Frozen-thawed embryo transfer (FET) cycles are widely used in the clinical setting. FET can reduce the number of ovulation-stimulating cycles in infertile patients and increase the cumulative pregnancy rate per ovulation-stimulating cycle, benefiting such patients (1). In FET, the preparation of the endometrium is critical for the success of embryo transfer. Studies have revealed that natural-cycle FET (NC-FET) and modified natural-cycle FET (mNC-FET) improve the perinatal outcome compared with the hormone replacement cycle (2); thus, NC-FET and mNC-FET, in which the timing of ovulation is triggered by human chorionic gonadotropin (hCG) (3), are more suitable for women with regular ovulation.
In assisted reproductive technology, luteal phase support is essential for the maintenance of pregnancy, and a previous Randomized controlled trial (4) revealed a significantly higher rate of live birth after administration of luteal phase support compared with non-administration in NC-FET. A single drug or a combination of drugs is commonly used as luteal phase support for the mNC-FET cycle in our center. The combined-drug protocol consists of progesterone soft capsules or progesterone vaginal sustained-release gel with dydrogesterone, and the single-drug protocol consists of dydrogesterone alone. However, it is necessary to establish whether the single drug and a combination of drugs, in addition to different routes of administration and medication frequencies, result in different pregnancy and perinatal outcomes.
Therefore, in this study we investigated differences in pregnancy and perinatal outcomes among the three luteal phase support schemes in the mNC-FET cycle to determine the most suitable protocol.
Materials and methodsStudy design and participantsIn this retrospective cohort study, we examined the clinical data of 3658 mNC-FET cycles from the Reproductive Center of the Third Affiliated Hospital of Zhengzhou University from January 2018 to December 2020. The inclusion criteria of the women were as follows: 1) age ≤35 years with a regular menstrual cycle, 2) body mass index (BMI) <28 kg/m2, 3) endometrial thickness ≥7 m on the hCG injection day, 4) transfer of one or two cleavage embryos or blastocysts, 5) no more than two cycles of transfer failure. The exclusion criteria of the women were as follows: 1) previously undergone preimplantation genetic testing (PGT), 2) previously accepted oocyte donation, 3) abnormal uterine environment such as intrauterine adhesions, submucosal fibroids, adenomyosis, or uterine malformations, 4) repeated implantation failure, 5) recurrent spontaneous abortion, 6) hydrops tubae profluens, 7) chromosome abnormalities. The study was approved by the ethics committee of the Third Affiliated Hospital of Zhengzhou University (2022-221-01).
Endometrial preparation protocolOn days 10 to 12 of the menstrual cycle, follicle size and endometrial thickness were assessed using vaginal ultrasonography until the superior follicle diameter exceeded 15 mm. This measurement, combined with urinary luteinizing hormone (LH), serum LH, estradiol (E2), and progesterone (P) levels, was used to determine whether the follicles met the maturation criteria. When the urinary LH was positive or the serum LH level exceeded 20 IU/L, and the serum P level less than 1.5 ng/ml, a subcutaneous injection of 10,000 IU hCG (hCG, 5000 IU; Lizhu Pharmaceutical Trading Co., Ltd., Zhuhai, Guangdong, China) was administered to induce ovulation, and luteal phase support was given the following day as the intimal transformation day.
Luteal phase support protocolPatients in group A received progesterone soft capsules (Utrogestan; Besins Healthcare, Brussels, Belgium) at 600 mg/day and oral dydrogesterone (Abbott Biologicals, Olst, The Netherlands) at 30 mg/day, those in group B received oral dydrogesterone (Abbott Biologicals) at 30 mg/day, and those in group C received progesterone vaginal sustained-release gel (Central Pharma Ltd., Bedford, UK) at 90 mg/day and oral dydrogesterone (Abbott Biologicals) at 30 mg/day. Pregnancy was confirmed by serum hCG level on 14 days after embryo transfer, and post-pregnancy luteal phase support was continued until 30 days post-transfer. Intrauterine pregnancy was determined on 30 days after embryo transfer, the vaginal luteal support drugs was stopped on 45 days after embryo transfer, and the oral luteal phase support drug was stopped on 65 days after embryo transfer.
Embryo transfer protocolCleavage-stage embryos were thawed on day4 after HCG injection, and blastocyst embryos were thawed on day6 after HCG injection. Up to two embryos were transferred. Cleavage-stage embryo scoring criteria were those of the Bourn Hall Clinic scoring system (5), with grades I–III classified as portable embryos and grades I and II classified as high-quality embryos. Blastocyst scoring criteria were those of the Gardner scoring system (5), with 4BC and above classified as portable blastocysts and 4AA, 4AB, 4BA, and 4BB classified as high-quality blastocysts.
Data collection and outcome definitionThe patient characteristics of age, duration of infertility, BMI, anti-Müllerian hormone (AMH) level, basal serum follicle stimulating hormone (FSH) level, infertility factor, endometrial thickness, number of IVF/ICSI attempts, number of previous ET cycles, number of transferred embryos, developmental stage of embryo, number of high-quality transferred embryos, pregnancy or live birth, and singletons or twins were collected from the electronic case system of our center. For patients with a gestational sac echo and singleton live birth after embryo transfer, information on pregnancy complications was retrieved during a telephone follow-up and recorded by a designated nurse at our center. Maternal and neonatal outcomes were recorded and classified according to the information provided by the patients.
The clinical outcome indicators of the three groups were observed, including hCG positive rate, clinical pregnancy rate, 12-week pregnancy rate, live birth rate, premature delivery rate, pregnancy complications rate, neonatal birth weight, and neonatal birth defects, among which the live birth rate per embryo transfer cycle was the primary outcome.
Serum hCG of 50 IU/L on 14 days after embryo transfer was defined as hCG positive. Vaginal ultrasonography on 30 days after embryo transfer was performed to confirm a clinical pregnancy (an ectopic pregnancy was also considered a clinical pregnancy), and 12-week pregnancy was defined as a clinical pregnancy that reached the 12th gestational week. Live birth was defined as the birth of a live child after 28 weeks of gestation per embryo transfer cycle. Premature delivery (PTD) was considered as a baby born before 37 weeks of gestation. For the neonatal birth weight, birth weight<2500 g was classified as low birth rate (LBW) and birth weight≥4000 g was classified as macrosomia.
Statistical analysisAll statistical analyses were performed using SPSS version 24.0 software. Measurement data were represented using mean ± standard deviation (X ± S), and count data were represented using the rate (%).
Measurement data (age, duration of infertility, BMI, basal FSH, endometrial thickness, and number of quality embryos) were analyzed using one-way analysis of variance (ANOVA) or the Kruskal–Wallis H test according to the homogeneity of variance, and pairwise comparisons using the Bonferroni method were used. Stage of embryo at transfer, number of embryos transferred, and clinical outcomes were analyzed using the chi-square test (χ2). The effect of the different luteal support protocols on the live birth rate was analyzed using univariate and multivariate logistic regression models, and the odds ratio (OR) and 95% confidence interval (95% CI) were calculated. A P-value of <0.05 was considered a statistically significant difference.
ResultsA total of 3658 cycles were included in the analyses. The patients in group A (882 cycles) received a combination of progesterone soft capsules and dydrogesterone, those in group B (627 cycles) received dydrogesterone only, and those in group C (2149 cycles) received a combination of progesterone vaginal sustained-release gel and dydrogesterone.
No significant difference was found in the duration of infertility and AMH among the three groups (P>0.05). Significant differences in age, infertility factor, number of IVF/ICSI attempts, number of previous ET cycles, basal FSH, BMI, and endometrial thickness were observed (P<0.05; Table 1).
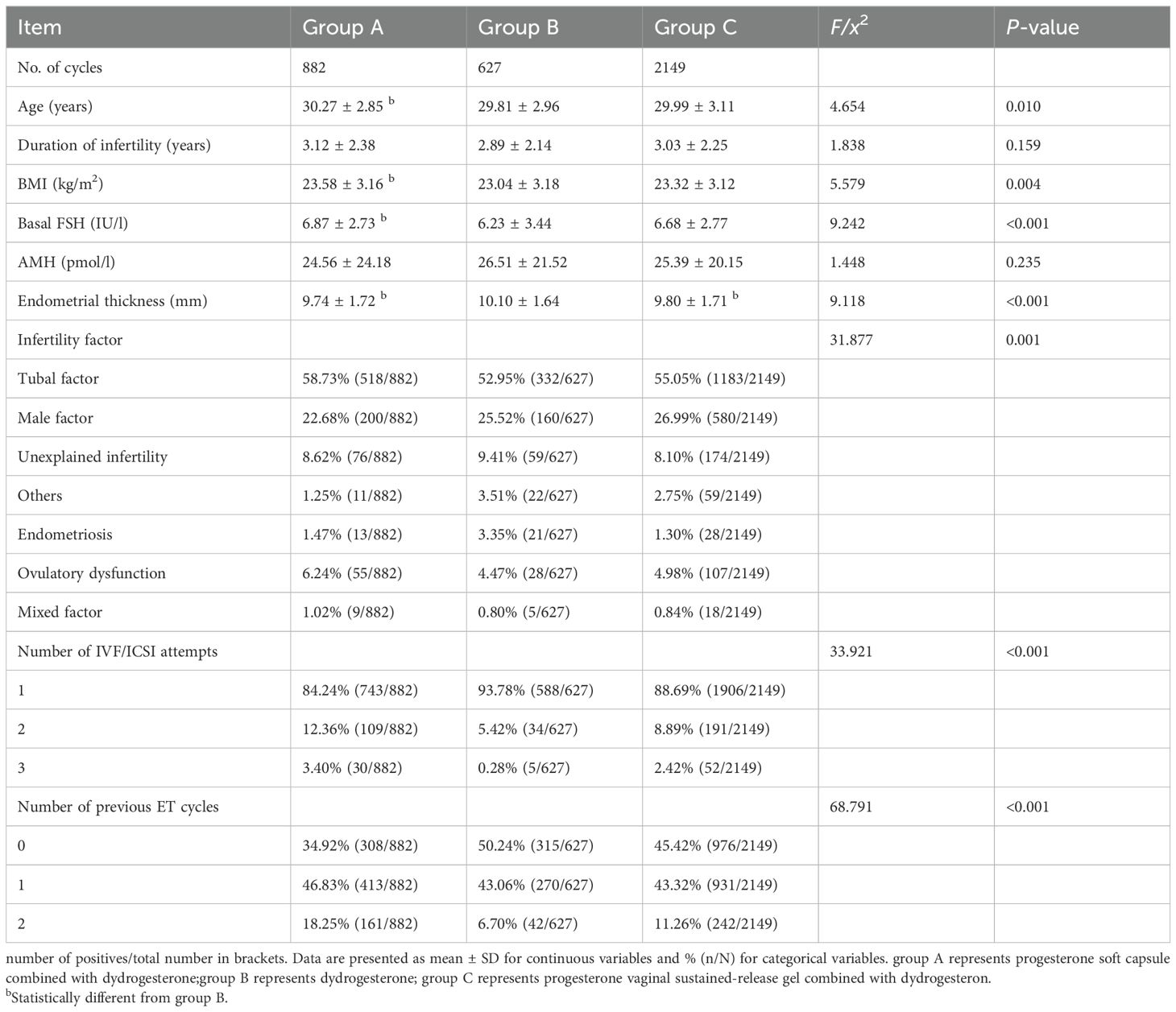
Table 1. Comparison of basic parameters among the three groups (X ± s).
No significant difference was found in the ectopic pregnancy rate and the multifetal pregnancy rate among the three groups (P>0.05). The hCG positive rate, clinical pregnancy rate, and implantation rate in group B were all higher than those in groups A and C, with statistical significance (P<0.001). The 12-week pregnancy rate and live birth rate in group B were higher than those in group A (P<0.05; Table 2).
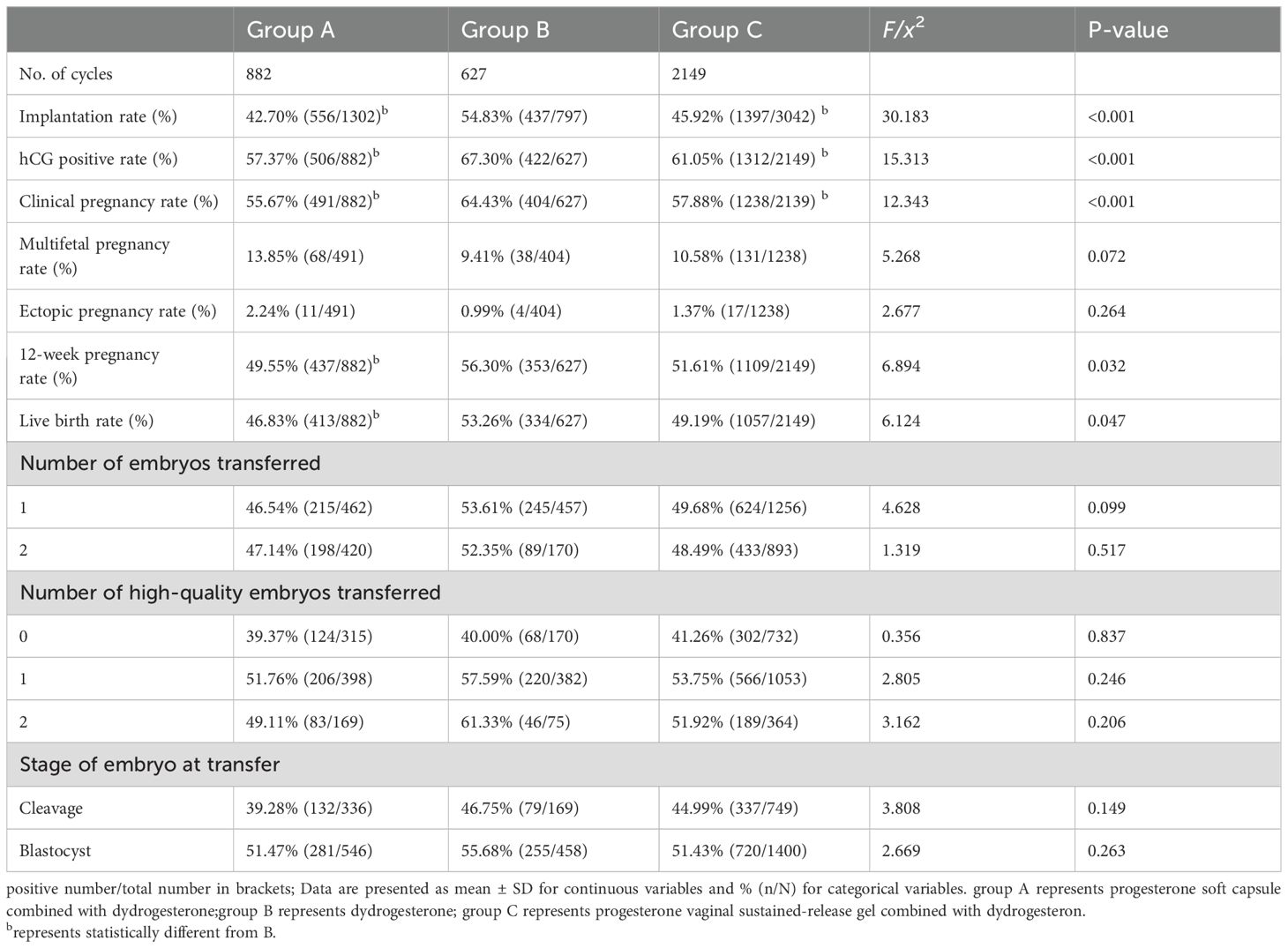
Table 2. Comparison of pregnancy outcomes among the three groups.
For the perinatal outcomes of singleton and twin births, no significant differences in gestational hypertension, gestational diabetes mellitus, premature rupture of membranes, placental abruption, placenta previa, newborn weight, and premature delivery rate were found among the three groups (P>0.05; Tables 3, 4).
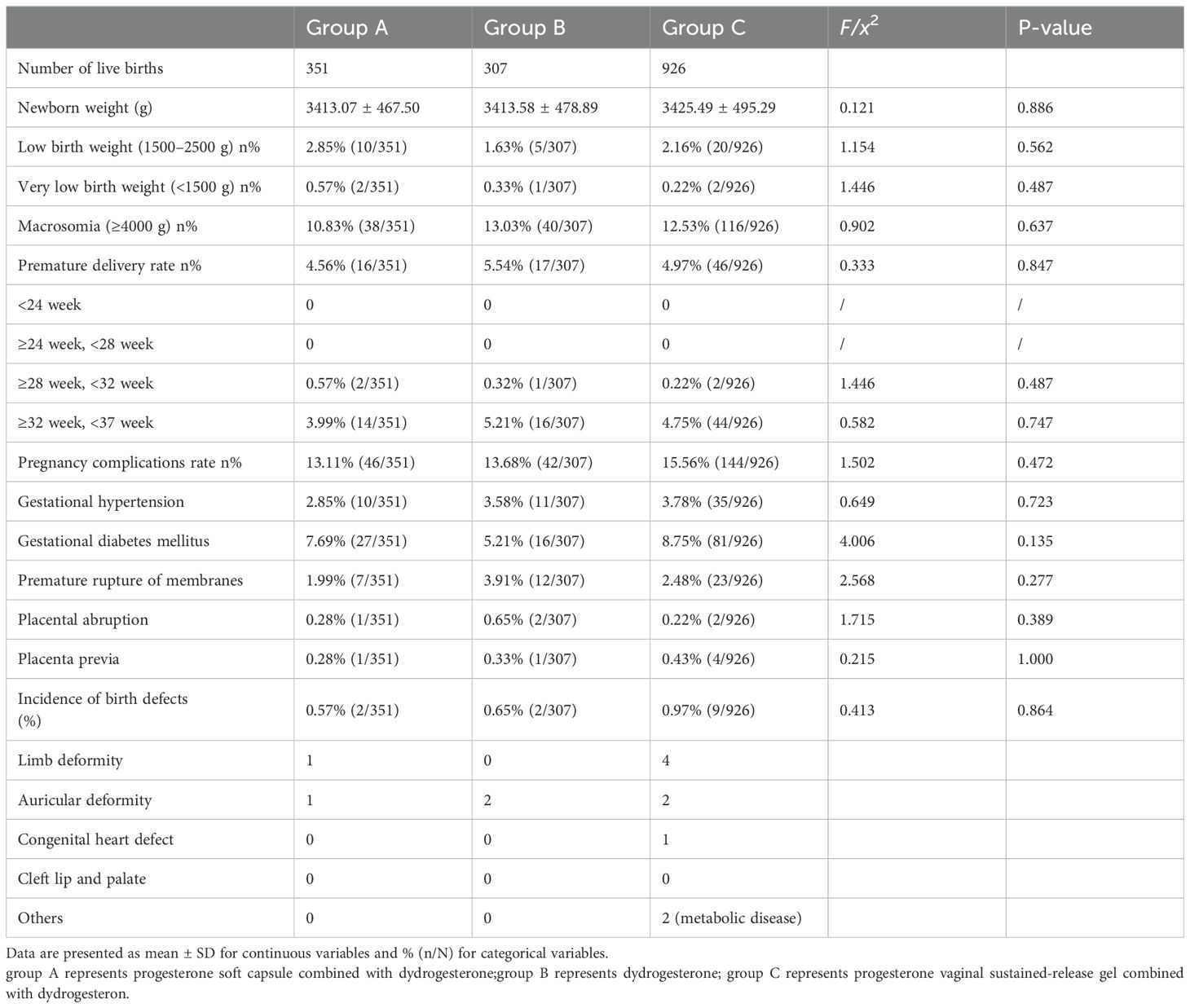
Table 3. Comparison of perinatal and neonatal outcomes of singleton live births among the three groups (X ± s).
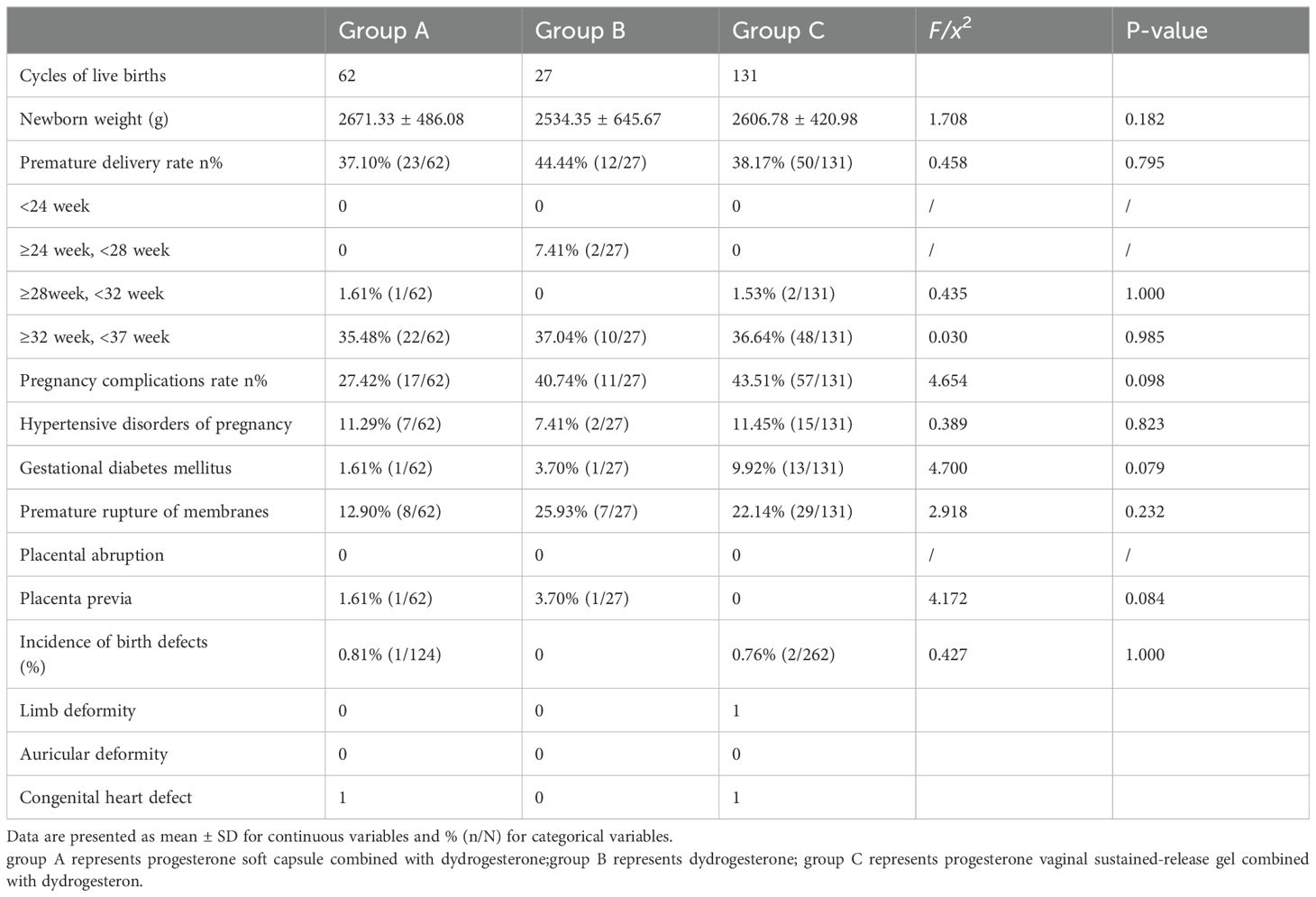
Table 4. Comparison of perinatal and neonatal outcomes of twin live births among the three groups (X ± s).
Univariate and multivariate regression analyses using age, BMI, basal FSH, endometrial thickness, infertility factor, number of IVF/ICSI attempts, number of previous ET cycles, and the luteal phase support drug used in the three different luteal phase support protocols were not independent factors of the live birth rate during the transfer cycle (Table 5).
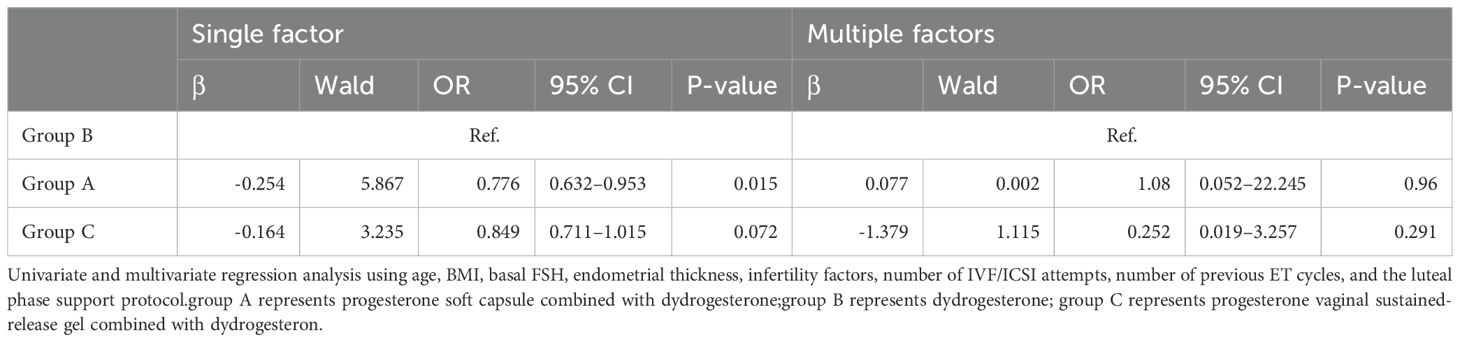
Table 5. Logistic analysis of single and multiple factors influencing live birth rate.
DiscussionOur study revealed that in the mNC-FET cycle, women of age ≤35 years who received dydrogesterone alone as the luteal support drug exhibited no difference in the live birth rate and perinatal outcome from those who received the progesterone soft capsules or progesterone vaginal sustained-release gel combined with dydrogesterone.
Luteal phase support drugs can be administered orally, by intramuscular injection, by vaginal medication, or subcutaneously, and the effect of luteal phase support has been reported to be similar across the different routes of administration (6–8). Intramuscular injection of progesterone requires daily injection, and the long-term use of injection can cause injection-site pain, hardening of the site, reduced drug absorption, and even the formation of a sterile abscess (7, 9). In contrast, vaginal medication, which avoids the disadvantages of intramuscular injection, has a uterine first-pass effect, where the local drug concentration is maintained (10) but an increase in vaginal secretions is stimulated, causing vulvar discomfort, increased risk of vaginal infection, and the possibility of sexual intercourse affecting drug absorption (11). Oral progesterone has low bioavailability (12) and may have adverse effects such as drowsiness. Dydrogesterone is a reverse-transcribed progesterone, is a more selective progesterone receptor agonist than progesterone, and has low affinity for androgens and glucocorticoid receptors (13), and its oral administration can avoid the inconvenience and side effects of vaginal medication or intramuscular injection (12, 14, 15).
The maintenance of pregnancy cannot be separated from normal luteal function. The role of luteal phase support in IVF fresh embryo transfer is widely recognized (7). FET in the hormone replacement cycle has itself does not induce luteal generation and is completely dependent on exogenous progesterone to maintain luteal function (16). It remains controversial whether luteal phase support is required for FET (4, 17–19). Previous studies suggested that the corpus luteum, produced by spontaneous ovulation, can maintain embryo implantation (20). Although luteal insufficiency may lead to implantation failure and abortion (21), a previous report revealed that the incidence of luteal insufficiency was 3.7–20% in infertile patients. Even in the normal ovulation of primary or secondary infertility, about 8.1% of patients exhibited luteal insufficiency (22), and progesterone regulated the immune mechanism to reduce the abortion rate (23). A systematic review and meta-analysis in 2021 of 15 studies involving 416 reports (24) suggested that luteal phase support using progesterone was significantly associated with a higher clinical pregnancy rate and live birth rate in NC-FET and mNC-FET. Furthermore, the LH level at the use of hCG to trigger, which may have affected endometrial events and the clinical pregnancy rate (25). In addition, luteal phase support may be able to correct synchrony at transfer. Therefore, we preferred to use progesterone for luteal phase support in mNC-FET.
There are no uniform criteria for luteal phase support in mNC-FET. Jin et al. used a combination of progesterone vaginal sustained-release gel and dydrogesterone as the luteal phase support in NC-FET (26), Shi et al. (27) and Hu et al. (28) used dydrogesterone alone as the luteal phase support in mNC-FET, and Peeraer et al. (29) used progesterone soft capsules in mNC-FET. Previous studies have indicated that dydrogesterone and vaginal drugs do not work well if used alone, and their combination should be used (30). If progesterone alone or vaginal luteal phase support alone has shown little effectiveness, their combined use may be not avoided (31), and the combination of vaginal medication and intramuscular administration exhibited a better clinical outcome than the use of each approach alone (32). However, studies were performed on FET in the hormone replacement cycle, and studies may have been related to the absence of auto-luteal formation of HRT-FET. Such absence increases the need for greater luteal phase support compared with the case of mNC-FET. Some investigators believed that the combination of luteal phase support drugs through the vagina and other routes is not supported by evidence, assuming that the clinician decided to combine different drugs the drug to exclude the possibility of unsatisfactory drug administration (8).
In our study, the luteal phase support in mNC-FET used dydrogesterone alone or in combination. Upon increasing luteal phase support drugs, the live birth rate did not improve, although it increased the cost and discomfort borne by the patient. A randomized, single-center, parallel controlled trial exhibited similar sustained pregnancy rates for mNC-FET with dydrogesterone and with vaginal sustained-release gel. In the same study, vaginal irritation, vaginal discharge, and interference of sexual intercourse with drug absorption were lower with oral medication than with vaginal medication (33). A systematic review and meta-analysis on luteal phase support protocol (34) explored fresh embryo transfer and frozen embryo transfer in mNC-FET, as well as the clinical outcome achieved using dydrogesterone, which was similar to that using progesterone soft capsules. These results were consistent with our study, in which satisfactory clinical outcomes were achieved with dydrogesterone alone.
The development of the placenta has a direct impact on perinatal outcomes (35) from the establishment of fetoplacental circulation at 3 weeks after fertilization until the complete formation of placental function at 12 weeks of pregnancy, during which progesterone improves the uterine environment and promotes the establishment of placental function (36). During normal placental development, estrogen and progesterone are critical, and altered sex steroid hormone levels may contribute to placenta-related complications (37–39). In early pregnancy, low progesterone levels may lead to placenta accreta (40), and high progesterone in early third trimester has been associated with the later development of pre-eclampsia (41). In our study, no difference was found in the premature delivery rate, newborn weight, gestational diabetes mellitus, gestational hypertension, premature rupture of membranes, placental abruption, placenta previa, and incidence of birth defects. The effect of a second luteal phase support drug on the perinatal outcome was not obvious in mNC-FET, but because the perinatal outcome is also influenced by many factors, such as both parental characteristics, ART treatment characteristics, after FET, maternal tubal factor, ovulatory dysfunction, and unexplained infertility (42, 43), so the impact of different luteal phase support protocols on the perinatal outcome requires further investigation.
This study had the following limitations. 1) The study was retrospective with some bias; therefore, additional prospective studies are required to validate our results. 2) Because maternal complications and offspring outcomes were obtained by telephone conversation and reported by patients, the data were incomplete. 3) Luteal phase support drugs were voluntarily selected by patients: most of the patients in the mNC-FET cycles did not choose to be treated with dydrogesterone alone, and the majority chose progesterone vaginal sustained-release gel and oral dydrogesterone, resulting in differences in basic indicators between the groups and in the sample size. Especially in the dydrogesterone group, high proportion of blastocyst transfer cycles, and a high proportion of high-quality embryos, these indicators will improve the pregnancy outcomes. Because of this, the live birth rate was studied as the dependent variable, and univariate and multivariate regression analyses using age, BMI, basal FSH, endometrial thickness, infertility factors, number of IVF/ICSI attempts, number of previous ET cycles, and luteal phase support drugs in different luteal phase support schemes did not independently influence the live birth rate.
ConclusionPatients with age ≤ 35 years who chose dydrogesterone alone as the luteal phase support drug in mNC-FET cycles exhibited clinically effective and safe maternal and infant outcomes. However, this finding requires further confirmation by large-sample prospective studies.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the ethics committee of the Third Affiliated Hospital of Zhengzhou University (protocol number: 2022-221-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsWZ: Writing – original draft, Writing – review & editing. SW: Writing – original draft. BR: Data curation, Writing – review & editing. RJ: Data curation, Writing – original draft, Writing – review & editing. WJZ: Software, Writing – review & editing. BW: Software, Writing – review & editing. XD: Software, Writing – review & editing. YG: Conceptualization, Investigation, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Joint Project of Medical Disciplines of Henan Province (LHGJ20200435, WZ and LHGJ20200428, BW), National Key R&D Program “Fertility Health and Health Security for Women and Children”: Clinical Cohort and Intervention Study on Genetic Problems in Assisted Reproduction Offspring (2021YFC2700602), and Henan Young and Middle-aged Health Science and Technology Innovation Leading Talent Training Project (YXKC2021020).
AcknowledgmentsThe authors are grateful to the physicians and coordinators who enrolled the patients and collected the data and to all the women who participated in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JZ declared a shared affiliation with the author(s) to the handling editor at the time of review.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Doody KJ. Cryopreservation and delayed embryo transfer-assisted reproductive technology registry and reporting implications. Fertil Steril. (2014) 102:27–31. doi: 10.1016/j.fertnstert.2014.04.048
PubMed Abstract | Crossref Full Text | Google Scholar
2. Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. (2019) 34:1567–75. doi: 10.1093/humrep/dez079
PubMed Abstract | Crossref Full Text | Google Scholar
3. Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, de Bruin JP, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. (2016) 31:1483–92. doi: 10.1093/humrep/dew120
PubMed Abstract | Crossref Full Text | Google Scholar
4. Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil Steril. (2011) 95:534–7. doi: 10.1016/j.fertnstert.2010.05.019
PubMed Abstract | Crossref Full Text | Google Scholar
5. Nasiri N, Eftekhari-Yazdi P. An overview of the available methods for morphological scoring of pre-implantation embryos in in vitro fertilization. Cell J. (2015) 16:392–405.
PubMed Abstract | Google Scholar
6. Shapiro D, Boostanfar R, Silverberg K, Yanushpolsky EH. Examining the evidence: progesterone supplementation during fresh and frozen embryo transfer. Reprod BioMed Online. (2014) 29 Suppl 1:S1–14; quiz S5-6. doi: 10.1016/S1472-6483(14)50063-6
PubMed Abstract | Crossref Full Text | Google Scholar
7. van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. (2015), CD009154. doi: 10.1002/14651858.CD009154.pub3
PubMed Abstract | Crossref Full Text | Google Scholar
8. Di Guardo F, Midassi H, Racca A, Tournaye H, De Vos M, Blockeel C. Luteal phase support in IVF: comparison between evidence-based medicine and real-life practices. Front Endocrinol (Lausanne). (2020) 11:500.
PubMed Abstract | Google Scholar
9. Penzias AS. Luteal phase support. Fertil Steril. (2002) 77:318–23.
10. Kaser DJ, Ginsburg ES, Missmer SA, Correia KF, Racowsky C. Intramuscular progesterone versus 8% Crinone vaginal gel for luteal phase support for day 3 cryopreserved embryo transfer. Fertil Steril. (2012) 98:1464–9.
11. Merriam KS, Leake KA, Elliot M, Matthews ML, Usadi RS, Hurst BS. Sexual absorption of vaginal progesterone: a randomized control trial. Int J Endocrinol. (2015) 2015:685281. doi: 10.1155/2015/685281
PubMed Abstract | Crossref Full Text | Google Scholar
12. Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update. (2000) 6:139–48. doi: 10.1093/humupd/6.2.139
PubMed Abstract | Crossref Full Text | Google Scholar
13. Rizner TL, Brozic P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, et al. Selectivity and potency of the retroprogesterone dydrogesterone in vitro. Steroids. (2011) 76:607–15. doi: 10.1016/j.steroids.2011.02.043
PubMed Abstract | Crossref Full Text | Google Scholar
14. Beltsos AN, Sanchez MD, Doody KJ, Bush MR, Domar AD, Collins MG. Patients' administration preferences: progesterone vaginal insert (Endometrin(R)) compared to intramuscular progesterone for Luteal phase support. Reprod Health. (2014) 11:78. doi: 10.1186/1742-4755-11-78
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lockwood G, Griesinger G, Cometti B, European C. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study. Fertil Steril. (2014) 101:112–9 e3. doi: 10.1016/j.fertnstert.2013.09.010
PubMed Abstract | Crossref Full Text | Google Scholar
16. Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. (2017) 32:2234–42. doi: 10.1093/humrep/dex285
PubMed Abstract | Crossref Full Text | Google Scholar
17. Horowitz E, Mizrachi Y, Finkelstein M, Farhi J, Shalev A, Gold E, et al. A randomized controlled trial of vaginal progesterone for luteal phase support in modified natural cycle - frozen embryo transfer. Gynecol Endocrinol. (2021) 37:792–7. doi: 10.1080/09513590.2020.1854717
PubMed Abstract | Crossref Full Text | Google Scholar
18. Seol A, Shim YJ, Kim SW, Kim SK, Lee JR, Jee BC, et al. Effect of luteal phase support with vaginal progesterone on pregnancy outcomes in natural frozen embryo transfer cycles: A meta-analysis. Clin Exp Reprod Med. (2020) 47:147–52. doi: 10.5653/cerm.2019.03132
PubMed Abstract | Crossref Full Text | Google Scholar
19. Haas J, Lantsberg D, Feldman N, Manela D, Machtinger R, Dar S, et al. Modifying the luteal phase support in natural cycle frozen-thawed embryo transfer improves cycle outcome. Gynecol Endocrinol. (2015) 31:891–3.
PubMed Abstract | Google Scholar
20. Rosenberg SM, Luciano AA, Riddick DH. The luteal phase defect: the relative frequency of, and encouraging response to, treatment with vaginal progesterone. Fertil Steril. (1980) 34:17–20. doi: 10.1016/S0015-0282(16)44831-4
PubMed Abstract | Crossref Full Text | Google Scholar
22. Eftekhar M, Rahsepar M, Rahmani E. Effect of progesterone supplementation on natural frozen-thawed embryo transfer cycles: a randomized controlled trial. Int J Fertil Steril. (2013) 7:13–20.
PubMed Abstract | Google Scholar
23. Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. Int Immunopharmacol. (2001) 1:1037–48. doi: 10.1016/S1567-5769(01)00035-2
PubMed Abstract | Crossref Full Text | Google Scholar
24. Mizrachi Y, Horowitz E, Ganer Herman H, Farhi J, Raziel A, Weissman A. Should women receive luteal support following natural cycle frozen embryo transfer? A systematic review and meta-analysis. Hum Reprod Update. (2021) 27:643–50. doi: 10.1093/humupd/dmab011
PubMed Abstract | Crossref Full Text | Google Scholar
25. Litwicka K, Mencacci C, Arrivi C, Varricchio MT, Caragia A, Minasi MG, et al. HCG administration after endogenous LH rise negatively influences pregnancy rate in modified natural cycle for frozen-thawed euploid blastocyst transfer: a pilot study. J Assist Reprod Genet. (2018) 35:449–55. doi: 10.1007/s10815-017-1089-x
PubMed Abstract | Crossref Full Text | Google Scholar
26. Jin Z, Li J, Yang E, Shi H, Bu Z, Niu W, et al. Effect of endometrial thickness changes on clinical pregnancy rates after progesterone administration in a single frozen-thawed euploid blastocyst transfer cycle using natural cycles with luteal support for PGT-SR- and PGT-M-assisted reproduction: a retrospective cohort study. Reprod Biol Endocrinol. (2021) 19:154. doi: 10.1186/s12958-021-00841-x
PubMed Abstract | Crossref Full Text | Google Scholar
28. Hu KL, Zhang D, Li R. Endometrium preparation and perinatal outcomes in women undergoing single-blastocyst transfer in frozen cycles. Fertil Steril. (2021) 115:1487–94. doi: 10.1016/j.fertnstert.2020.12.016
PubMed Abstract | Crossref Full Text | Google Scholar
29. Peeraer K, Couck I, Debrock S, De Neubourg D, De Loecker P, Tomassetti C, et al. Frozen-thawed embryo transfer in a natural or mildly hormonally stimulated cycle in women with regular ovulatory cycles: a RCT. Hum Reprod. (2015) 30:2552–62. doi: 10.1093/humrep/dev224
PubMed Abstract | Crossref Full Text | Google Scholar
30. Zarei A, Sohail P, Parsanezhad ME, Alborzi S, Samsami A, Azizi M. Comparison of four protocols for luteal phase support in frozen-thawed Embryo transfer cycles: a randomized clinical trial. Arch Gynecol Obstet. (2017) 295:239–46. doi: 10.1007/s00404-016-4217-4
PubMed Abstract | Crossref Full Text | Google Scholar
32. Feinberg EC, Beltsos AN, Nicolaou E, Marut EL, Uhler ML. Endometrin as luteal phase support in assisted reproduction. Fertil Steril. (2013) 99:174–8 e1. doi: 10.1016/j.fertnstert.2012.09.019
PubMed Abstract | Crossref Full Text | Google Scholar
33. Ozer G, Yuksel B, Yucel Cicek OS, Kahraman S. Oral dydrogesterone vs. micronized vaginal progesterone gel for luteal phase support in frozen-thawed single blastocyst transfer in good prognosis patients. J Gynecol Obstet Hum Reprod. (2021) 50:102030. doi: 10.1016/j.jogoh.2020.102030
PubMed Abstract | Crossref Full Text | Google Scholar
34. Barbosa MWP, Valadares NPB, Barbosa ACP, Amaral AS, Iglesias JR, Nastri CO, et al. Oral dydrogesterone vs. vaginal progesterone capsules for luteal-phase support in women undergoing embryo transfer: a systematic review and meta-analysis. JBRA Assist Reprod. (2018) 22:148–56. doi: 10.5935/1518-0557.20180018
PubMed Abstract | Crossref Full Text | Google Scholar
35. Asserhoj LL, Spangmose AL, Aaris Henningsen AK, Clausen TD, Ziebe S, Jensen RB, et al. Adverse obstetric and perinatal outcomes in 1,136 singleton pregnancies conceived after programmed frozen embryo transfer (FET) compared with natural cycle FET. Fertil Steril. (2021) 115:947–56. doi: 10.1016/j.fertnstert.2020.10.039
PubMed Abstract | Crossref Full Text | Google Scholar
36. Nakashima A, Araki R, Tani H, Ishihara O, Kuwahara A, Irahara M, et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertil Steril. (2013) 99:450–5. doi: 10.1016/j.fertnstert.2012.09.027
PubMed Abstract | Crossref Full Text | Google Scholar
37. Chen JZ, Sheehan PM, Brennecke SP, Keogh RJ. Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol Cell Endocrinol. (2012) 349:138–44. doi: 10.1016/j.mce.2011.10.014
PubMed Abstract | Crossref Full Text | Google Scholar
38. Schatz F, Guzeloglu-Kayisli O, Arlier S, Kayisli UA, Lockwood CJ. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum Reprod Update. (2016) 22:497–515. doi: 10.1093/humupd/dmw004
留言 (0)