Bone remodeling is a continuous process that regulates the mass and strength of bones. By means of this process, old bone is substituted by new bone. In addition to supporting bone growth, bone remodeling also functions as a reparative mechanism for damaged bones following the rule of fractures and micro-injuries (1). Bone remodeling involves the coordinated activity of osteoblasts and osteoclasts (2). Bone tissue undergoes constant remodeling. The imbalances in this process are closely associated with various skeletal disorders (3), such as osteoporosis, a prevalent degenerative bone disease characterized by decreased bone quality and increased turnover resulting from an imbalance between resorption and replacement during remodeling (4). Other skeletal disorders, such as osteoarthritis and rheumatoid arthritis, involve disruptions in both cartilage resorption and formation. Cancer-associated bone diseases can lead to calcium loss in bones and diffuse absorption due to hypercalcemia (5). Therefore, targeting healthy and diseased bones through remodeling behavior represents the fundamental approach to prevent and treating bone diseases (6).
1.2 Many factors systematically regulate bone remodelingMany factors systematically regulate bone remodeling. The equilibrium of bone remodeling is dynamic and influenced by numerous factors, including ATP as the core component of energy supply, post-translational modification of proteins, metabolic programming, immune and inflammatory factors, among others indicating that the homeostasis of bone remodeling is not only influenced by bone resorption but also by systemic factors. ATP plays a crucial role in bone remodeling as it serves as the central component for the energy supply. Osteoblasts are essential in creating new bone mass and enhancing bone density throughout the processes of bone growth, development, and continuous remodeling; these processes require a substantial amount of energy to which ATP significantly contributes (7). Osteoclasts are responsible for bone resorption and also need to generate significant quantities of ATP through glycolysis and oxidative phosphorylation (OXPHOS) (8). Studies have indicated impaired key enzymes for ATP production in patients with osteoporosis (9), suggesting that disrupted ATP-facilitated energy provision plays a crucial part in disturbed bone remodeling. Metabolic reprogramming also plays an important role in disorders related to bone remodeling. The metabolic reprogramming of osteoblasts and osteoclasts has emerged as a critical approach for promoting bone regeneration and managing osteoporosis (10, 11). Bone remodeling is significantly influenced by the immune system. Inflammation in the bone leads to a higher rate of bone absorption compared to formation, resulting in overall bone loss (inflammation-induced osteolysis) (12). The immune system primarily relies on immune-inflammatory factors to regulate bone remodeling; therefore, targeting these factors is crucial for treating disorders related to bone remodeling. Additionally, histone modification also plays a critical role in the process of bone remodeling. Research has demonstrated that abnormalities in histone modification are significant contributors to this process (13), and targeting histone modification has become an important approach for treating osteoporosis (14). In conclusion, multiple factors systematically regulate bone remodeling. The fundamental solution lies in exploring the systemic pathogenesis of bone disorders, identifying key nodes within the system’s dysfunctions, and implementing targeted treatments.
1.3 Citrate and bone remodelingAmong the numerous metabolic products, citrate is a very unique metabolite. On the one hand, it can enter the TCA cycle to participate in its functions; on the other hand, during this process, the cleavage of citrate produces a large amount of ace-CoA, which serves as the sole substrate for histone acetylation modification (15). Therefore, citrate may be considered an essential metabolite involved in the multi-level regulation of bone remodeling. Citrate serves as a crucial substrate for cellular energy metabolism, being generated in the mitochondria and utilized in the Krebs cycle or transported into the cytoplasm by the dedicated mitochondrial carrier CIC(mitochondrial citrate transporter). Within the cytoplasm, citrate and its derivatives, such as acetyl-CoA and oxaloacetate, are implicated in both regular and pathological processes (16). In addition to its role as an energy regulator, citrate also plays diverse roles including maintenance of protein acetylation, lipid synthesis and breakdown, amino acid production, and immune responses (17). Firstly, citrate acts as a key metabolite in the TCA cycle to maintain OXPHOS, ultimately leading to ATP production through complex V or ATP synthase (18). Moreover, citrate derivatives like acetyl-CoA serve as substrates for acetylation modifications that significantly contribute to histone acetylation. Additionally, citrate is believed to play a crucial role in metabolic reprogramming under various physiological and pathological conditions such as inflammation, Behcet’s syndrome, and heart development (19–21), while also modulating the release of inflammatory factors by the immune system (22). Abnormal levels of citrate can lead to an imbalance in bone remodeling, resulting in bone metabolism-related diseases, which further underscores the importance of citrate in the process of bone remodeling. In conclusion, citrate is now recognized not only as a solitary energy metabolite but also as a pivotal component contributing to systemic homeostasis. Furthermore, citrate holds significance in regulating bone remodeling with disrupted levels observed in bone tissue and plasma of individuals with osteoporosis (23). Notably, citrate has demonstrated efficacy against osteoporosis (24). However, the mechanisms underlying how citrate regulates bone remodeling along with therapeutic effects on bone metabolic disorders remain unclear.
In this review, we will explore the role of citrate in bone remodeling, elucidate its molecular mechanisms, and discuss its potential clinical applications. This review will introduce the innovative concept that citrate regulates bone remodeling by influencing ATP energy supply, histone acetylation, metabolic programming, and immune-inflammatory factors.
2 Citrate in the circulation of the human systemThe balance between availability and elimination of citrate is maintained by physiological requirements. Citrate in the human body exists as citrate ions and solid salts. Citrate ions are primarily generated through two pathways: direct ingestion via the gastrointestinal tract and cellular production through the metabolism of various energy substances. Solid citrate is predominantly stored in mineralized tissues, including bones and teeth. Renal metabolism plays a crucial role in the clearance of citrate to uphold body citrate homeostasis. In summary, nutrient intake, renal clearance, cellular metabolism, and bone remodeling collectively determine citrate homeostasis (Figure 1).
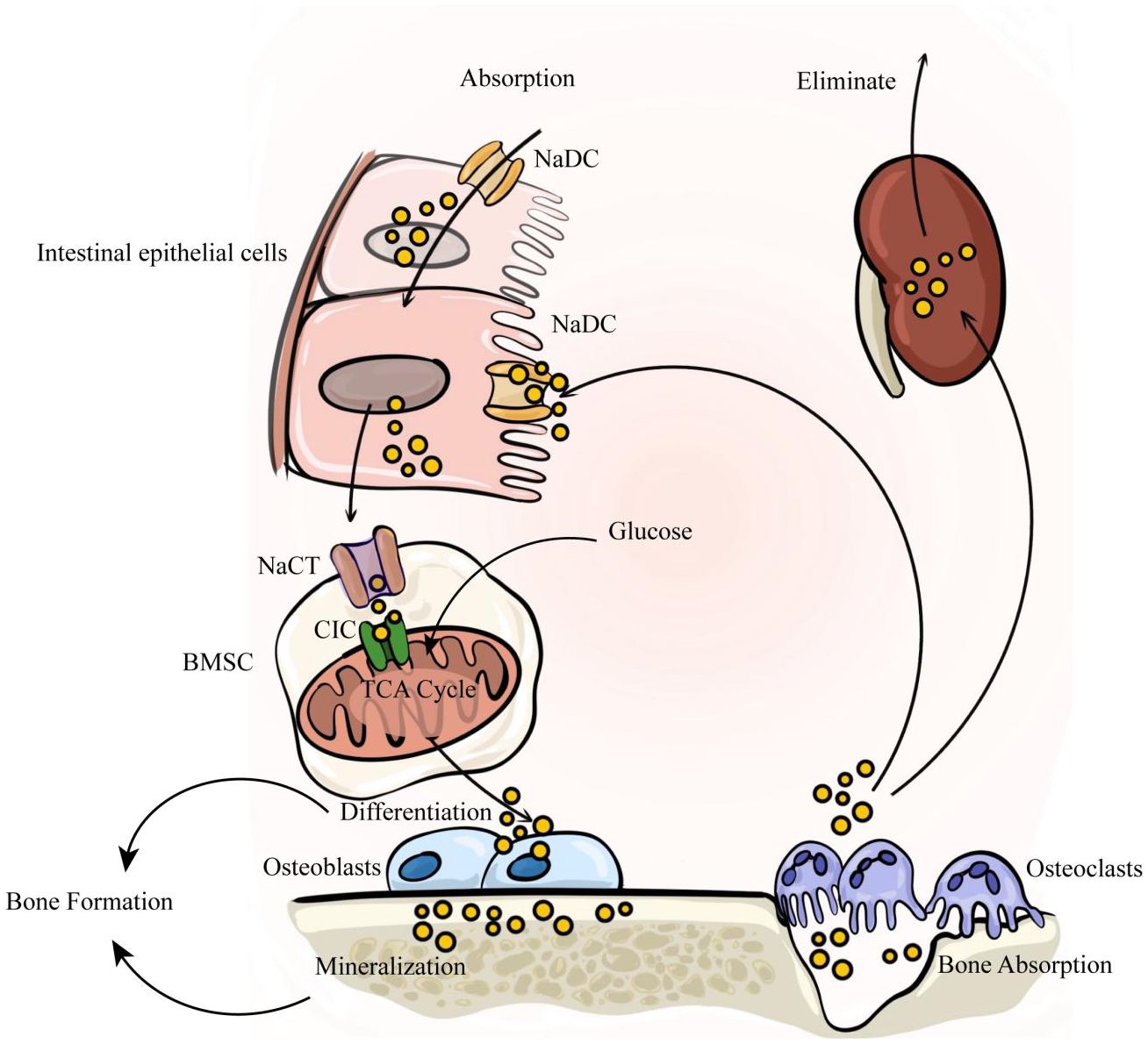
Figure 1. The internal circulation of citrate relies on four factors: nutrient intake, renal clearance, cellular metabolism, and bone remodeling. Citrate is primarily absorbed through dietary intake, enters the bloodstream via intestinal absorption, and is subsequently eliminated by the kidneys to maintain a dynamic balance of citrate in the body. Intestinal absorption and renal reabsorption depend on NaDC. At the same time, citrate within bone tissue serves as a crucial component of bone mineralization. It is stored in the bone matrix and released during osteoclast-mediated bone resorption. The released citrate enters the blood circulation, with a portion being taken up by bone marrow stromal cells via NaCT to activate osteogenic differentiation of stem cells. Subsequently, osteoblasts secrete citrate to participate in new bone formation.
2.1 Citrate intake and clearanceThe sources of citrate intake are diverse, including citrus fruits, food and beverage additives, etc. Industrial-grade citrate serves various functions such as preventing food spoilage and regulating acidity (25–27). The typical daily nutritional intake of citrate is approximately 4 grams (28). Over 95% of citrate is absorbed by the small intestinal epithelial cells through the Na+dependent dicarboxylate cotransporter (NaDC). The rapidly increasing levels of citrate in plasma are promptly filtered by the kidneys, which also rely on NaDC (27, 29–31). Around 99% of plasma citrate exists as a tricarboxylate or dicarboxylate complexed with divalent ions like calcium and magnesium. In summary, the balance of plasma citrate depends on its intake through the small intestine and renal clearance, with an important role played by NaDC. However, studies have shown that the intake and clearance of citrate salts cannot fully maintain stable plasma concentrations. Therefore, it is necessary to identify factors affecting the stability of plasma citrate concentrations. Abundant amounts of citrate are also found in cells and bone tissue (30, 31).
2.2 The citrate cycle in cellsCellular metabolism has minimal impact on citrate availability, as it is predominantly sequestered within the mitochondria and not exchanged with the extracellular space. Citrate is synthesized in the mitochondrial matrix through the enzymatic action of citrate synthase (CS), which catalyzes the condensation of acetyl-CoA and oxaloacetate to form citrate. This generated citrate then enters the TCA cycle to provide cellular energy. To proceed further in the Krebs cycle, citrate undergoes isomerization to isocitrate facilitated by aconitase (ACO2). Abundant mitochondrial citrate can be transported to the cytosol via the mitochondrial CIC. In the cytosol, ACLY (ATP citrate lyase) cleaves citrate into acetyl-CoA and oxaloacetate. A portion of acetyl-CoA is subsequently carboxylated into malonyl-CoA, which participates directly in lipid metabolism by condensing into long-chain fatty acids. The remaining acetyl-CoA serves as a substrate for histone acetylation and provides acetyl groups for protein modification under the influence of acetyltransferase enzymes. Furthermore, intracellular citrate also regulates glycolysis, gluconeogenesis, and fatty acid oxidation (16). Only select cell types absorb or release extracellular citrate due to various physiological reasons (17), such as osteocytes secreting citrates involved in bone mineralization or intestinal epithelial cells and renal tubules absorbing extracellular citrates through NaDC family transporters.
2.3 Citrate in bone tissueStudies indicate that approximately 90% of citrate in the human body is stored in mineralized tissues, playing a crucial role in regulating metabolic functions and maintaining the structural integrity of bones (32). The primary constituents of bone tissue consist of both organic and inorganic substances. The inorganic component, referred to as bone salts, primarily comprises hydroxyapatite, which aligns along the elongated axis of collagen fibers and contains a high concentration of calcium and phosphorus (33). Osteoblasts synthesize and secrete the organic portion, which includes around 10% amorphous bone matrix and approximately 90% collagen. Collagen forms a gel-like substance rich in glycine, alanine, proline, and hydroxyproline with neutral or weakly acidic glycosaminoglycans predominantly composed of type I collagen alongside a small amount of type V collagen. The amorphous bone matrix mainly consists of proteoglycans, polysaccharide complexes, as well as osteocalcin-like osteonectin (34). Within the organic component lies about 1-5% citrate content while over 15% surface area of apatite within bones is occupied by citrate molecules (35). These findings suggest that citrate not only plays an essential role in cellular metabolism but also actively participates in vital processes associated with bone matrix development and mineralization.
3 Citrate regulates bone mineralizationThe citrate-hydroxyapatite combination serves as the fundamental constituent of bone mineral salt, with approximately 70% of bone mass primarily composed of nano-scale citrate-hydroxyapatite crystals measuring 5 × 25 × 50 nm in diameter, exhibiting a delicate plate-like structure (36). Citrate plays a crucial role in the formation of plate-like structures within bone crystals and is tightly bound to hydroxyapatite as an integral component (37). This suggests that citrate cannot be substituted in the process of apatite-based bone tissue formation.
In general, citrate plays four major roles in bone mineralization: it slows down the deposition of calcium and phosphate, promotes stable nucleation of calcium phosphate(CaP), maintains the lamellar growth of apatite, and limits excessive formation of apatite crystals to maintain optimal mechanical conditions. The initiation of bone mineralization begins with the creation of amorphous CaP within highly saturated solutions containing CaP (38). During the initial phases, a limited number of citrate molecules can adhere to the surface of small amorphous CaP clusters, effectively impeding particle aggregation. Research has demonstrated that citrate inhibits hydroxyapatite nucleation by interacting with calcium ions and attaching to crystal surfaces (39, 40). Recent studies suggest that citrate’s capacity to stabilize CaP formations is essential in postponing the emergence of liquid or solid phases required for the formation of the CaP liquid precursor phase. Furthermore, by stabilizing early CaP precursors including nucleating precursor material and the liquid precursor phase, citrate significantly delays solid CaP nucleation (41). This suggests that citrate plays a significant role in the formation of stable CaP precursor materials with appropriate dimensions during initial bone mineralization. In the subsequent stage, nucleation of CaP precursors occurs and non-collagenous proteins secreted by bone cells guide the attachment of CaP to collagen surfaces (42), while citrate facilitates plate-like arrangement of CaP for normal bone tissue formation (43). During the final stage, citrate salts completely envelop the surface of CaP crystals, resulting in unique geometric shapes of hydroxyapatite and forming nanocrystals as mentioned earlier. Citrate coverage prevents further crystal growth and contributes to optimal mechanical structure formation in bone tissue (43).
Citrate has been used to explain changes observed in various bone diseases. Sodium-coupled citrate transporter (NaCT) is responsible for extracellular-to-cell transport of citrate. Research indicates that SLC13A5(the gene encoding NaCT) deficiency leads to decreased BMD and impaired bone formation in homozygous and heterozygous knockout mice (44). A subsequent study revealed that mice lacking SLC13A5 exhibited structural and biomechanical properties indicative of abnormal mineralization. Additionally, the researchers observed excessive citrate accumulation in the bones of SLC13A5 deficient mice, which may have contributed to reduced cortical thickness and impaired cortical strength (45). This could be attributed to citrate deposition affecting hydroxyapatite formation and decreased citrate coverage impacting water-mineral integrity bound to the bone surface. In brief, Citrate serves as a vital component of hydroxyapatite, where its incorporation into bone minerals and spatial arrangement within the mineral structure is indispensable for preserving the biomechanical properties of bone, including stability, strength, and fracture resistance.
4 Citrate circulates during bone remodelingAs previously mentioned, bone remodeling represents the dynamic balance between bone formation and resorption in mature bone tissue, with citrate playing a crucial role in mediating this interplay (Figure 1). Recently, research has shown that osteoblasts are the primary origin of citrate in bone tissue. A study by Costello et al. (2012) revealed the secretion of citrate by osteoblasts in mice (46). While calcium citrate acts as an intermediary for calcium exchange between bone and blood, the exact origin of plasma citrate remains uncertain. Experiments utilizing C13 isotope-labeled glucose tracers have suggested that mitochondrial citrate derived from glucose deposition occurs during the later stages of osteogenic differentiation in bone marrow mesenchymal stem cells (BMSC) (47) Interestingly, undifferentiated BMSCs do not possess the capacity to secrete citrate (48). This suggests that citrate found in bone is derived from differentiated BMSCs rather than plasma citrate. BMSCs have the ability to differentiate into various cell types, including osteoblasts within bone tissue (49). Research indicates that osteoblasts are specifically responsible for producing citrate in bone tissue (50), highlighting the significant role of citrate in osteogenic differentiation. In vitro studies demonstrate an increase in citrate production with osteoblast differentiation, accompanied by changes in protein expression related to citrate secretion. The deposition of citrate into the bone matrix relies on the net production of citrate by osteoblasts and involves molecular activities such as: Citrate synthetase CS, mitochondrial aconitase (m-acon, which converts citrate to isocitrate), CIC and NaCT. Citrates produced by osteoblasts during mineralization are subsequently released upon breakdown by osteoclasts. Osteoclasts originate from monocyte-macrophages and play a crucial role in degrading the bone matrix. They are formed through fusion of monocytic precursors belonging to monocyte/macrophage lineage and serve as primary resorptive cells within bones (51, 52) Mature osteoclasts adhere to the bone surface through αvβ integrin, establishing an F-actin sealing zone for efficient absorption of the bone matrix. The acidic microenvironment in the resorption area is generated by carbonic anhydrase II, which produces HCO and H ions that are transported by vacuolar H adenosine triphosphatase (V-ATPase) within the folded structure of osteoclasts (53, 54). In this acidic environment of the resorption lacuna, inorganic minerals dissolve while exposing the organic collagen matrix to enzymatic degradation facilitated by proteolytic enzymes such as collagenase, cathepsin K (CTSK), and matrix metalloproteinases (MMP) (54, 55). Subsequently, these enzymes proceed with degrading the exposed collagen and other organic components (56–58), meanwhile, citrate is also released during this process (59). To summarize, within intact bone tissue, osteoblasts secrete citrate to assist in bone matrix formation whereas osteoclasts break down released citrate from the bone matrix, which further provides energy for stem cells and supports their differentiation.
5 Citrate as a fuel for bone remodelingAs mentioned above, citrate circulates among osteoblasts and osteoclasts during bone remodeling to participate in the formation of bone matrix and osteoblastic differentiation. During this process, bone formation is highly energy-consuming, necessitating the substantial production of ATP. Meanwhile, the differentiation process of osteoclasts requires the rearrangement of the cytoskeleton from mononuclear to multinuclear cells and cell fusion, which also necessitates a significant amount of ATP (60). Moreover, the migration of osteoclasts along the bone surface facilitates ongoing bone resorption, involving dynamic rearrangements of the actin and microtubule cytoskeleton, which necessitates significant ATP consumption (61, 62). The TCA cycle is essential for supplying the energy needed for numerous cellular functions, which requires the involvement of cirtrate (63). Abnormal ATP synthesis could result in imbalances in bone metabolism (64–66). Therefore, in addition to its role in bone mineralization, citrate can enter the TCA cycle to provide a substantial amount of ATP, suggesting that citrate may act as a fuel in the process of bone remodeling (Figure 2).
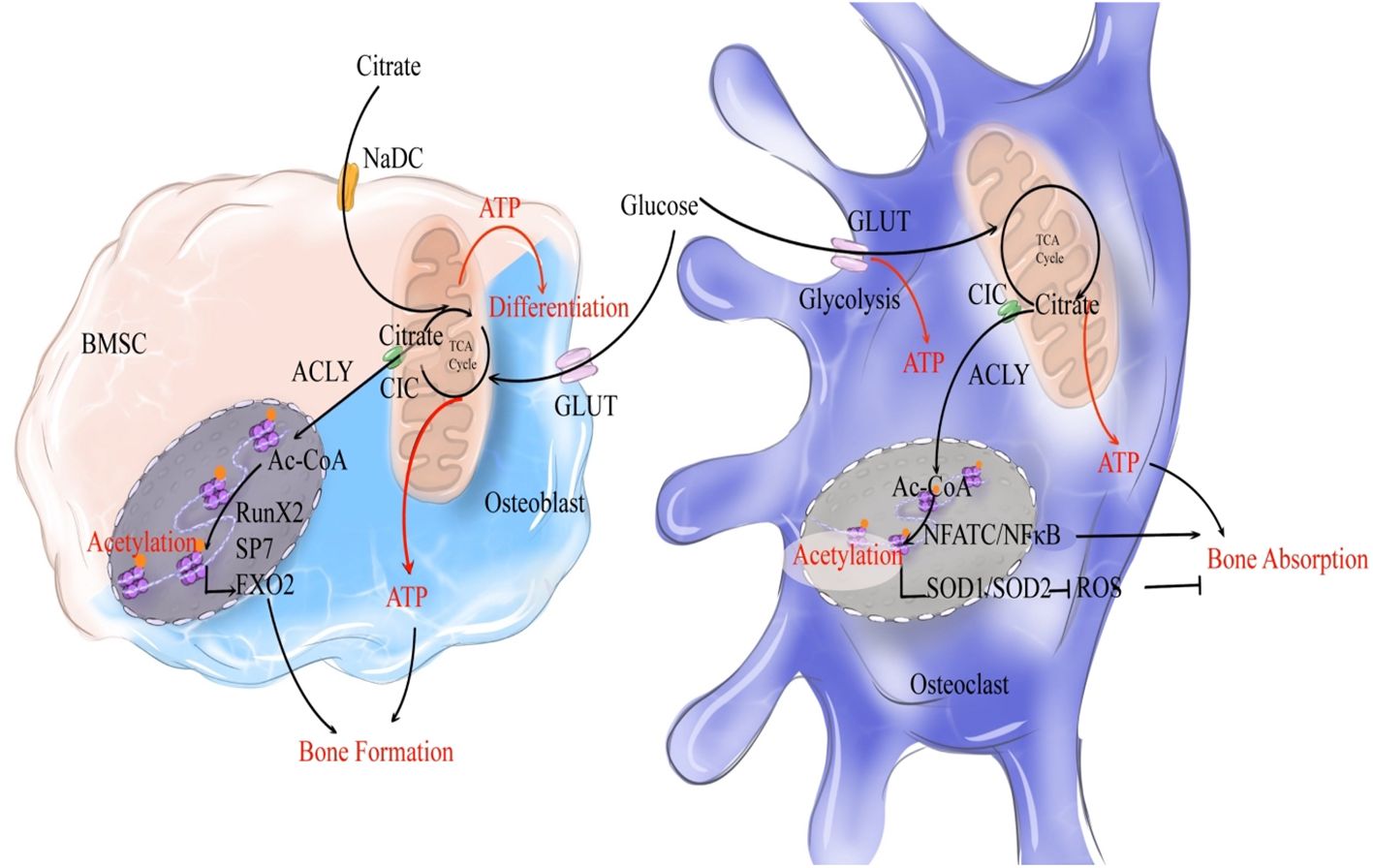
Figure 2. Citrate regulates bone remodeling through ATP production and histone acetylation. During the process of bone formation, NaCT-mediated uptake of exogenous citrate by bone marrow stromal cells (BMSCs, indicated by a light pink cell) activates their differentiation into osteoblasts. Exogenous citrate serves a dual role: it generates ATP via TCA cycle to fuel osteoblastic differentiation, while also being cleaved into acetyl-CoA to enhance the expression of osteogenic genes. Once activated and differentiated into osteoblasts (indicated by a light blue cell), glucose metabolism becomes the primary source of citrate production, as glucose undergoes OXPHOS to generate citrate that provides both ATP and acetyl-CoA for promoting osteogenic gene expression and subsequent acetylation. In terms of bone resorption, glucose metabolism produces citrate that supplies ATP for osteoclastic bone resorption. Moreover, citrate enhances acetylation to stimulate NFATC/NFκB expression and induce osteoclast (indicated by a dark blue cell) differentiation. Simultaneously, it promotes oxidase expression to inhibit the pro-resorptive effect of ROS on bones. Overall, citrate plays a crucial role in promoting bone remodeling.
5.1 Citrate participates in TCA cycle to produce substantial ATPIn the TCA cycle, citrate plays a crucial role in sustaining reduced FADH2 and NADH (nicotinamide adenine dinucleotide) levels, which are utilized in the electron transport chain for oxidative phosphorylation and ATP production through complex V (67). Numerous proteins contribute to the regulation of citrate-mediated ATP, and disturbances in these associated proteins can lead to disorders in osteogenesis and osteoclast function, resulting in various diseases.
5.2 Citrate as a fuel in bone remodelingDuring this process, citrate acts as a key intermediate product in the energy supply and plays a critical role as an energy source for bone tissue. Upon initiation of osteogenic differentiation, there is an increased demand for metabolic energy in MSCs leading to elevated production of citrate regulated by various enzymes related to citrate metabolism (68).
5.2.1 Extracellular citrate as a fuel in early stage of bone formationResearch has demonstrated that extracellular citrate significantly influences the osteogenic differentiation of MSCs, confirming its role as a fuel for stem cell differentiation (69). This “metabolic” regulation begins with the absorption of citrate through NaCT, activating the energy production pathway that enhances cellular energy status. Consequently, this facilitates meeting high metabolic demands during MSC differentiation into osteoblasts. Notably, this effect appears to be both time- and dose-dependent; however, the dose-dependent impact is more significant during early stages of osteogenesis (69). Additionally, ATP generated from exogenous citrate was found to promote the early stages of osteogenic differentiation (70, 71).
However, the expression and functionality of NaCT following the differentiation of MSCs into osteoblasts remain controversial, suggesting that the demand for extracellular citrate may decrease during the later stages of osteoblastic differentiation. In contrast to the significant expression of various types of transporters in HepG2 cells, Costello et al. found that NaCT transporters are absent in mature osteoblasts (50). On the contrary, studies have demonstrated the presence of NaCT mRNA in bone and primary osteoblasts. Furthermore, reduced bone mineral density and impaired bone formation have been linked to diminished expression of SLC13A5 which codes NaCT in 13-week-old mice, as well as overall growth retardation, shortened body length, and reduced bone size (44). Additionally, Mantila Roosa SM et al.’s study revealed that local mechanical loading stimulation can upregulate bone NaCT expression (72), thereby increasing bone tissue content. In summary, during early stages of osteogenic differentiation, exogenous citrate intake provides a substantial amount of ATP for BMSCs.
5.2.2 Intracellular citrate as a fuel in late stage of bone formationAs BMSC activation occurs, intracellular glucose metabolism generates citrate which eventually produces ATP - becoming the main energy supply pathway. Intracellular citrate production is reliant on the activity of citrate synthestase (CS) (73). Research findings suggest that rats with postmenopausal osteoporosis exhibit a decrease in CS activity (74). In our previous study, we observed a reduction in citrate content in osteoporotic mice through energy metabolism sequencing analysis (75). Moreover, the heightened production of citrate is associated with diminished aconitase (ACO2) activity, which facilitates the conversion of citrate to isocitrate. ACO2 has not been extensively studied as a rate-limiting enzyme in the TCA cycle. However, its function is known to be inhibited by Zn2+, and zinc finger protein 1 (ZIP1) aids in Zn2+ transportation (48, 76). Furthermore, knockdown of ZIP1 resulted in the prevention of intracellular citrate accumulation, demonstrating that ZIP1 could increase the intracellular levels of citrate (50). Additionally, research on ZIP1 has also revealed that the expression of ZIP1 and intracellular zinc levels increase during the late stages of osteoblastic differentiation, as osteogenic precursors into bone-forming cells (50). Hence, increased levels of intracellular citrate, rather than that extracellular citrate, is required for the late stage of bone formation.
Meanwhile, intracellular citrates is also essential for the normal function of mitochondria. With assistance from mitochondrial complex II (CII), also known as succinate dehydrogenase-ubiquinone oxidoreductase, ATP is generated to support the osteogenic process. Research has identified mutations in succinate dehydrogenase complex A (SDHA) within CII present in osteoblasts from individuals with age-related osteoporosis,SDHA which is nuclear-encoded is a subunit of CII and it is the marker of CII (77), underscoring the significance of energy produced by mitochondria. Abnormal mitochondrial function can adversely affect ATP synthesis and consequently impact osteogenesis. Furthermore, an in vitro study demonstrated that induction of osteoblast differentiation progressively enhances the activity of mitochondrial complexes I and II (78). In addition, the absence of mitochondrial citrate may impair the energy supply within the Krebs cycle, which could lead to a failure to meet the bioenergetic energy demands of proliferating and differentiated cells, thereby inhibiting their proliferation and differentiation (48). This suggests that citrate also plays an essential role as a fuel in mitochondria and thereby participates in the osteogenic differentiation of BMSCs.
5.2.3 Citrate as a fuel in bone absorptionMoreover, citrate also holds significance in providing energy for osteoclasts. Mature osteoclasts, rich in mitochondrial DNA, transfer ATP from mitochondria to the cytoplasm. A subsequent increase in ATP levels results in enhanced bone resorption (79). Research has also indicated that during RANKL-stimulated osteoclast differentiation, there is upregulation of CS and other metabolic enzymes to increase citrate synthesis, which is associated with increased production of ATP (80). In another study, it was also demonstrated that the addition of 1-2mM sodium citrate significantly enhances osteoclastogenesis, highlighting the essential role of citrate during osteoclastic differentiation (81). However, there have been relatively few studies on how citrate is involved in the osteoclastic differentiation. More studies are needed to fully understand how energy is utilized during the process of bone resorption.
6 Citrate regulates bone remodeling through histone acetylation modificationCitrate not only serves as an energy source but also regulates bone remodeling through the modulation of histone acetylation. Histone protein acetylation refers to the addition of an acetyl group (CH3CO-) to specific amino acid residues, typically lysine, within a histone molecule. This modification process is tightly controlled by enzymes called acetyltransferases and deacetylases. Acetyltransferases transfer acetyl-CoA to amino acid residues, playing a crucial role in modulating histone protein function. The production of acetyl-CoA occurs in mitochondria and its transport to the cytosol heavily relies on the citrate-malate-pyruvate shuttle (82). Mitochondrial citrate carrier (CIC) facilitates the exchange of citrate from mitochondria to cytoplasm with malate. Once in the cytoplasm, citrate is converted into acetyl-CoA by ACLY enzyme, thereby participating in the process of histone acetylation. Research has shown that additional application of citrate promotes higher levels of acetylation (83). Hydroxy-citrate (HCA), which competitively inhibits citrate, hinders this process (84). Acetyl-CoA modulates protein activity through acetylation, particularly influencing histones involved in transcription maintenance during G1 phase and estrogen receptor proteins (ER) responsible for regulating protein homeostasis (85). Unlike nonhistone proteins, histones undergo lysine residue modification at their N-terminal tails through acetylation, causing them to protrude from nucleosomes. Consequently, negatively charged DNA is repelled leading to chromatin relaxation. This open chromatin conformation facilitates easier binding of transcription factors and subsequently influences gene expression (86, 87) (Figure 2).
6.1 Citrate promotes osteogenic differentiation of BMSC through histone acetylationRunx2, Sp7, and FoxO1 are crucial transcription factors essential for osteoblast differentiation and maturation. They stimulate the expression of key genes such as ALP, osteocalcin, osteopontin, and COL-1, thereby promoting the maturation and mineralization of osteoblasts. Research indicates that histone H3 acetylation plays a facilitative role in osteoblast differentiation and maturation by enhancing Runx2 transcription (88, 89). Decreased histone acetylation caused by HDAC leads to the suppression of Runx2, SP7, and FoxO1 transcription, ultimately inhibiting osteoblast differentiation and maturation. This suggests that citrate may enhance osteogenic differentiation through modulation of histone acetylation (90–93). In the aging process, mitochondrial structural abnormalities result in reduced CIC levels. Impaired transport of citrate from mitochondria to cytoplasm along with decreased histone acetylation contributes to senile osteoporosis. Exogenous supplementation of acetyl-CoA has been shown to effectively treat osteoporosis; thus, highlighting the importance of citrate decomposition into acetyl-CoA as a critical pathway for regulating acetylation modification during osteogenic differentiation (15).
6.2 Citrate promotes osteoclast differentiation through histone acetylationCitrate regulates histone acetylation to modulate osteoclasts, serving as a crucial mediator of bone resorption. NFATc1 and NF-κB govern osteoclast differentiation (94). Histone H3 acetylation by CBP/p300 enhances the expression of NFATc1 and NF-κB, thereby promoting osteoclast differentiation; conversely, HDAC-mediated H3 deacetylation inhibits this process (88, 89). Furthermore, inhibition of ACLY suppresses osteoclast differentiation and function through its influence on histone acetylation, suggesting that citrate has the potential to enhance osteoclastogenesis via modulation of histone acetylation (95). Osteoclasts’ ability to resorb bone is influenced by their production of reactive oxygen species (ROS). ROS plays a beneficial role in facilitating osteoclast differentiation (96). The scavenging of ROS relies on the action of antioxidant enzymes. One study demonstrated that histone acetylation modification may promote the expression of superoxide dismutase (SOD) (97), while another study showed that histone acetylation can regulate the expression of antioxidant enzyme genes SOD1 and SOD2 (98). Conversely, research suggests that the SOD family contributes to maintaining bone homeostasis by promoting osteoblast differentiation and inhibiting osteoclast differentiation (99–101) This suggests that citrate may promote osteoclast differentiation by modulating histone acetylation, up-regulating transcription factors associated with osteoclastogenesis, and potentially inducing the expression of antioxidants, thereby attenuating oxidative stress and impeding osteoclast differentiation. Overall, citrate significantly contributes to osteoclast formation; however, its inhibition of ROS through histone acetylation may restrict further enhancement of bone resorption. Nevertheless, as mentioned earlier, citrate promotes osteoclast formation in vitro, indicating its predominantly promoting effect on bone resorption. In summary, citrate serves as a crucial energy source for ATP production and a substrate for histone acetylation while playing a role in bone remodeling processes mediated by both osteoblasts and osteoclasts. The simultaneous impact on bone formation and resorption poses challenges in assessing its influence on bone mass. Nonetheless, research has demonstrated that exogenous citrate can enhance bone mineral density and mitigate excessive bone resorption in patients (102). Another study demonstrated that exogenous citrate effectively reversed bone resorption in mice (102). As previously mentioned, citrate promotes the proliferation of osteoclasts, indicating its contrasting effects in both in vivo and in vitro settings. In vitro, citrate enhances bone resorption, whereas in vivo it suppresses this process. These findings suggest that the primary impact of citrate on bone tissue lies within osteogenesis.
7 Citrate regulates disordered bone remodeling through energy reprogramming7.1 Connection between bone remodeling and energy metabolismThe skeletal system is influenced by systemic metabolic processes, including glucose, lipid, and amino acid metabolism. Among the body’s organs, bones rank fourth in terms of glucose consumption, which plays a crucial role in bone development (103). In the presence of oxygen, differentiated cells typically respond to OXPHOS by metabolizing glucose into CO2 and maximizing 5’-adenosine triphosphate (ATP) production (104) to provide energy for cellular activities. Lipid metabolism encompasses the biological processes of fat digestion, absorption, synthesis, and decomposition that are essential for maintaining cellular homeostasis (105). Glutamine metabolism holds particular importance among amino acid metabolisms due to its prevalence in plasma. Apart from facilitating protein biosynthesis directly, glutamine also serves as a vital carbon source and nitrogen donor for nucleotide synthesis, amino acid synthesis, glutathione production, and other essential compound formation (106). Cell metabolism forms the core of the metabolic cycle; thus maintaining a balance between osteoblast and osteoclast metabolism ensures metabolic homeostasis within bone tissue. Any disruption to this balance will result in an imbalance of bone metabolism.
7.1.1 Bone remodeling and glucose metabolismThe regulation of glucose metabolism primarily relies on estrogen and glucose transporters. Estrogen plays a crucial role in maintaining glucose homeostasis (107). Research has demonstrated that osteoporotic rats exhibit decreased systemic glucose metabolism (108). During menopause, estrogen-triggered cellular pathways activate PI3K/AKT-mediated glucose uptake, leading to glucose deprivation (109, 110), which is mediated by the estrogen receptors ESR1 and ESR2 as well as the glucose transporter. Furthermore, the function of transporting glucose into cells cannot be separated from the role of glucose transporters. Glucose is subsequently metabolized in the cytoplasm through glycolysis to produce two molecules of pyruvate, two ATPs, and two NADHs. Among significant glucose transporters facilitating glucose uptake in osteoblast lineage cells are Glut-1, along with Glut-3 and Glut4 (111, 112). Unlike muscle cells, both osteoblasts and osteoclasts take up glucose independently of insulin (113). Glut1 acts as a facilitator for insulin-independent uptake of glucose by transporting it across a concentration gradient. Loss of Glut1 in osteoblast precursors inhibits their differentiation into mature osteoblasts both in vitro and in vivo (114). Interestingly, estrogen can also regulate glycometabolism by activating AKT signaling through its receptor ESR1 (115), directly enhancing transcription of the SLC2A4 gene encoding Glut4 (116), while deficiency in ESR2 reduces AKT expression (117); thus estrogen deficiency may lead to impaired intake disorder mainly due to reduced activation of the AKT pathway mediated by ESR1 and ESR2.
7.1.2 Fatty acid metabolism and bone remodelingLipids, including fatty acids, cholesterol, triglycerides (TG), and phospholipids, have increasingly been associated with bone metabolism. Recent research suggests that lipids and their derivatives are significant sources of energy for osteoblasts, shifting the focus from glucose alone. Osteoblasts possess the necessary receptors and catabolic enzymes to uptake and utilize circulating lipids (118). Fatty acids and their derivatives play a crucial role in maintaining bone health, with their levels in the bone microenvironment being linked to osteoporosis (119). Furthermore, fatty acids are known to significantly contribute to osteogenic differentiation as previous studies have demonstrated the ability of osteoblasts to oxidize fatty acids (120). In vitro studies have shown a substantial increase in fatty acid oxidation during osteoblast maturation, with mineralized osteoblasts exhibiting three times higher fatty acid catabolic activity compared to proliferating cells This research highlights the crucial role of fatty acids in promoting osteogenic differentiation and mineralization. The uptake and utilization of fatty acids by osteoblasts are primarily facilitated by specific receptors for fatty acid uptake and enzymes involved in fatty acid catabolism. Osteoblasts express the CD36 receptor to facilitate free fatty acid uptake, and studies conducted on mice have demonstrated that depletion of CD36 leads to reduced bone mass due to impaired osteoblast-mediated bone formation (121). Furthermore, osteoblasts possess numerous metabolic enzymes responsible for processing fatty acids. Upon cellular entry, fatty acids are predominantly utilized through fat oxidation and β-oxidation pathways. In the cytoplasm, they are converted into fatty acyl-CoA, which then binds to CPT1—an enzyme located on the outer mitochondrial membrane—to generate acylcarnitine that is subsequently transported into the mitochondrial matrix. Once inside the mitochondria, CPT2 converts acylcarnitine back into acyl-CoA for β-oxidation. Research findings have revealed that knockout of CPT2 results in impaired bone formation (122) Subsequently, acetyl-CoA, NADH, and FADH2 undergo β-oxidation, leading to the generation of fuel for various metabolic pathways. Research findings have demonstrated that pharmacological inhibition of β-oxidation in vitro impedes osteoblast differentiation (123). In summary, the transportation and metabolism of fatty acids play a significant role in osteoblasts, while disruptions in fatty acid metabolism in osteoporosis impact the bone formation process of osteoblasts.
7.1.3 Glutamine metabolism and bone remodelingThe regulation of glutamine primarily relies on the cysteine transporter 2 (ASCT2, also known as SLC1A5) and aminidase (GLS). ASCT2 facilitates the transport of glutamine from the bloodstream into cells to maintain cellular glutamine homeostasis, while GLS deaminates glutamine to form glutamate, which further undergoes deamination to produce αKG. Studies have indicated that the absence of sodium-dependent amino acid exchanger SLC1A5 affects the uptake of essential glutamine and asparagine required for maintaining amino acid balance in osteoblasts (124). Research has demonstrated that both glutamine metabolism and GLS activity play a role in mediating osteoblast differentiation (125). Genetic inactivation of GLS1 leads to the elimination of PTH-induced osteoblast generation (126). Previous studies have described age-related changes in glutamine metabolism in osteoporosis, which may disrupt the balance between osteogenic and adipocyte differentiation of BMSC due to impaired key enzymes involved in glutamine metabolism or declining mitochondrial function (127, 128). Recent research has emphasized the potential impact of glutamine metabolism on osteoblast development. Glutamine is crucial for matrix mineralization in osteoblast calvaria cultures. It has been observed that as BMSCs age, their uptake of glutamine significantly decreases, leading to a reduction in osteoblast formation. Isotopic tracing studies have revealed that glutamine is converted into citrate through the TCA cycle, thereby assisting osteoblasts in energy production (129). Furthermore, glutamine exerts an inhibitory effect on osteoclasts, and α- ketoglutarate(α-KG) serves as a metabolite of glutamine. Administration of exogenous α-ketoglutarate has been shown to significantly increase trabecular bone mineral density, cortical bone mineral density, and bone mechanical properties. Moreover, it has been observed to alleviate symptoms associated with osteopenia and osteoporosis in animals and postmenopausal women (130–133). A study discovered that treatment with αKG reduced H3K9me3 levels, resulting in chromatin opening. This subsequently led to decreased RANKL-induced ROS production and inhibition of osteoclast differentiation. These findings indicate the crucial role played by glutamine in regulating osteoclast differentiation (134). In summary, glutamine promotes bone remodeling by stimulating both the proliferation and differentiation of osteoblasts while suppressing the differentiation of osteoclasts through α-KG. Impaired glutamine metabolism due to disorders in bone remodeling can lead to abnormal functioning of both osteoblasts and osteoclasts, thus influencing overall bone remodeling.
7.2 Citrate and energy metabolismOrdinarily, the three metabolic types in normal individuals are interconnected. However, osteoporosis-related bone remodeling disorders can disrupt glucose, fatty acid, and glutamine metabolism, resulting in a metabolic disorder known as reprogramming. Reprogramming involves altering cellular or tissue metabolic pathways that lead to changes in cell function and physiological status. Citrate plays a critical role in this process (19). Citrate-mediated metabolic reprogramming can treat bone metabolic disorders by supplying essential intermediates for glucose and fatty acid metabolism. In glucose metabolism, citrate serves as a crucial intermediate in the TCA cycle while contributing to fatty acid synthesis by transporting acetyl-CoA into the cytoplasm through its involvement in the citrate-pyruvate cycle (135). In the context of glutathione metabolism, citrate can participate in the synthesis of glutamine despite being a crucial intermediate in glutamine metabolism (136). Furthermore, citrate also exerts regulatory control over metabolic reprogramming by modulating estrogen receptors and key metabolic proteins (Figure 3).
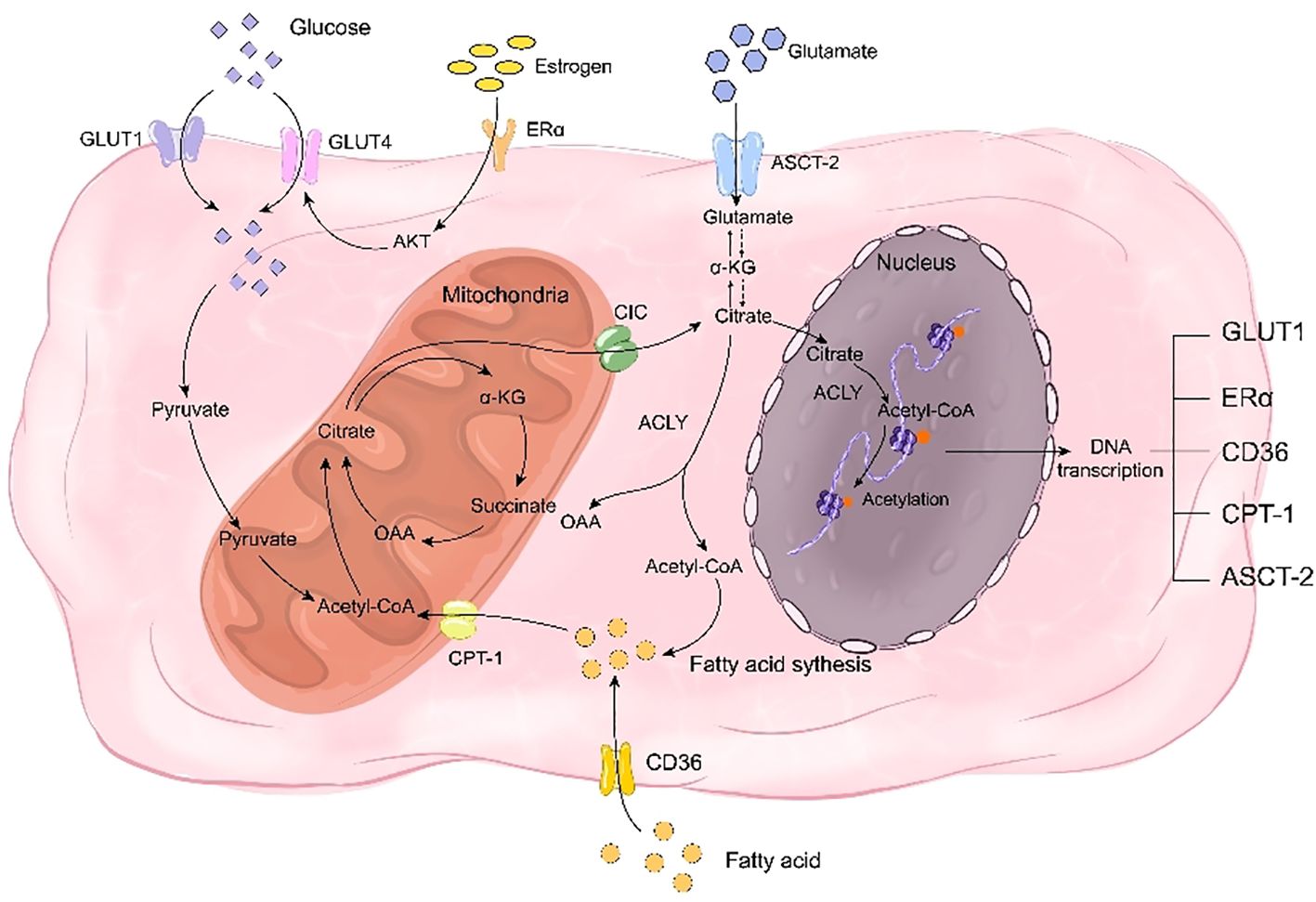
Figure 3. Citrate regulates bone remodeling through energy reprogramming. On one hand, citrate acts as a crucial intermediate in glucose metabolism, while on the other hand, it facilitates the synthesis of fatty acids and glutamine to compensate for deficiencies in glucose, fatty acids, and glutamine during bone remodeling disorders. Citrate promotes the transcription of transporters for these three nutrients, including GLUT1, CD36, and ASCT-2, thereby facilitating their uptake. Additionally, citrate enhances histone acetylation to promote the transcription of estrogen receptor ERα and mitigate the impact of estrogen deficiency on glucose transporter GLUT4. Simultaneously, citrate upregulates CPT-1 transcription through histone acetylation to enhance fatty acid metabolism and play a pivotal role in rescuing energy metabolism disorders.
7.2.1 Citrate regulates glucose metabolism to mediate bone remodelingExogenous citrate has the potential to ameliorate glucose metabolic disorders. As an intermediate in the TCA cycle, citrate can partially compensate for energy metabolic disorders caused by glucose deficiency. Moreover, it enhances glucose homeostasis by augmenting glucose uptake. A study demonstrated that exogenous chromium citrate significantly increased glucose intake in rats and upregulated Glut4 expression and AMPK transcription, indicating that citrate could enhance cellular glucose uptake and improve bone homeostasis through increased expression of glucose transporters (137). Another study revealed that HDAC inhibitors promote GLUT1 acetylation to facilitate glucose uptake, suggesting another mechanism by which citrate directly promotes glucose uptake via acetylation to enhance transporter expression (138). Additionally, a separate study illustrated that estrogen receptor ESR undergoes acetylation and histone H3 lysine 27 (H3K27ac) acetylation can regulate promoter regions of ER pathway genes encoding ERα and ESR1 (139). Therefore, the upregulation of ESR estrogen receptor expression induced by exogenous citrate may also serve as a possible mechanism through which it improves glucose uptake in osteoporotic bone tissue.
7.2.2 Citrate-mediated fatty acid metabolism regulates bone remodelingCitrate is a crucial substrate for fatty acid synthesis and serves as the initial component, acetyl-CoA, in the pathway. After being transported out of the cell by CIC, citrate undergoes breakdown into acetyl-CoA. Subsequently, through successive reactions involving enzymes such as acetyl-CoA carboxylase, dehydratase, reductase, and acylase (140), it can further synthesize long-chain fatty acids. CICs have been proven to promote fatty acid biosynthesis (141). Additionally, the expression of SLC25A1, a type of mitochondrial citrate transporter, was found to be most prominent in adipogenic tissues such as the liver, renal cortex, and pancreas (142). As fatty acids are essential substrates for adipogenesis and CIC transports citrate accordingly plays a significant role in fatty acid production. Furthermore, citrate has the ability to influence histone acetylation and thereby impact fatty acid transport and metabolism. A proteomic examination of lysine acetylation sites in mouse and rat tissues identified CD36 as being acetylated at lysine 52, 166, 231, and 403 (143). Mass spectrometry analysis confirmed the acetylation of these sites on human CD36 (144). This finding suggests that histone acetylation-mediated upregulation of CD36 transcription may contribute to the elevated intracellular fatty acid levels. Additionally, another study demonstrated that enhanced acetylation of mitochondrial fatty acid β-oxidase promotes fatty acid breakdown (145). Moreover, it was observed that CPT-1, an enzyme responsible for transporting fatty acids into mitochondria, is susceptible to acetylation. These findings collectively indicate that citrate not only serves as a precursor for fatty acid synthesis but also plays a crucial role in regulating fatty acid uptake, breakdown, and β-oxidation to support osteoblast differentiation and mineralization. In summary, citrate acts as a modulator for proteins involved in fatty acid transport and metabolism in osteoblasts, facilitating processes such as absorption, synthesis, breakdown, and oxidation of fatty acids while maintaining their homeostasis.
7.2.3 Citrate-mediated glutamine metabolism regulates bone remodelingCitrate can enhance the regulation of amino acid metabolism disorder by modulating glutamine synthesis and transport. CIC deficiency has been shown to impair glutamine synthesis, indicating a positive correlation between cytoplasmic citrate content and glutamine levels (142, 146). Additionally, SLC25A1 mutant mice with hydroxyglutaric aciduria exhibited altered glutamine remodeling (147). In Huh7 cells deprived of glutamine, supplementation with exogenous citrate rescued cell viability that was reduced by NaCT inhibition, suggesting that exogenous citrate could restore depleted glutamine levels via NaCT (148). Furthermore, studies have demonstrated that exogenous citrate generates derivatives of both glutamine and glutamate as well as promotes fatty acid synthesis (138), implying its potential in regulating the content of glutamine to ameliorate amino acid metabolism disorders in osteoporosis patients. Moreover, citrate can modulate ASCT2-mediated uptake of glutamine; previous research indicated that bortezomib (BTZ)-induced peripheral neuropathy led to decreased histone acetylation which may silence SLC1A5 expression (149), while another study suggested upregulation of ASCT2 in mice subjected to chronic social defeat stress due to excessive histone acetylation (150). These findings suggest that citrate can promote ASCT2 expression and enhance the uptake of glutamine. Therefore, citrate plays a regulatory role in bone remodeling by increasing the content of intracellularly available glutamine and modulating its intake as well as metabolism.
8 Citrate regulates bone remodeling through the immune systemThe link between bones and the immune system holds significant importance. The immune system comprises immune organs, immune cells, and immune factors, while bone homeostasis involves osteoblasts and osteoclasts. Extensive descriptions have been provided regarding the role of immune inflammation in bone loss. In cases of pathological immune dysfunction, such as immune deficiency or inflammatory response to infection/disease, the bone is impacted by the immune response, potentially leading to osteoporosis and an increased risk of fracture (151, 152). Moreover, various prevalent inflammatory conditions exacerbate bone loss including rheumatoid arthritis, periodontal infection, and inflammatory bowel disease. Immune-inflammatory factors are considered as the primary means through which the immune system regulates bone remodeling. Postmenopausal women with osteoporosis often exhibit a chronic mild inflammatory state characterized by altered cytokine expression (153). Citrate plays a crucial role in modulating the release of inflammatory factors; thus suggesting that citrate’s impact on these factors may serve as a mechanism for regulating bone remodeling.
8.1 Bone immune factors and bone remodelingThe immunophenotyping clinical evidence in postmenopausal patients indicates that women in the postmenopausal stage exhibit elevated levels of inflammatory cytokines, specifically interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNFα) (154–157). This applies to both circulating blood cells and cells within the bone microenvironment (158, 159). Previous experimental evidence confirms the heightened presence of inflammatory mediators, such as IL-1β, IL-6, and TNFα, in the bloodstream and bone marrow of ovariectomized rodents (160, 161). In summary, postmenopausal women with osteoporosis exhibit a persistent mild inflammatory state characterized by altered cytokine expression. This suggests that estrogen-mediated immune cell-induced changes in inflammatory cytokines play a crucial role in bone formation.
The regulation of bone immunity primarily involves three inflammatory factors, namely IL-1β, IL-6, and TNF-α. IL-1β, a prominent member of the IL-1 ligand family, is recognized as a primary therapeutic target for various inflammatory conditions (162). Multiple studies have demonstrated that IL-1 directly enhances osteoclast formation, multinucleation, pit-forming activity, and survival (163). Additionally, IL-1 also affects osteoblasts. In a study by Zhang YZ et al., downregulation of TLR4 reduced the levels of IL-1, TNF-α, and IL-6 in osteoblasts leading to improved cell viability attributed to the suppression of inflammatory pathways (164). Furthermore, IL-1β stimulates bone resorption and inhibits bone formation while IL-6 promotes T cell growth and differentiation and enhances the differentiation of osteoclasts, macrophages, and megakaryocytes (165). Moreover, IL‐6 has dual effects on osteoblast activity by promoting initial differentiation but impeding subsequent differentiation at later stages (166–168) TNF-α, an inflammatory factor, can be produced by various cell types such as macrophages, NK cells, mast cells, and T and B lymphocytes (169). The impact of TNF-α on osteogenic differentiation remains a subject of debate. Some studies suggest that lower concentrations of TNFα enhance the levels of Runx2, Osx, OCN, and ALP in MSCs (170, 171), while higher concentrations of TNFα have been shown to decrease these levels (170–173). In terms of the relationship between TNF-α and osteoclasts, it is considered a crucial stimulator for osteoclast differentiation (174). Treatment with TNFα alone (without RANKL) has been found to increase the number of TRAP-positive osteoclasts in WT mice by activating the NF-κB signaling pathway both locally and systemically (175–177). Overall, inflammatory factors predominantly regulate bone remodeling by inhibiting osteogenic differentiation and promoting osteoclast differentiation.
8.2 Citrate regulates immune inflammatory factors to regulate bone remodelingCitrate plays a pivotal role in modulating immune factors. Numerous studies have demonstrated that exogenous citrate influences the secretion of IL-1, TNF-α, and IL-6 (Figure 4). A study indicated that the introduction of exogenous citrate resulted in elevated expression of proinflammatory cytokines TNF-α, IL-1β, and IL-6 (178). Research conducted on gastric cancer epithelial cells demonstrated that citrate could enhance the expression of IL-1β and TNF-α (179). Furthermore, another study revealed a positive correlation between increased plasma levels of ACLY and the expression of IL-6, suggesting that citrate may promote the expression of immune factors through ACLY. ACLY breaks down citrate into acetyl-CoA, which serves as a substrate for acetylation - a fundamental mechanism for regulating immune factors. In one study, it was demonstrated that the absence of ACLY led to reduced secretion of IL-6 and TNF-α in macrophages primarily due to decreased macrophage response to IL-4 stimulation caused by reduced levels of histone acetylation-dependent genes targeted by IL-4 (180, 181). Another study also demonstrated that silencing of SLC25A1 suppressed the production of TNF-α induced by LPS (182). The results indicated a positive correlation between cytoplasmic citrate levels and the expression of IL-1β, TNF-α, and IL-6. Ethanol-exposed monocyte-macrophages convert it to acetate, which metabolizes into acetyl-CoA, leading to enhanced histone acetylation and generation of proinflammatory cytokines such as IL-6, IL-8, and TNF-α. These cytokines were influenced by the downregulation of ACSS1 and ACSS2 from the short-chain family members of acyl-CoA synthetase (183, 184). These findings suggested that ACLY activity resulted in the synthesis of acetyl-CoA from citrate, thereby facilitating the expression of inflammatory factors while regulating bone formation and resorption. In brief,the inhibition of bone formation is facilitated by citrat
留言 (0)