4. doi: 10.1001/jama.1972.03210120041010
Crossref Full Text | Google Scholar
36. Rosenthal SR. Cancer precursors and their control by BCG. Dev Biol Stand. (1986) 58:401–16.
37. Singh S, Kishore D, Singh RK. Trained Immunity” from Mycobacterium spp. exposure (BCG vaccination and environmental) may have an impact on the incidence of early childhood leukemia. Front Immunol. (2023) 14:1193859. doi: 10.3389/fimmu.2023.1193859
PubMed Abstract | Crossref Full Text | Google Scholar
39. Marron M, Brackmann LK, Kuhse P, Christianson L, Langner I, Haug U, et al. Vaccination and the risk of childhood cancer-a systematic review and meta-analysis. Front Oncol. (2021) 10:610843. doi: 10.3389/fonc.2020.610843
PubMed Abstract | Crossref Full Text | Google Scholar
40. Morra ME, Kien ND, Elmaraezy A, Abdelaziz OAM, Elsayed AL, Halhouli O, et al. Early vaccination protects against childhood leukemia: a systematic review and meta-analysis. Sci Rep. (2017) 7:15986. doi: 10.1038/s41598-017-16067-0
PubMed Abstract | Crossref Full Text | Google Scholar
41. Cardwell CR, McKinney PA, Patterson CC, Murray LJ. Infections in early life and childhood leukaemia risk: a UK case-control study of general practitioner records. Br J Cancer. (2008) 99:1529–33. doi: 10.1038/sj.bjc.6604696
PubMed Abstract | Crossref Full Text | Google Scholar
42. Hwee J, Tait C, Sung L, Kwong JC, Sutradhar R, Pole JD. A systematic review and meta-analysis of the association between childhood infections and the risk of childhood acute lymphoblastic leukaemia. Br J Cancer. (2018) 118:127–37. doi: 10.1038/bjc.2017.360
PubMed Abstract | Crossref Full Text | Google Scholar
45. Singh S, Diwakar A, Singh RK. BCG vaccination policy, natural boosting and pediatric brain and CNS tumor incidences. Front Immunol. (2023) 14:1174006. doi: 10.3389/fimmu.2023.1174006
PubMed Abstract | Crossref Full Text | Google Scholar
47. Lancione S, Alvarez JV, Alsdurf H, Pai M, Zwerling AA. Tracking changes in national BCG vaccination policies and practices using the BCG World Atlas. BMJ Glob Health. (2022) 7:e007462. doi: 10.1136/bmjgh-2021-007462
PubMed Abstract | Crossref Full Text | Google Scholar
48. Global Burden of Disease Collaborative Network. Global burden of disease study 2017 (GBD 2017) results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME (2018). Available at: http://ghdx.healthdata.org/gbd-results-tool (Accessed June 09, 2020).
51. Singh S, Maurya RP, Singh RK. Trained immunity” from Mycobacterium spp. exposure or BCG vaccination and COVID-19 outcomes. PloS Pathog. (2020) 16:e1008969. doi: 10.1371/journal.ppat.1008969
PubMed Abstract | Crossref Full Text | Google Scholar
52. Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. (2019) 25:1473–8. doi: 10.1016/j.cmi.2019.04.020
PubMed Abstract | Crossref Full Text | Google Scholar
53. Rakshit S, Ahmed A, Adiga V, Sundararaj BK, Sahoo PN, Kenneth J, et al. BCG revaccination boosts adaptive polyfunctional Th1/Th17 and innate effectors in IGRA+ and IGRA–Indian adults. JCI Insight. (2019) 4:e130540. doi: 10.1172/jci.insight.130540
留言 (0)