The incidence of mid-trimester preterm premature rupture of membranes (PPROM) is reported to be 0.4%–0.7% of pregnancies (1). This results in the loss of amniotic fluid (AF), and oligohydramnios ensues. In the embryonic period, AF is contributed by the maternal serum, coelomic fluid, and amnion. Later, with renal function beginning at 10 weeks of gestation, amniotic fluid is primarily produced by fetal urine, with contributions from lung secretions (2, 3). Animal studies have shown the need for adequate lung fluid secretion for the optimal growth and development of fetal lungs (4–6). The lung luminal pressure is higher than the AF pressure and the vocal cords act as a one-way valve. The lung fluid is secreted into the lumen against a resistance imparted by the laryngeal abduction, nasopharynx, and chest wall recoil pressure (7–9). This luminal distending pressure is crucial for lung growth.
While there are diverse etiologies for oligohydramnios in the mid-trimester gestation, PPROM poses a significant challenge as prolonged oligohydramnios hinders fetal development. The available evidence suggests that the volume of lung fluid at term equals the functional residual capacity (FRC) of the lungs, which determines the compliance of the lungs, chest wall, and the distending pressure, which are critical factors determining lung growth (10–12). Thus, prolonged leaking of lung fluid hinders the development of liquid FRC, resulting in hypoplastic lungs (4). Pulmonary hypoplasia (PH) presents as a neonatal emergency with severe respiratory compromise resulting from a reduced number of alveoli or bronchopulmonary segments. PH secondary to mid-trimester PPROM has been reported with a high perinatal mortality rate (1). We present a favorable respiratory outcome in an infant born at 32 weeks with maternal oligohydramnios following PPROM from 16 weeks of gestation.
Case presentationA primigravida mother was booked for antenatal care at 5 weeks of gestation in our center. Her routine antenatal tests, incorporating fetal aneuploidy screening, including cell-free fetal DNA non-invasive prenatal screening, were normal and reassuring. However, a screening fetal anomaly scan performed at 20 + 3 weeks showed a significantly reduced amniotic fluid index (AFI) of 1.8 cm. Fetal biometry was appropriate for gestational age (GA). The kidneys and bladder were visualized. The mother had experienced leaking liquor from 16 weeks of gestation, and this was confirmed on vaginal speculum examination with a pool of liquor in the vaginal fornix. There was no maternal pyrexia or foul-smelling vaginal discharge.
The examination was unremarkable, with a soft, non-tender abdomen and uterus. An Actin PROM swab test was positive for AF. The couple was counseled on the diagnosis and the poor prognosis by the obstetric team. Fetal medicine specialists and the neonatal team gave a guarded prognosis. The team explained oligohydramnios and related risks of fetal loss, PH, limb contractures, and chorioamnionitis. The couple was also informed of the complications of prematurity and the need for a prolonged Neonatal Intensive Care Unit (NICU) stay if the pregnancy continued past the threshold of neonatal viability. Their options were discussed, including termination of pregnancy, and the couple opted for expectant management.
The mother was initiated on antibiotics and strict bed rest was advised. Blood counts and infective markers were unremarkable. The vaginal swab was positive for Gardnerella vaginalis, negative for Group B Streptococcus, and the urine culture was sterile.
She was reviewed in the outpatient clinic weekly and advised to get admitted if she developed symptoms or signs of sepsis or preterm labor. Her infective markers were serially monitored, trended, and remained negative. At 24 weeks of gestation, antenatal corticosteroids were given. The AFI remained persistently low, between 1.0 and 1.7 cm, but maintained fetal growth.
At 31 + 4 weeks, the mother complained of abdominal pain and blood-stained discharge. The cervix was closed, and there was no foul-smelling discharge. An ultrasound scan (US) showed the fetus in breech presentation and oligohydramnios. The clinical impression was chorioamnionitis. She was admitted and was given antibiotics. The cardiotocograph showed a reassuring trace. A multidisciplinary team discussion was held to discuss delivery plans, and the team reached a consensus on delivering the baby. She underwent a cesarean section after a second course of corticosteroids and magnesium sulfate at 32 weeks of gestation. The neonatal team was on standby. Placental swabs and histopathology were negative for chorioamnionitis. The mother was discharged home on day 4.
A male infant weighing 1,550 g [26th percentile, −0.65 standard deviation scores (SDS)] with a head circumference of 28.5 cm (27th percentile, −0.60 SDS) and a length of 38 cm (6th percentile, −1.58 SDS) was born with an Apgar score of 5 and 9 at 1 and 9 min, respectively. Cord blood gas showed a pH of 7.2 and a base excess of +0.3. The baby had an initial weak cry. He quickly became apneic and was given positive pressure ventilation. He required positive inspiratory pressure/positive end-expiratory pressure (PIP/PEEP) of 25/5 cm H2O with a fraction of inspired oxygen (FiO2) of 1. With the return of spontaneous respiratory effort at 5 min, he was given continuous positive airway pressure (CPAP, cm H2O) support of +5. A chest radiograph showed bilateral small radiolucent lungs (Figure 1). However, oxygen saturations remained in the low 80s, and the baby was intubated at 8 min of life. A blood culture was taken, and antibiotics (penicillin and gentamicin) were initiated. There were no dysmorphic features, limb contractures, or chest deformity.
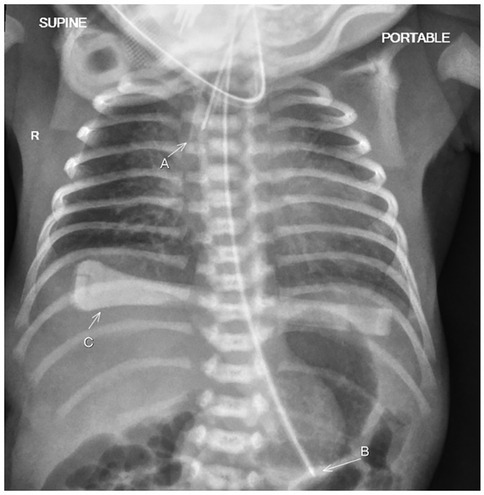
Figure 1. The chest radiograph taken just after birth shows bilateral small radiolucent lungs in keeping with bilateral pulmonary hypoplasia. The patient has been intubated with the endotracheal tube seen in the trachea (A). A feeding tube (B) and an umbilical cord clamp (C) are also present.
In the NICU, the baby remained ventilated with two doses of surfactant at 49 min and 11 h of life, respectively, with inadequate response. At 14 h of life, he had oxygenation failure. An echocardiography revealed severe pulmonary hypertension (PHT) and a patent ductus arteriosus (PDA) of 2.6 mm with a right-to-left shunt. The respiratory support was escalated to high-frequency oscillatory ventilation (HFOV) with a graded-up setting to a mean airway pressure of 18, frequency of 12, amplitude of 36, and FiO2 of 1. Inhaled nitric oxide (iNO) of 20 ppm was added at 15 h of life. The team counseled the parents regarding the severity of the illness and possible poor outcomes. Blood counts, septic markers, and the blood culture were unremarkable, and antibiotics were discontinued on day 3 of life.
The pre/post-ductal saturations and blood gases improved over 8 h of iNO. By day 8 of life, he was weaned off iNO and converted to a conventional ventilator. On day 15, he was extubated to a non-invasive nasal mask with bilevel positive airway pressure (BiPAP) ventilation using a PIP/PEEP of 22/8 cm H2O and FiO2 of 0.3. He was then converted to CPAP of +8 on day 17 of life with stable blood gases while on FiO2 of 0.3.
On day 50, he had an increasing oxygen requirement; his BiPAP pressures were increased to 22/9 cm H2O, respiratory rate (RR) of 40/min, and FiO2 to 0.5. His chest x-ray showed increased bilateral diffuse peribronchial airspace opacification and cuffing, suggesting bronchopulmonary dysplasia. Subsequently, he was commenced on oral prednisolone for 14 days as per the Bhandari protocol (13). He responded well; on day 78, he was converted to CPAP + 7 and FiO2 of 0.24. The pediatric respiratory team reviewed him. He underwent an overnight pulse oximetry test (POT) on day 87 of life, which revealed suboptimal results (Table 1). A POT was performed using a Masimo Radical-7 oximeter with a signal averaging three seconds. Profox™ software was used to download and analyze the oximetry indices. The oximetry data were analyzed and reported by a pediatric respiratory consultant. The respiratory specialist team recommended investigating gastroesophageal reflux (GER). Medical management of GER was unsuccessful. The pediatric gastrointestinal specialist team advised an upper gastrointestinal contrast study that confirmed moderate GER and a nasojejunal (NJ) tube was inserted. On day 93, a POT was conducted, and the results warranted an escalation of respiratory support to BiPAP. On day 100, the POT showed the percentage of time SpO2 was <90% improved from 6.2% to 1.7%.
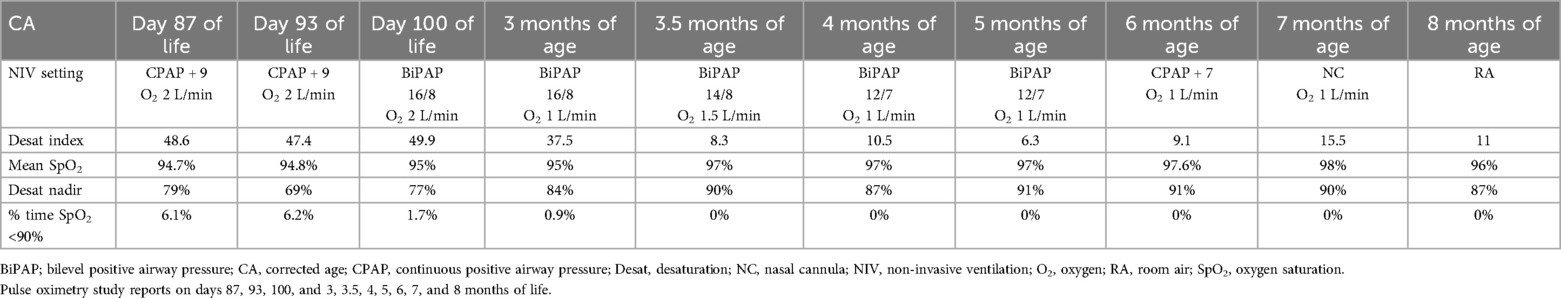
Table 1. Serial pulse oximetry test data during the treatment period.
Home care training for the parents was initiated by the neonatal home care team, which included a respiratory therapist, physiotherapist, dietitian, speech and language therapist (SLT), and specialized trained nurses for infant home care. The parents/carers were trained and certified in home non-invasive ventilation machines, tube feeding, vital sign monitoring devices, infant cardio-pulmonary resuscitation, and foreign body airway obstruction management. The parents were educated on red flags related to NJ feeding. The carers were allowed to contact the home care team whenever necessary. Periodic home visits by our nursing team recorded the progress. The baby was discharged home on day 108 of life on continuous NJ tube feeding, home BiPAP machine support of 16/8, RR of 30/min, and an oxygen flow of 2 L/min. The neonatal home care team followed up with the baby. The respiratory and neonatal consultants made weekly phone calls to the parents and conducted fortnightly/monthly clinical reviews. The dietitian and SLT assessed him during joint clinic sessions. He was also enrolled in a preterm neurodevelopmental follow-up program.
A series of POTs (Table 1) performed at home showed improvement in oximetry indices, enabling the gradual weaning of respiratory support to CPAP + 7 by 5 months of age. As the baby remained stable with good oximetry indices at 6 months, we gradually weaned him onto nasal cannula oxygen.
Subsequently, the nasal cannula oxygen was weaned to room air by a corrected age of 8 months. Following this, his oximetry indices in room air were satisfactory (Figure 2).
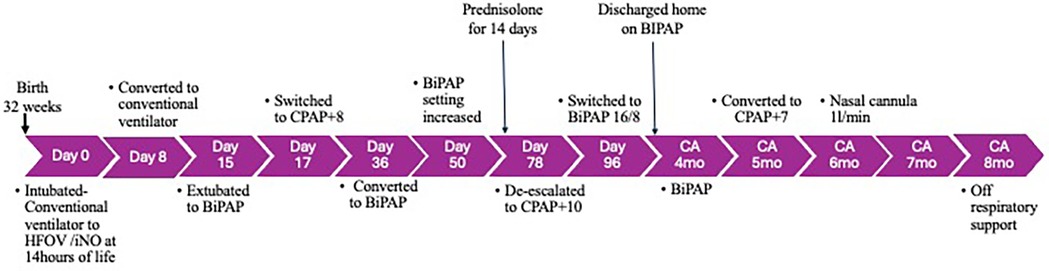
Figure 2. Timeline of events from birth to 8 months of age. BiPAP, bilevel positive airway pressure; CA, corrected age; CPAP, continuous positive airway pressure; HFOV, high-frequency oscillatory ventilation; NIV, non-invasive ventilation.
A serial cardiac evaluation documented the spontaneous closure of the PDA with no PHT at 2 months of age. He was initiated on palivizumab prophylaxis before discharge. His hearing and retinopathy of prematurity screenings were normal. He had a normal cranial US at birth and 2 and 4 months. His renal and liver function tests from birth remained stable. At 4 months, he was converted to bolus feeding and was bottling by 6 months of age.
He was periodically assessed by neonatal, respiratory, and cardiac specialists; an SLT; and a dietitian at 3–6 weekly intervals. At the corrected age of 14 months, his growth parameters were as follows: weight 9,300 g (100th percentile, +73.77 SDS), head circumference 45 cm (11th percentile, −1.22 SDS), and height 79.3 cm (69th percentile, +0.50 SDS). His periodic neurodevelopmental assessment was conducted using the “Ages and Stages Questionnaires” (ASQ-3 and ASQ: SE-2) appropriate for his age and was within the normal range.
DiscussionWe presented a mother who had PPROM and oligohydramnios from 16 weeks of gestation. The infant was delivered at 32 weeks of gestation after two courses of antenatal steroids. The preterm infant had a turbulent immediate neonatal period. Endotracheal intubation, surfactant administration, ventilation using HFOV/iNO, postnatal steroids, non-invasive ventilation, and home care support resulted in a favorable outcome at 8 months. Sequential POTs monitored his progress in respiratory status.
Oligohydramnios is characterized by an AFI <5 cm or a single deepest pocket (SDP) <2 cm (14). Isolated oligohydramnios occurs without any fetal and maternal underlying conditions, and the incidence is 1%–5% of all pregnancies (15). Pulmonary hypoplasia is evident in 18% of cases with oligohydramnios (14).
The etiology of oligohydramnios can be antenatal factors or fetal abnormalities. Fetal abnormalities resulting in oligohydramnios include chromosomal and renal anomalies, impairing urine production, which is essential for maintaining AF volume (14, 16). Mid-trimester PPROM results in acute and dramatic oligohydramnios. The rupture of membranes can potentially trigger uterine contractions and cervical dilatation, resulting in either miscarriage or preterm birth, and increases the risk of chorioamnionitis, leading to maternal sepsis and perinatal infective morbidity. Rahman and Pervin (17) reported a study in which 5.2% of patients had oligohydramnios, with 66% attributed to PPROM, with unfavorable neonatal outcomes including perinatal death (14%), low birth weight (28%), respiratory depression (32%), and NICU admissions (29%). The First Look Study performed in low- and middle-income countries reported an association of oligohydramnios with higher rates of adverse maternal, fetal, and neonatal outcomes, suggesting the need for effective prediction, diagnosis, and interventions (18). Antenatally, the prediction and diagnosis of PH using fetal lung-to-bodyweight ratio and 2D and 3D USs has been reported by Ruano et al. (19). Messerschmidt et al. (20) reported that lung volumetric assessments using MRI in 16–27-week fetuses can predict neonatal mortality with a sensitivity of 80% and specificity of 86%.
The pathophysiology of PH secondary to oligohydramnios involves disruption of fetal pulmonary development in or before the canalicular stage. The reduced volume of AF can lead to compression of the fetal chest cavity, limiting the expansion of the lungs and impeding their optimal development (1). This compression restricts the growth of lung tissue and interferes with alveolarization, resulting in decreased lung volume and compromised respiratory function. The mechanical constraint on lung development may also lead to PH with fewer, smaller alveoli and reduced airway arborization (16). Consequently, neonates born with PH face significant challenges in gas exchange, often requiring intensive medical intervention to support respiratory function. There is a strong correlation between the duration of oligohydramnios and the severity of PH, with longer durations of PPROM leading to more pronounced lung underdevelopment (21, 22).
The consequences of oligohydramnios extend beyond PH to affect other neonate organ systems. Kim et al. (16) found that PPROM onset <25 weeks gestation with severe oligohydramnios increased the odds of neonatal death, PH, severe PHT, and retinopathy of prematurity (ROP). Other complications may include growth restriction, neonatal sepsis, musculoskeletal contractures, umbilical cord compression resulting in fetal distress, and meconium aspiration syndrome. In addition, severe oligohydramnios may increase the risk of cesarean delivery and NICU admission (23, 24).
McIntosh (25) coined the term dry lung syndrome as a milder end of the oligohydramnios spectrum. He described this as a temporary “functional hypoplasia” due to a leak of the lung fluid and collapse of the airway, necessitating high inflation pressures to reopen them. The other end of the spectrum is PH, a postmortem pathological diagnosis defined by lung DNA/bodyweight ratio and radial alveolar counts. PH occurs with severe deformations, but mild or no deformations are also seen with severe PH. The clinical diagnosis of PH in an infant rests on oligohydramnios, low Apgar scores, deformities, and an unexpected respiratory course (26).
Williams et al. (23) indicated that survival with short-term morbidity among high-risk patients was 73%, with a favorable response to HFOV and iNO therapy, highlighting the reversibility of PHT in infants with PH with iNO therapy. Linehan et al. (27) reported a neonatal survival rate of 20% when PPROM was diagnosed between 14 and 23 + 6 weeks. Winn et al. (24), in a prospective study, found PH in 12.9% of PPROM cases and a perinatal mortality rate of 54%. Logistic regression analysis revealed that the GA at the rupture of the membranes, latency period, and initial or average AFI were critical, independent predictors of PH and the former two were predictors of perinatal death. All three predictors were observed in our case. Blott and Greenough (28) reviewed outcomes of pregnancies complicated by PPROM in the second trimester, reporting a neonatal mortality rate of 36%, mainly attributed to PH.
After parental training, survivors can be discharged home on non-invasive ventilation with home care support. Multidisciplinary care with sequential POTs and echocardiography for PHT can facilitate successful weaning of respiratory support, as noted in our case.
Long-term outcome studies of infants born to mothers with PPROM and oligohydramnios are limited. The 2-year outcome of a cohort with PPROM at <25 weeks gestation and oligohydramnios showed no statistically significant difference in neurodevelopmental outcomes compared to an aged-matched group of infants (29). Conversely, Simons et al. (30) conducted a prospective cohort study of 86 singleton pregnancies with previable PPROM at a median GA of 20 + 2 weeks, and delivery was at a median of 22 + 6 weeks. In total, 52 women (60.5%) delivered at <24 weeks of gestation. The perinatal mortality rate was 63/86 (73.3%). None of the four fetuses <16 weeks survived to discharge. Of the 23/86 (26%) who survived to discharge, long-term follow-up was available for 13/23(56.5%), of whom 50% at 2 years and 57.1% at 5 years were experiencing respiratory issues.
In our case, the respiratory and hemodynamic consequences of PH were severe in the immediate neonatal period. The impaired morphological maturation of the lungs with severe PHT complicated the neonatal transition. A tailored approach to mechanical ventilation using HFOV with iNO helped our patient overcome hypoxemic failure and PHT.
Strengths and limitationsThe comprehensive evaluation process and step-by-step management approach by a multidisciplinary team outlined in this report serve as a valuable reference for practitioners. The vital feature lies in using POTs for the longitudinal evaluation of his respiratory status. The outcome showcases the possibility of achieving a favorable outcome in patients with severe oligohydramnios from a mid-trimester PPROM. However, as a single case report, our findings cannot be generalizable to all patients who present this way.
ConclusionOver the years, neonatal mortality following mid-trimester PPROM and oligohydramnios has shown improvement. Factors contributing to this trend include early diagnosis, rigorous antenatal care, fetal monitoring, antenatal steroids, surfactant therapy, HFOV/iNO, postnatal steroids, and non-invasive respiratory support with periodic POTs. This case report highlights the importance of vigilant monitoring and timely intervention by a multidisciplinary team in pregnancies affected by PPROM and oligohydramnios. Early detection and management of PPROM through interventions such as strict bed rest, antibiotics, close fetal surveillance, and antenatal steroids at the limit of viability can help to optimize outcomes.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statementWritten informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributionsAS: Writing – original draft, Writing – review & editing. OZ: Writing – original draft, Writing – review & editing. AP: Writing – original draft, Writing – review & editing. VR: Writing – original draft, Writing – review & editing. LT: Writing – original draft, Writing – review & editing. SC: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank our doctors, nurses, and respiratory therapists from the Department of Neonatology for their contribution to the management of this baby and Associate Professor Teo Eu-Leong Harvey James, Department of Diagnostic and Interventional Imaging, KK Women's and Children's Hospital, Singapore, for providing the images and legends for this report. We are grateful to our Fetal Medicine, Pediatric Respiratory, and Cardiology colleagues, and home care team. We sincerely thank Professor Fabian Yap, Department of Endocrinology, KK Hospital, Singapore, for his valuable input regarding this manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Waters TP, Mercer BM. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am J Obstet Gynecol. (2009) 201:230–40. doi: 10.1016/j.ajog.2009.06.049
PubMed Abstract | Crossref Full Text | Google Scholar
3. Suliburska J, Kocyłowski R, Komorowicz I, Grzesiak M, Bogdański P, Barałkiewicz D. Concentrations of mineral in amniotic fluid and their relations to selected maternal and fetal parameters. Biol Trace Elem Res. (2016) 172(1):37–45. doi: 10.1007/s12011-015-0557-3
PubMed Abstract | Crossref Full Text | Google Scholar
4. Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. (1977) 123(Pt 3):649–60.885780
PubMed Abstract | Google Scholar
5. Fisk NM, Parkes MJ, Moore PJ, Hanson MA, Wigglesworth JS, Rodeck CH. Mimicking low amniotic pressure by chronic pharyngeal drainage does not impair lung development in the sheep. Am J Obstet Gynecol. (1992) 166:991–6. doi: 10.1016/0002-9378(92)91378-N
PubMed Abstract | Crossref Full Text | Google Scholar
7. Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of Adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. J Physiol. (1983) 344:137–52. doi: 10.1113/jphysiol.1983.sp014929
PubMed Abstract | Crossref Full Text | Google Scholar
9. Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. (1990) 86:1270–7. doi: 10.1172/JCI114834
PubMed Abstract | Crossref Full Text | Google Scholar
10. Wallen LD, Kulisz E, Maloney JE. Main pulmonary artery ligation reduces lung fluid production in fetal sheep. J Dev Physiol. (1991) 16:173–9.1797925
PubMed Abstract | Google Scholar
11. Harrison MR, Bressack MA, Churg AM, de Lorimiere AA. Correction of congenital diaphragmatic hernia in utero. II. Simulated correction permits fetal lung growth survival at birth. Surgery. (1980) 88:260–8.6893089
PubMed Abstract | Google Scholar
12. Moessinger AC, Singh M, Donnelly DF, Haddad GG, Collins MH, James LS. The effect of prolonged oligohydramnios on fetal lung development, maturation and ventilatory patterns in the newborn guinea pig. J Dev Physiol. (1987) 9:419–27.3693821
PubMed Abstract | Google Scholar
13. Bhandari A, Schramm CM, Kimble C, Pappagallo M, Hussain N. Effect of a short course of prednisolone in infants with oxygen-dependent bronchopulmonary dysplasia. Pediatrics. (2008) 121:e344–9. doi: 10.1542/peds.2006-3668
PubMed Abstract | Crossref Full Text | Google Scholar
14. Shumway JB, Al-Malt A, Amon E, Cohlan B, Amini S, Abboud M, et al. Impact of oligohydramnios on maternal and perinatal outcomes of spontaneous premature rupture of the membranes at 18–28 weeks. J Matern Fetal Med. (1999) 8:20–3. doi: 10.1002(SICI)1520-6661(199901/02)8:1%3C20::AID-MFM5%3E3.0.CO;2-7
PubMed Abstract | Crossref Full Text | Google Scholar
15. Hou L, Wang X, Hellerstein S, Zou L, Ruan Y, Zhang W. Delivery mode and perinatal outcomes after diagnosis of oligohydramnios at term in China. J Matern Fetal Neonatal Med. (2020) 33:2408–14. doi: 10.1080/14767058.2018.1553944
PubMed Abstract | Crossref Full Text | Google Scholar
16. Kim MS, Kim S, Seo Y, Oh MY, Yum SK. Impact of preterm premature rupture of membranes and oligohydramnios on in-hospital outcomes of very-low-birthweight infants. J Matern Fetal Neonatal Med. (2023) 36:2195523. doi: 10.1080/14767058.2023.2195523
PubMed Abstract | Crossref Full Text | Google Scholar
17. Rahman J, Pervin S. Maternal complications and neonatal outcomes in oligohydramnios. Int J Reprod Contracept Obstet Gynecol. (2022) 11:310–4. doi: 10.18203/2320-1770.ijrcog20220153
Crossref Full Text | Google Scholar
18. Figueroa L, McClur EM, Swanson J, Nathan R, Garces AL, Moore JL, et al. Oligohydramnios: a prospective study of fetal, neonatal and maternal outcomes in low-middle income countries’. Reprod Health. (2020) 17:19. doi: 10.1186/s12978-020-0854-y
PubMed Abstract | Crossref Full Text | Google Scholar
19. Ruano R, Takashi E, da Silva MM, Campos JA, Tannuri U, Zugaib M. Prediction and probability of neonatal outcome in isolated congenital diaphragmatic hernia using multiple ultrasound parameters. Ultrasound Obstet Gynecol. (2012) 39:42–9. doi: 10.1002/uog.10095
PubMed Abstract | Crossref Full Text | Google Scholar
20. Messerschmidt A, Pataraia A, Helmer H, Kasprian G, Sauer A, Brugger PC, et al. Fetal MRI for prediction of neonatal mortality following preterm premature rupture of the fetal membranes. Pediatr Radiol. (2011) 41:1416–20. doi: 10.1007/s00247-011-2199-8
PubMed Abstract | Crossref Full Text | Google Scholar
21. van Dongen PW, Antonissen J, Jongsma HW, Sporken JM, Hein PR. Lethal lung hypoplasia in infants after prolonged rupture of membranes. Eur J Obstet Gynecol Reprod Biol. (1987) 25:287–92. doi: 10.1016/0028-2243(87)90138-9
PubMed Abstract | Crossref Full Text | Google Scholar
22. Shenker L, Reed KL, Anderson CF, Borjon NA. Significance of oligohydramnios complicating pregnancy. Am J Obstet Gynecol. (1991) 164:1597–600. doi: 10.1016/0002-9378(91)91442-Y
PubMed Abstract | Crossref Full Text | Google Scholar
23. Williams O, Hutchings G, Debieve F, Debauche C. 1,22 neonatal outcome following rupture of membranes prior to 25 weeks with prolonged oligohydramnios. Early Hum Dev. (2009) 85:273–7. doi: 10.1016/j.earlhumdev.2008.11.003
PubMed Abstract | Crossref Full Text | Google Scholar
24. Winn HN, Chen M, Amon E, Leet TL, Shumway JB, Mostello D. Neonatal pulmonary hypoplasia and perinatal mortality in patients with midtrimester rupture of amniotic membranes—a critical analysis. Am J Obstet Gynecol. (2000) 182:1638–44. doi: 10.1067/mob.2000.107435
PubMed Abstract | Crossref Full Text | Google Scholar
26. Thibeault DW, Beatty EC Jr, Hall RT, Bowen SK, O’Neill DH. Neonatal pulmonary hypoplasia with premature rupture of fetal membranes and oligohydramnios. J Pediatr. (1985) 107:273–7. doi: 10.1016/S0022-3476(85)80148-7
PubMed Abstract | Crossref Full Text | Google Scholar
27. Linehan LA, Walsh J, Morris A, Kenny L, O’Donoghue K, Dempsey E, et al. Neonatal and maternal outcomes following midtrimester preterm premature rupture of the membranes: a retrospective cohort study. BMC Pregnancy Childbirth. (2016) 16:25. doi: 10.1186/s12884-016-0813-3
PubMed Abstract | Crossref Full Text | Google Scholar
28. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. (1988) 63:1146–50. doi: 10.1136/adc.63.10_Spec_No.1146
PubMed Abstract | Crossref Full Text | Google Scholar
29. Williams O, Michel B, Hucthings G, Debauche C, Hubinont C. Two-year neonatal outcome following PPROM prior to 25 weeks with prolonged period of oligohydramnios. Early Hum Dev. (2012) 88:657–61. doi: 10.1016/j.earlhumdev.2012.01.012
PubMed Abstract | Crossref Full Text | Google Scholar
30. Simons NE, de Ruigh AA, van der Windt LI, Kazemier BM, van Wassenaer-Leemhuis AG, van Teeffelen AS, et al. Maternal, perinatal and childhood outcomes of the PPROMEXILIII cohort: pregnancies complicated by previable prelabor rupture of membranes. Eur J Obstet Gynecol Reprod Biol. (2021) 265:44–53. doi: 10.1016/j.ejogrb.2021.08.007
留言 (0)