Retinoblastoma (RB) is a highly invasive tumor and the most common primary intraocular malignancy in children. It arises from the developing retina, which originates from the neuroectoderm, a tissue responsible for forming the central nervous system (CNS) (1). The disialoganglioside GD2 is overexpressed in tumors of neuroectodermal origin, including RB and neuroblastoma (NB) (2). GD2 plays an important role in malignant transformation and is a well-established therapeutic target (3).
Anti-GD2 monoclonal antibodies (mAbs) have significantly improved outcomes in high-risk NB (4–10). Naxitamab, a humanized version of m3F8 (hu3F8), received FDA breakthrough designation in 2018 and full approval in 2020. It is indicated, in combination with GM-CSF, for pediatric and adult patients with high-risk NB in the bone or bone marrow who demonstrate partial response, minor response, or stable disease following standard induction therapy (11). This approval was based on data from the pivotal phase II trial (Study 201, NCT03363373), which evaluated naxitamab in patients with high-risk NB refractory to initial standard treatments or demonstrated insufficient response to therapies for progressive or relapsed disease (12).
In high-income countries offering timely diagnosis and specialized care, RB overall survival rates have reached 95%, however, survival rates are significantly lower in low-income countries (13, 14). Treatment of metastatic RB presents formidable challenges, with intensive, multimodal regimens associated with significant toxicities (15). Metastatic disease frequently involves regional lymph nodes, bone and bone marrow (BM) and the CNS (16). CNS metastasis resulting from optic nerve infiltration is associated with a dismal prognosis (17).
In a recent study of patients with metastatic RB treated with high-dose chemotherapy and autologous hematopoietic stem cell transplant (HDC-ASCT), overall survival for patients with metastatic disease outside of the CNS was 76.7%, decreasing to less than 10% for those with CNS involvement (18). The treatment of RB with CNS metastasis lacks effective systemic agents that can penetrate the blood brain barrier (BBB). However, intrathecal topotecan has improved long-term survival in patients with metastatic CNS disease (19, 20).
Complications of metastatic RB and associated treatments include toxic deaths, hearing loss, neurocognitive impairment, and second malignant neoplasms (SMNs) (21–24). Germline cancer-predisposing mutations, present in 40% of RB patients, heighten the risk of SMNs (1). In high-income settings, metastatic retinoblastoma often arises after unsuccessful attempts at eye-preserving treatments in patients who have undergone extensive prior therapies. This further underscores the need for alternative, less toxic treatment options.
Here, we report two cases of metastatic RB managed with the anti-GD2 mAb naxitamab as consolidation after reduced-intensity chemotherapy and ASCT. In both cases the detection of CRX (cone-rod-homeobox) in the cerebrospinal fluid (CSF) prompted the use of CNS targeted therapy with intrathecal topotecan. Despite the historically challenging prognosis of systemic and CNS relapsed RB (25–27), both patients are alive and disease-free 3 years after the metastatic relapse.
2 Cases descriptionA summary of the cases information can be found in Table 1.
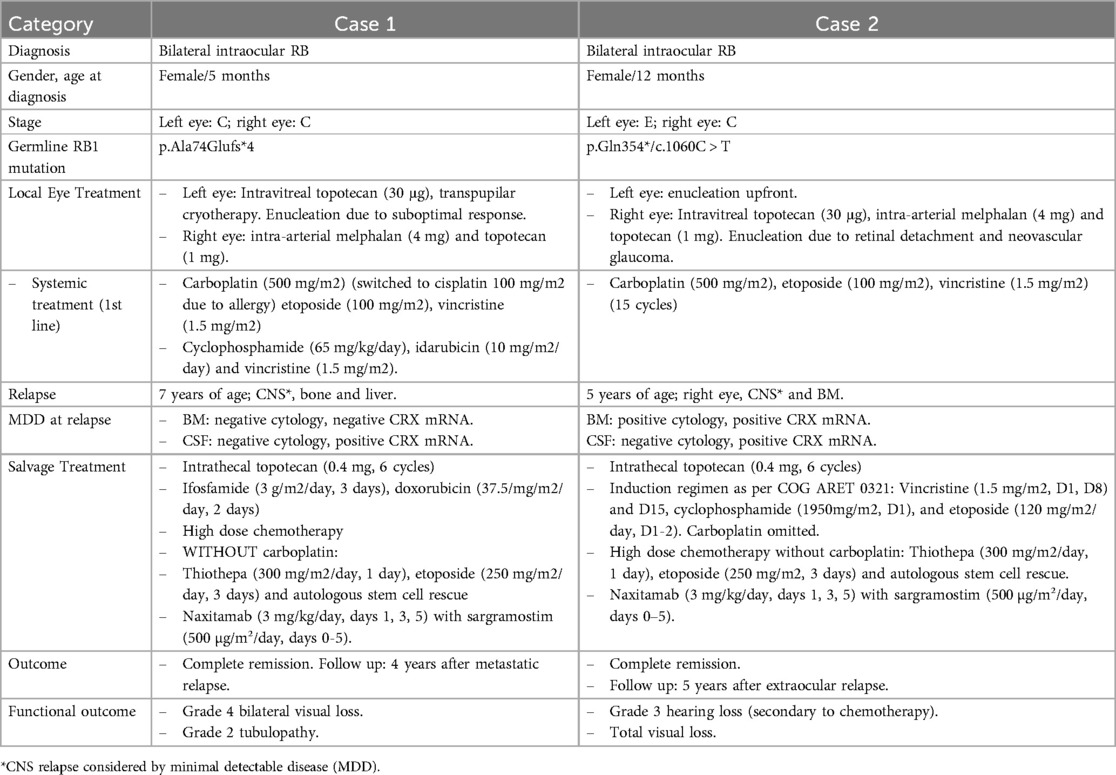
Table 1. F, female; D, day.
2.1 Case 1The first patient was diagnosed with bilateral group C RB at 5 months of age, as per international classification of intraocular RB International Classification for Intraocular Retinoblastoma (ICRB). The initial treatment included an undetermined number of cycles of systemic vincristine, cisplatin, and etoposide (28, 29) causing grade 3 allergy to both cisplatin and carboplatin (See Figure 1A for additional details). Information regarding the patient's response to this initial treatment is not available. At 2 years of age, the patient arrived at our institution with active bilateral intraocular RB, subsequently confirmed to carry an RB1 germline mutation. Six tandem intra-arterial chemotherapy cycles were administered, yielding a favorable response in the right eye but suboptimal results in the left eye, requiring enucleation. Histological analysis of the enucleated eye revealed disease spread to the ciliary body and sclera, prompting adjuvant therapy with systemic alkylating agents, anthracyclines, and vincristine. Disease evaluation showed CRX mRNA positivity in the CSF along with normal cytology and no CRX detection in the BM (30–32). Intrathecal topotecan (0.4 mg) was added to systemic chemotherapy for 6 total monthly doses. After the first cycle, CRX mRNA status became negative and remained negative throughout subsequent cycles.
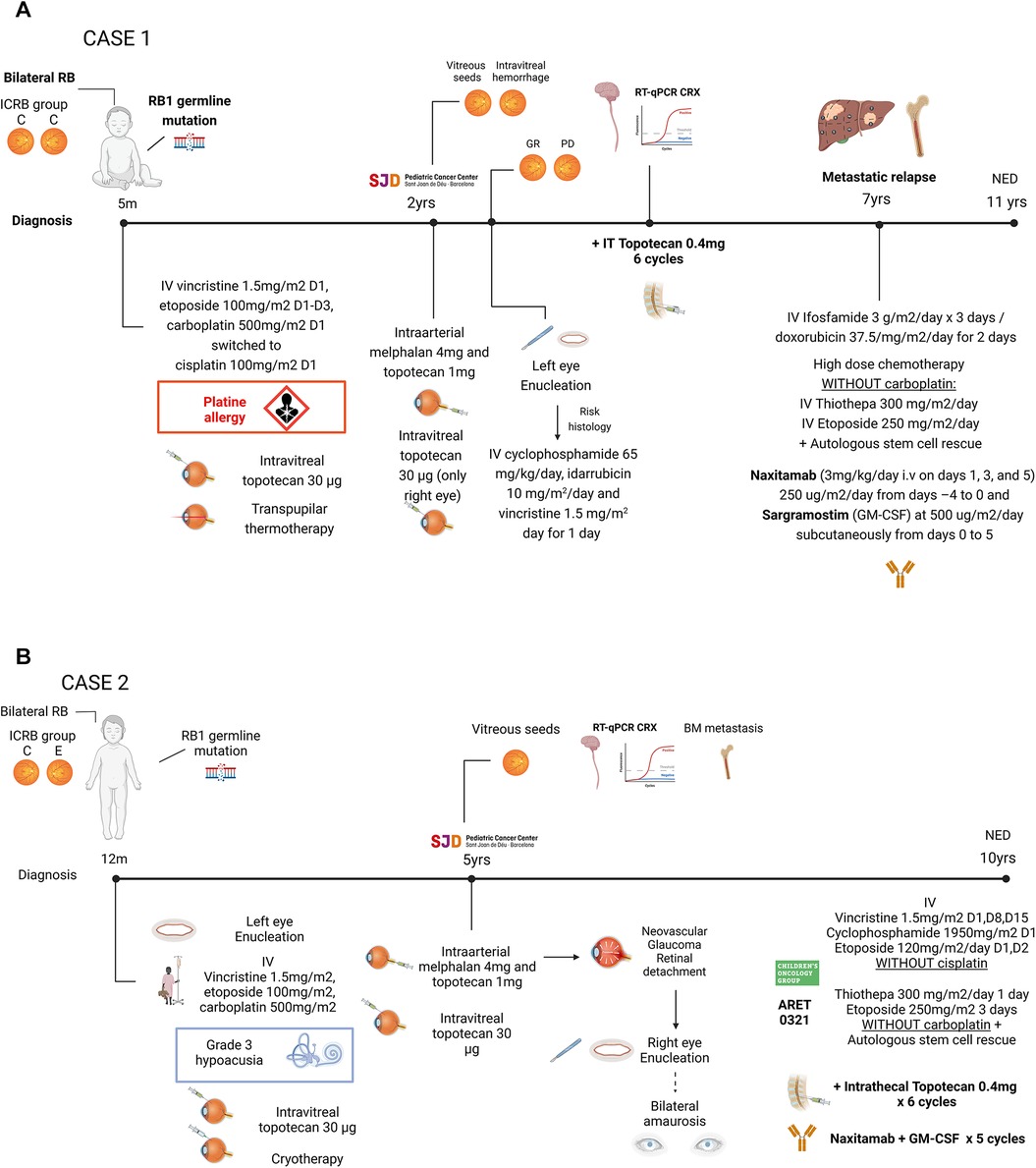
Figure 1. (A) ICRB, international classification of retinoblastoma; IV, intravenous; IT, intrathecal. (B) IV, intravenous; IT, intrathecal; NED, no evidence of disease. Created in BioRender.
Four years later, the patient presented with metastatic lesions in the BM, bone and liver. Rescue treatment comprised two cycles of ifosfamide and doxorubicin, followed by reduced-dose of myeloablative therapy (etoposide and thiotepa, no carboplatin due to prior allergy) and ASCT, achieving complete remission (CR). Because of reduced myeloablative therapy, 5 cycles of naxitamab (3 mg/kg/day i.v on days 1, 3, and 5) 250 ug/m2/day from days –4 to 0 and sargramostim (GM-CSF) at 500 ug/m2/day subcutaneously from days 0 to 5 (33) were added for further consolidation (34).The patient experienced no major acute toxicities, but reported grade 2 pain, hypotensiona and urticarial reaction.
The patient underwent regular disease surveillance, including ophthalmological exams, total body and craniospinal MRI, and morphological and molecular (CRX mRNA) monitoring in BM and CSF during the first 2 years after the end of treatment (EoT). The patient remains alive and disease-free 10 years post initial diagnosis and 4 years after metastatic relapse. Current sequelae include ifosfamide-related nephropathy requiring magnesium supplementation, grade 1 hearing loss, and bilateral grade 4 vision loss.
2.2 Case 2The second patient was diagnosed with bilateral RB at one year of age, with the left eye classified as group E and the right eye as group C according to the ICRB (35) (Figure 1B). The left eye was enucleated, and the right eye received conservative treatment. Initial therapy with systemic carboplatin, etoposide and vincristine (28, 29) included 15 cycles, achieving remission but leading to grade 3 hypoacusia.
Five years later, the patient presented with vitreous seeding in the right eye and was referred to our center. Comprehensive disease work-up, including craniospinal MRI, PET-FDG, BM examination, showed no extraocular dissemination and confirmed the presence of RB1 germline mutation. Intra-arterial chemotherapy to the right eye caused retinal detachment and neovascular glaucoma, necessitating enucleation. Histological studies revealed scleral infiltration, while BM examination demonstrated RB infiltration, and qRT-PCR detected CRX mRNA in the CSF. Systemic therapy followed the ARET0321, including induction chemotherapy and reduced intensity (thiotepa and etoposide only) myeloablative therapy and ASCT (18). Platins were omitted in both induction and myeloablative regimen due to prior ototoxicity. As CRX remained detectable in repeated CSF samples, 6 monthly cycles of intrathecal topotecan (0.4 mg) were administered, normalizing CRX levels after the first cycle. After achieving systemic and CNS CR, consolidation therapy with naxitamab and GM-CSF were administered with manageable toxicity, consisting of grade 2 pain and grade 1 urticarial reactions.
Patient underwent regular monitoring, including ophtalmological exams, craniospinal MRI, and molecular surveillance in BM and CSF. She remains alive and disease-free 8 years after diagnosis and 5 years post extraocular relapse. Current sequelae include total visual loss due to bilateral enucleation and moderate bilateral sensorineural hearing loss secondary to chemotherapy.
3 DiscussionWe hereby report, two patients diagnosed with heritable RB receiving intensive, multimodal treatment over the course of their care. Following extraocular relapse and reduced intensity myeloablative therapy and ASCT, both patients were treated with GD2 mAbs and GM-CSF and remain disease-free over 4 and 5 years, respectively, after extraocular and CNS metastasic relapse.
Limited treatment options exist for extraocular RB. Although studies have shown that HDC-ASCT significantly improves survival (18), substantial acute and long-term complications, including toxic deaths and SMNs, highlight an urgent unmet medical need (18, 21).
Platinum-based systemic chemotherapy remains a cornerstone for patients with high-risk retinoblastoma (HR-RB) (18). However, platinum-related ototoxicity, associated with bilateral high-frequency sensorineural hypoacusia (14), compounds the disease-related visual impairment already affecting RB survivors, further reducing their quality of life (36). One of the reported patients underwent bilateral enucleation, resulting in complete vision, and developed severe hearing loss following systemic therapy. This precluded treatment with high-dose carboplatin to prevent further functional deterioration.
Targeted therapies aim to improve outcomes while reducing long-term side effects. Current and emerging research suggests a potential role for mAbs directed against GD2, a glycosphingolipid highly expressed during embryogenesis, and in developmental cancers such as NB and RB (Figure 1) (2, 37) Following extensive clinical development, several anti-GD2 mAbs have been integrated into first-line treatment for HR-NB (4–6, 38). Naxitamab, a humanized version of mu3F8, is FDA-approved for bone and bone marrow refractory/relapsed HR-NB (11).
Despite their proven efficacy in HR-NB, limited research exists on the role of anti-GD2 mAbs in RB. Preclinical experiments suggest that GD2-targeted therapy is effective against RB cell lines; however, there are few studies in the clinical setting. In a recent case series of 4 patients treated with the anti-GD2 mAb dinutuximab beta (ch14.18/CHO) after ASCT, Eichholz et al. demonstrated clinical responses in 2 patients with residual extraocular RB lesions after ASCT, radiotherapy and immunotherapy. However, the overlapping treatment timelines confounded the analysis of the individual contributions of each modality to these outcomes (39). Another report described a 9-year-old male with relapsed single bone metastatic RB treated with two cycles of dinutuximab beta with GM-CSF, aldesleukin (IL-2), and spironolactone after ASCT, demonstrating safety and no disease progression for up to 18 months post-treatment (40). Our experience provides further clinical evidence that long-term survival is achievable even with reduced chemotherapy intensity. While our findings do not support attributing the outcomes solely to naxitamab, it is notable that historical survival rates for metastatic RB patients before the advent of myeloablative chemotherapy were dismal (41, 42). Furthermore, in patients with recurrent metastatic extraocular RB treated with HDC and ASCT, 5-year survival rates remain at only 31.3% (43). Our results suggest that naxitamab may have contributed to the favorable outcomes observed in our patients, underscoring the potential of anti-GD2 immunotherapy to reduce the chemotherapy burden in extraocular RB.
Importantly, anti-GD2 mAbs exhibit acute and reversible toxicities but no long-term side effects have been reported to date. However, this observation is limited by the relatively short follow-up period since their approval. Additionally, as chemotherapy is typically administered prior to or alongside anti-GD2 therapy, overlapping toxicities may complicate the assessment of anti-GD2-specific effects in the future (44). In this case report, patients responded well to naxitamab, with adverse events consistent with previous reports and manageable using appropriate protocols (45, 46). Although preliminary, these results should guide future studies evaluating anti-GD2 mAbs and their potential to reduce reliance on highly toxic HDC-ASCT in RB patients.
Since anti-GD2 mAbs do not cross the BBB, patients with CNS metastasis also require CNS-targeted treaments, as CNS remains the primary site of RB metastatic relapse (16, 47). Recent reports support the use of intrathecal therapy to address CNS dissemination in RB, but timely detection remains challenging (20). Traditional methods, including CSF cytology and neuroimaging, only detect overt CNS disease, which is incurable (27, 48). Early detection and pre-emptive treatment of MDD, by contrast, may improve outcomes (30). Emerging evidence identifies CRX, a transcription factor upregulated in RB, as a specific biomarker for MDD (31, 32, 49). Although its prognostic role has yet to be confirmed (48), detectable CRX MDD in BM and CSF in non-metastatic RB patients with high-risk features is associated with decreased event-free survival (50) and CNS relapse (51), respectively. We routinely monitor CRX mRNA levels in the BM and CSF of high-risk RB patients. In this report, both patients achieved CR while successfully clearing CRX-positive MDD in the CSF using intrathecal topotecan. Continued monitoring has demonstrated no subsequent CNS progression. This approach underscores the importance of integrating molecular diagnostics with targeted CNS therapies to improve outcomes for high-risk RB patients.
Future therapeutic strategies may leverage CRX monitoring as a tool for early detection and to stratify patients who might benefit from intensified local CNS treatment, including novel combinations of intrathecal agents.
4 ConclusionsOur experience provides preliminary evidence for the potential role of anti-GD2 mAbs in the multimodality management of extraocular RB, especially for heavily pre-treated patients with significant cumulative prior toxicities and susceptibility to SMNs. Moreover, the results suggest that intrathecal topotecan may improve outcomes in patients with detectable CRX in the CSF. Taken together, the data support further investigation of anti-GD2 mAbs and CNS-directed therapy for MDD in well-defined subsets of patients with metastatic RB.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributionsCL: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MS-R: Writing – review & editing. NS: Investigation, Writing – review & editing. JM: Data curation, Writing – review & editing. CL: Supervision, Writing – review & editing. GC: Data curation, Writing – original draft, Writing – review & editing. JM: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe are grateful to the families of our patients at PCCB for their unwavering support.
Conflict of interestJM Consulting fees from Y-mAbs Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statementThe author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI assisted in correcting the English language.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Nazha B, Inal C, Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. (2020) 10:1000. doi: 10.3389/fonc.2020.01000
PubMed Abstract | Crossref Full Text | Google Scholar
4. Cheung NK V, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte- macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. (2012) 30(26):3264–70. doi: 10.1200/JCO.2011.41.3807
PubMed Abstract | Crossref Full Text | Google Scholar
5. Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. (2010) 363(14):1324–34. doi: 10.1056/NEJMoa0911123
PubMed Abstract | Crossref Full Text | Google Scholar
6. Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19(12):1617–29. doi: 10.1016/S1470-2045(18)30578-3
PubMed Abstract | Crossref Full Text | Google Scholar
7. Mody R, Yu AL, Naranjo A, Zhang FF, London WB, Shulkin BL, et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the children's oncology group. J Clin Oncol. (2020) 38(19):2160–9. doi: 10.1200/JCO.20.00203 32343642
PubMed Abstract | Crossref Full Text | Google Scholar
8. Gray J, Moreno L, Weston R, Barone G, Rubio A, Makin G, et al. BEACON-Immuno: Results of the dinutuximab beta (dB) randomization of the BEACON-Neuroblastoma phase 2 trial-A European Innovative Therapies for Children with Cancer (ITCC-International Society of Paediatric Oncology Europe Neuroblastoma Group (SIOPEN) trial. (2022).
9. Modak S, Kushner BH, Mauguen A, Castañeda A, Varo A, Gorostegui M, et al. Naxitamab-based chemoimmunotherapy for resistant high-risk neuroblastoma: results of “HITS” phase II study. J Clin Oncol. (2022) 40(16_suppl):10028–10028. doi: 10.1200/JCO.2022.40.16_suppl.10028
Crossref Full Text | Google Scholar
10. Furman WL, Federico SM, McCarville MB, Shulkin BL, Davidoff AM, Krasin MJ, et al. A phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res. (2019) 25(21):6320–8. doi: 10.1158/1078-0432.CCR-19-1452
PubMed Abstract | Crossref Full Text | Google Scholar
12. Mora J, Bear M, Chan G, Morgenstern DA, Nysom K, Tornøe K, et al. 891P Naxitamab treatment for relapsed or refractory high-risk neuroblastoma: outcomes from the first prespecified analyses of the pivotal 201 trial. Ann Oncol. (2022) 33:S956. doi: 10.1016/j.annonc.2022.07.1017
Crossref Full Text | Google Scholar
13. Mattosinho CCDS, Moura ATMS, Oigman G, Ferman SE, Grigorovski N. Time to diagnosis of retinoblastoma in Latin America: a systematic review. Pediatr Hematol Oncol. (2019) 36(2):55–72. doi: 10.1080/08880018.2019.1605432
PubMed Abstract | Crossref Full Text | Google Scholar
14. Global Retinoblastoma Study Group, Fabian ID, Abdallah E, Abdullahi SU, Abdulqader RA, Adamou Boubacar S, et al. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. (2020) 6(5):1. Available online at:/pmc/articles/PMC7047856 (cited September 10, 2024).
16. Gündüz K, Müftüoglu O, Günalp I, Ünal E, Taçyildiz N. Metastatic retinoblastoma clinical features, treatment, and prognosis. Ophthalmology. (2006) 113(9):1558–66. doi: 10.1016/j.ophtha.2006.03.039
PubMed Abstract | Crossref Full Text | Google Scholar
17. Hu H, Zhang W, Wang Y, Huang D, Shi J, Li B, et al. Characterization, treatment and prognosis of retinoblastoma with central nervous system metastasis. BMC Ophthalmol. (2018) 18(1):107. doi: 10.1186/s12886-018-0772-8
PubMed Abstract | Crossref Full Text | Google Scholar
18. Dunkel IJ, Piao J, Chantada GL, Banerjee A, Abouelnaga S, Buchsbaum JC, et al. Intensive multimodality therapy for extraocular retinoblastoma: a children's oncology group trial (ARET0321). J Clin Oncol. (2022) 40(33):3839–47. doi: 10.1200/JCO.21.02337
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sandri A, Besenzon L, Acquaviva A, Marino C, Cordero Di Montezemolo L, Madon E. “Eight drugs in one day” chemotherapy in a nonfamilial bilateral retinoblastoma with recurrent cerebrospinal fluid metastases. Pediatr Hematol Oncol. (1998) 15(6):557–61. doi: 10.3109/08880019809018319
PubMed Abstract | Crossref Full Text | Google Scholar
20. Rodriguez A, Zugbi S, Requejo F, Deu A, Sampor C, Sgroi M, et al. Combined high-dose intra-arterial and intrathecal chemotherapy for the treatment of a case of extraocular retinoblastoma. Pediatr Blood Cancer. (2018) 65(12):e27385. doi: 10.1002/pbc.27385
PubMed Abstract | Crossref Full Text | Google Scholar
21. Friedman DN, Sklar CA, Oeffinger KC, Kernan NA, Khakoo Y, Marr BP, et al. Long-term medical outcomes in survivors of extra-ocular retinoblastoma: the Memorial Sloan-Kettering cancer center (MSKCC) experience. Pediatr Blood Cancer. (2013) 60(4):694–9. doi: 10.1002/pbc.24280
PubMed Abstract | Crossref Full Text | Google Scholar
22. Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. (2007) 114(7):1378–83. doi: 10.1016/j.ophtha.2007.03.074
PubMed Abstract | Crossref Full Text | Google Scholar
23. MacCarthy A, Bayne AM, Brownbill PA, Bunch KJ, Diggens NL, Draper GJ, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951–2004. Br J Cancer. (2013) 108(12):2455–63. doi: 10.1038/bjc.2013.228
PubMed Abstract | Crossref Full Text | Google Scholar
24. Marees T, Moll AC, Imhof SM, De Boer MR, Ringens PJ, Van Leeuwen FE. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. (2008) 100(24):1771–9. doi: 10.1093/jnci/djn394
PubMed Abstract | Crossref Full Text | Google Scholar
25. Gianotti Antoneli CB, Steinhorst F, Braga Ribeiro KDC, Novaes PERS, Chojniak MMM, Arias V, et al. Extraocular retinoblastoma: a 13-year experience. Cancer. (2003) 98(6):1292–8. doi: 10.1002/cncr.11647
PubMed Abstract | Crossref Full Text | Google Scholar
26. Dunkel IJ, Chan HSL, Jubran R, Chantada GL, Goldman S, Chintagumpala M, et al. High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4b retinoblastoma. Pediatr Blood Cancer. (2010) 55(1):149–52. doi: 10.1002/pbc.22491
PubMed Abstract | Crossref Full Text | Google Scholar
27. Hu H, Zhang W, Wang Y, Huang D, Shi J, Li B, et al. Characterization, treatment and prognosis of retinoblastoma with central nervous system metastasis. BMC Ophthalmol. (2018) 18(1):1–7. doi: 10.1186/s12886-017-0645-6
PubMed Abstract | Crossref Full Text | Google Scholar
28. Shields CL, Jorge R, Say EAT, Magrath G, Alset A, Caywood E, et al. Unilateral retinoblastoma managed with intravenous chemotherapy versus intra-arterial chemotherapy. Outcomes based on the international classification of retinoblastoma. Asia-Pacific J Ophthalmol. (2016) 5(2):97–103. doi: 10.1097/APO.0000000000000172
PubMed Abstract | Crossref Full Text | Google Scholar
29. Graff Z, Giron V, Miller K, Pixtun D, Alejos A, Luna-Fineman S. Toxicity and feasibility of vincristine, etoposide, and carboplatin alternating with vincristine, doxorubicin, and cyclophosphamide in children with advanced retinoblastoma in Guatemala. Pediatr Blood Cancer. (2023) 70(7):e30392. doi: 10.1002/pbc.30392
PubMed Abstract | Crossref Full Text | Google Scholar
30. Torbidoni AV, Laurent VE, Sampor C, Ottaviani D, Vazquez V, Gabri MR, et al. Association of cone-rod homeobox transcription factor messenger RNA with pediatric metastatic retinoblastoma. JAMA Ophthalmol. (2015) 133(7):805–12. doi: 10.1001/jamaophthalmol.2015.0900
PubMed Abstract | Crossref Full Text | Google Scholar
31. Santagata S, Maire CL, Idbaih A, Geffers L, Correll M, Holton K, et al. CRX Is a diagnostic marker of retinal and pineal lineage tumors. PLoS One. (2009) 4(11):e7932. doi: 10.1371/journal.pone.0007932
PubMed Abstract | Crossref Full Text | Google Scholar
32. Meredith DM, Charville GW, Fletcher CDM, Hornick JL. Distantly metastatic retinoblastoma to soft tissue and bone: a challenging diagnosis highlighting the utility of CRX. Am J Surg Pathol. (2021) 45(6):820–4. doi: 10.1097/PAS.0000000000001620
PubMed Abstract | Crossref Full Text | Google Scholar
34. Mora J, Castañeda A, Gorostegui M, Santa-María V, Garraus M, Muñoz JP, et al. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatr Blood Cancer. (2021) 68(10):e29121. doi: 10.1002/pbc.29121
PubMed Abstract | Crossref Full Text | Google Scholar
35. Fabian ID, Reddy A, Sagoo MS. Classification and staging of retinoblastoma. Community Eye Health. (2018) 31(101):11–3.29915461
PubMed Abstract | Google Scholar
36. Belson PJ, Eastwood JA, Brecht ML, Hays RD, Pike NA. A review of literature on health-related quality of life of retinoblastoma survivors. J Pediatr Oncol Nurs. (2020) 37(2):116–27. doi: 10.1177/1043454219888805
PubMed Abstract | Crossref Full Text | Google Scholar
37. Chang HR, Cordon-Cardo C, Houghton AN, Cheung NK V, Brennan MF. Expression of Disialogangliosides GO2 and GD3 on Human Soft Tissue Sarcomas. (1992).
38. Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. (1985) 45(6):2642–9.2580625
PubMed Abstract | Google Scholar
39. Eichholz T, Heubach F, Arendt AM, Seitz C, Brecht IB, Ebinger M, et al. Targeted therapies in retinoblastoma: gD2-directed immunotherapy following autologous stem cell transplantation and evaluation of alternative target B7-H3. Cancer Immunol Immunother. (2024) 73:19. doi: 10.1007/s00262-023-03587-0
PubMed Abstract | Crossref Full Text | Google Scholar
40. Chan WYK, Fu NW, Fu ECH, Liu APY, Yan CLS, Yau JPW, et al. Autologous hematopoietic stem cell transplantation followed by quadruple immunotherapy with dinutuximab beta, sargramostim, aldesleukin, and spironolactone for relapsed metastatic retinoblastoma. Pediatr Blood Cancer. (2024) 71(7):e31044. doi: 10.1002/pbc.31044
PubMed Abstract | Crossref Full Text | Google Scholar
41. Schvartzman E, Chantada G, Fandiño A, de Dávila MT, Raslawski E, Manzitti J. Results of a stage-based protocol for the treatment of retinoblastoma. J Clin Oncol. (1996) 14(5):1532–6. doi: 10.1200/JCO.1996.14.5.1532
PubMed Abstract | Crossref Full Text | Google Scholar
43. Li N, Wang YZ, Zhang Y, Zhang WL, Huang DS. Characteristics of patients with recurrent retinoblastoma: a survival analysis. BMC Cancer. (2024) 24(1):1–7. doi: 10.1186/s12885-023-11764-8
PubMed Abstract | Crossref Full Text | Google Scholar
44. Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, et al. Long-term follow-up of a phase III study of ch14.18 (dinutuximab)+cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin Cancer Res. (2021) 27(8):2179–89. doi: 10.1158/1078-0432.CCR-20-3909
PubMed Abstract | Crossref Full Text | Google Scholar
45. Mora J, Chan GC, Morgenstern DA, Nysom K, Bear MK, Tornøe K, et al. Outpatient administration of naxitamab in combination with granulocyte-macrophage colony-stimulating factor in patients with refractory and/or relapsed high-risk neuroblastoma: management of adverse events. Cancer Rep (Hoboken). (2023) 6(1):e1627. doi: 10.1002/cnr2.1627
PubMed Abstract | Crossref Full Text | Google Scholar
46. Castañeda A, Gorostegui M, Miralles SL, Chamizo A, Patiño SC, Flores MA, et al. How we approach the treatment of patients with high-risk neuroblastoma with naxitamab: experience from the Hospital Sant Joan de Déu in Barcelona. Spain. ESMO Open. (2022) 7(2):100462. doi: 10.1016/j.esmoop.2022.100462
Crossref Full Text | Google Scholar
47. Cozza R, De Ioris MA, Ilari I, Devito R, Fidani P, De Sio L, et al. Metastatic retinoblastoma: single institution experience over two decades. Br J Ophthalmol. (2009) 93(9):1163–6. doi: 10.1136/bjo.2008.148932
PubMed Abstract | Crossref Full Text | Google Scholar
48. Aschero R, Torbidoni A, Sampor C, Laurent V, Zugbi S, Winter U, et al. Minimally disseminated disease and outcome in overt orbital retinoblastoma. Pediatr Blood Cancer. (2019) 66(6):e27662. doi: 10.1002/pbc.27662
PubMed Abstract | Crossref Full Text | Google Scholar
49. Terry J, Calicchio ML, Rodriguez-Galindo C, Perez-Atayde AR. Immunohistochemical expression of CRX in extracranial malignant small round cell tumors. Am J Surg Pathol. (2012) 36(8):1165–9. doi: 10.1097/PAS.0b013e3182601d84
PubMed Abstract | Crossref Full Text | Google Scholar
50. Laurent VE, Torbidoni AV, Sampor C, Ottaviani D, Vazquez V, Gabri MR, et al. Minimal disseminated disease in nonmetastatic retinoblastoma with high-risk pathologic features and association with disease-free survival. JAMA Ophthalmol. (2016) 134(12):1374–9. doi: 10.1001/jamaophthalmol.2016.4158
PubMed Abstract | Crossref Full Text | Google Scholar
51. Torbidoni AV, Sampor C, Laurent VE, Aschero R, Iyer S, Rossi J, et al. Minimal disseminated disease evaluation and outcome in trilateral retinoblastoma. Br J Ophthalmol. (2018) 102(11):1597–601. doi: 10.1136/bjophthalmol-2018-312263
留言 (0)