The increasing prevalence of type 2 diabetes mellitus (T2DM) has become a primary global public health concern in recent years. In 2019, there were 463 million people with diabetes; by 2045, this number is expected to rise by 51%, reaching 700 million (1). Prediabetes is a condition in which plasma glucose levels are elevated but not high enough to be diagnosed as diabetes. According to the World Health Organization (WHO) criteria, IGT (2-h glucose 7.8-11.0 mmol/L) and IFG (fasting glucose 6.1-6.9 mmol/L) are used to define prediabetes (2). Previous studies found that the prevalence of prediabetes is gradually increasing and reached 38.1% in 2018 in China (3). Approximately 10% of individuals with prediabetes progress to diabetes annually in the U.S. Prediabetes is a significant risk factor for the development of diabetes, cardiovascular events, and increased mortality (4). Therefore, early detection and management through lifestyle changes, medication, and regular monitoring can prevent or delay diabetes onset and its complications.
Genome-wide association studies (GWAS) have been used to identify links between human phenotypes and genetic variations. Thousands of GWAS summary statistics are available, covering a wide range of human traits and diseases. T2DM and prediabetes are complex, multifactorial conditions influenced by both lifestyle risk factors and genetic susceptibility (5). While GWAS has identified various genetic variants associated with an increased risk of T2DM across different populations (6, 7), studies specifically targeting genetic variants linked to prediabetes remain limited, particularly in Chinese populations. This study aims to contribute to addressing this gap by exploring genetic variants associated with prediabetes risk. From a pathophysiological perspective, major procedures in the progression to T2DM include dysfunction of β-cells and insulin resistance (8). Similarly, prediabetes is characterized by increased inflammation and islet dysfunction (9), emphasizing the importance of assessing islet function indicators in individuals with prediabetes. Consequently, we also explored the associations between genetic variations and clinical indicators of islet function in this study.
Melatonin receptor 1B (MTNR1B), a member of the G protein-coupled receptor family encoded by the MTNR1B gene, was initially identified as the regulator of glucose levels and insulin secretion binding with melatonin (10). Over the past decade, GWAS studies have identified several risk variants in MTNR1B associated with impaired glucose regulation and related conditions. The leading variant, rs10830963 (11), has been linked to T2DM (12), diabetic peripheral neuropathy (13), gestational diabetes mellitus (14), HbA1c (15), and insulin secretion (16). Similarly, rs2166706 has been associated with elevated plasma glucose levels, reduced islet function, and an increased risk of T2DM (17), while rs1447352 has been linked to fasting plasma glucose levels (18). Another variant, rs724030, which is in moderate linkage disequilibrium with rs10830963, rs2166706, and rs1447352 (r² = 0.61, 0.23, and 0.23, respectively), has been associated with T2DM (12, 19), HbA1c (15, 20), and islet function (16) in European populations. However, the role of rs724030 in individuals with prediabetes, a condition with a high risk of progressing to T2DM, remains unexplored, particularly in East Asian populations. To address this gap, we selected rs724030 as a representative locus to investigate its genetic association with prediabetes risk and related clinical indicators.
Therefore, our study aimed to investigate the relationships between rs724030 in MTNR1B and clinical features in both NGT and IFG/IGT individuals among Chinese people, including plasma glucose and serum insulin levels during OGTT, islet function, and islet resistance. Furthermore, after stratifying by WHR, we explore sex dimorphisms in the genetic effects of rs724030 in MTNR1B on islet function.
Materials and methodsStudy design and participantsThis study includes unrelated 3415 glucose-tolerant healthy and 1744 IFG/IGT individuals, and their clinical characteristics are shown in Supplementary Table S1. This study included participants from Nanjing, one of the 25 communities in the REACTION study (21). Participants with a history of other diseases, positive thyroid peroxidase, or thyrotropin receptor antibodies were excluded. All participants measured plasma glucose and serum insulin levels at fasting, 30, and 120 minutes after a standard 75-g OGTT. Based on the WHO criteria (2), IGT (2-h glucose 7.8–11.0 mol/L) and IFG (fasting glucose 6.1–6.9 mmol/L) are commonly used to define prediabetes. NGT is defined as fasting glucose <6.1 mmol/L and 2-h glucose < 7.8 mol/L. The study population was determined to be Chinese Han by questionnaire. All samples were collected with appropriate informed consent from all participants and/or their guardians in a written way. Approval for the study was granted from the relevant ethics committees in the First Affiliated Hospital of Nanjing Medical University. All participants provided written informed consent at the time of recruitment.
Study measurementsThe homeostasis model assessment of β-cell function (HOMA-β), homeostasis model assessment of insulin resistance (HOMA-IR), IGI, CIR, DIo, and Matsuda’s insulin sensitivity index (ISIMatsuda) were calculated according to the formula, and methods in Supplementary Table S4. Plasma glucose was measured with the hexokinase method (AU5400, Olympus). An insulin radioimmunoassay kit measured serum insulin levels (BNIBT, China). The chemiluminescence method was used to evaluate serum C-peptide levels and serum lipid levels such as high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total cholesterol (TC), and triglycerides (TG) (RocheDiagnostics, Switzerland). In addition, a questionnaire was used to obtain their basic information, including age, sex, height, weight, waist circumference, and hip circumference.
Genotyping assayHuman genomic DNA was extracted using a standard method. The SNP genotyping work was performed using a custom-by-design 48-Plex SNPscanTM Kit (Cat#:G0104; Genesky Biotechnologies Inc., Shanghai, China). This kit was developed according to patented SNP genotyping technology by Genesky Biotechnologies Inc., which was based on double ligation and multiplex fluorescence polymerase chain reactions (PCR) (22). Genotyping was conducted without any knowledge regarding the subject’s case or control status. Concordance for duplicate samples, which accounted for 4% of the total samples, was >99% for all assays. The genotype distribution in healthy and pre-diabetic individuals was consistent with Hardy-Weinberg equilibrium (P > 0.05).
Statistical analysisMean ± SD was used for normally distributed data and median (interquartile range) for non-normal data. Values of serum insulin, HOMA-β, HOMA-IR, IGI, CIR, DIo, and ISIMatsuda were log-transformed. Under an appropriately adjusted additive model, a binary logistic regression analysis was performed for the relationship between rs724030 in MTNR1B and the IFG/IGT individuals. The associations between the MTNR1B rs724030 variant and glycemic-related quantitative traits were analyzed by multiple linear regression. All the analyses were adjusted by age, sex, and BMI. All P-values were two-sided, and P < 0.05 was considered significant. The heterogeneity was considered significant with I2 >75% and P<0.05. Statistical analyses were performed using STATA 18.0.
ResultsMTNR1B Rs724030 A>G variant is not associated with the risk of IFG/IGT individualsSupplementary Table S2 shows no association between the MTNR1B rs724030 variant and IFG/IGT risk in total individuals (P > 0.05). Obesity is a significant risk factor for the dysregulation of glucose metabolism. Therefore, we stratified participants by BMI according to Chinese criteria (23). However, we did not find any association between the MTNR1B rs724030 variant and IFG/IGT risk in any of the BMI subgroups (P > 0.05).
MTNR1B Rs724030 A>G variant significantly correlates with islet function but not insulin resistance in the NGT individuals with normal BMI LevelsAfter stratifying people by BMI, we analyzed OGTT-derived indicators of glycemic-related traits in the NGT individuals and IFG/IGT individuals. As shown in Table 1, the MTNR1B rs724030 variant is associated with higher fasting and 30-minute plasma glucose levels in the NGT individuals with normal BMI levels (BMI < 24kg/m2) (P = 0.009 and 0.001, respectively). We further investigate the associations between this variant and islet function indices. In the NGT individuals with normal BMI levels, the G allele of this variant is significantly associated with decreased IGI and DIo (P = 0.001 and 0.007, respectively) but not associated with insulin resistance indices. Except for the similar correlation between the variant and IGI in individuals who are overweight (24 ≤ BMI < 28kg/m2) in the NGT individuals (P = 0.035), no other significant relationships exist in both the NGT and IFG/IGT individuals. Among individuals who are overweight in the NGT individuals, although the variant was positively correlated with glycated hemoglobin (HbA1c) levels (P = 0.003), this correlation was lower with significant heterogeneity to the individuals with normal BMI levels (Phet = 0.037). Similarly, among individuals who are obese (BMI ≥ 28kg/m2) in the NGT individuals, this variant had a negative association with fasting insulin levels and HOMA-β (P = 0.016 and 0.003), but these associations were also lower due to significant heterogeneity to the individuals with normal BMI levels (Phet = 0.039 for fasting insulin levels, Phet = 0.022 for HOMA-β).

Table 1. The associations between the MTNR1B rs724030 variant and glycemic quantitative traits, islet function/insulin resistance, and serum lipid levels stratified by BMI.
Significant sexual heterogeneities for the effect size on the associations between MTNR1B Rs724030 variant and islet function after stratifying by the WHR in the NGT individualsWe stratify all participants by WHR according to central obesity diagnosis criteria in WHO consultation (24). We grouped WHR according to 0.85 and assumed that individuals with WHR greater than 0.85 had a higher risk of abdominal obesity. As shown in Table 2, in the NGT individuals, significant associations were found between this variant in MTNR1B rs724030 and IGI, CIR, and DIo (All P < 0.001) in female individuals whose WHR are more remarkable than 0.85, and these associations had significant heterogeneity (Phet = 0.009, 0.030, and 0.049, respectively) to the corresponding male subgroup. This suggests that the effect of this variant on islet function exhibits sex dimorphism, with differing effect sizes between males and females. In the NGT individuals, this variant did not correlate with islet function/resistance indices among individuals whose WHR is less than or equal to 0.85. We also found no associations between this variant and related indices in the IFG/IGT individuals as shown in Table 3.
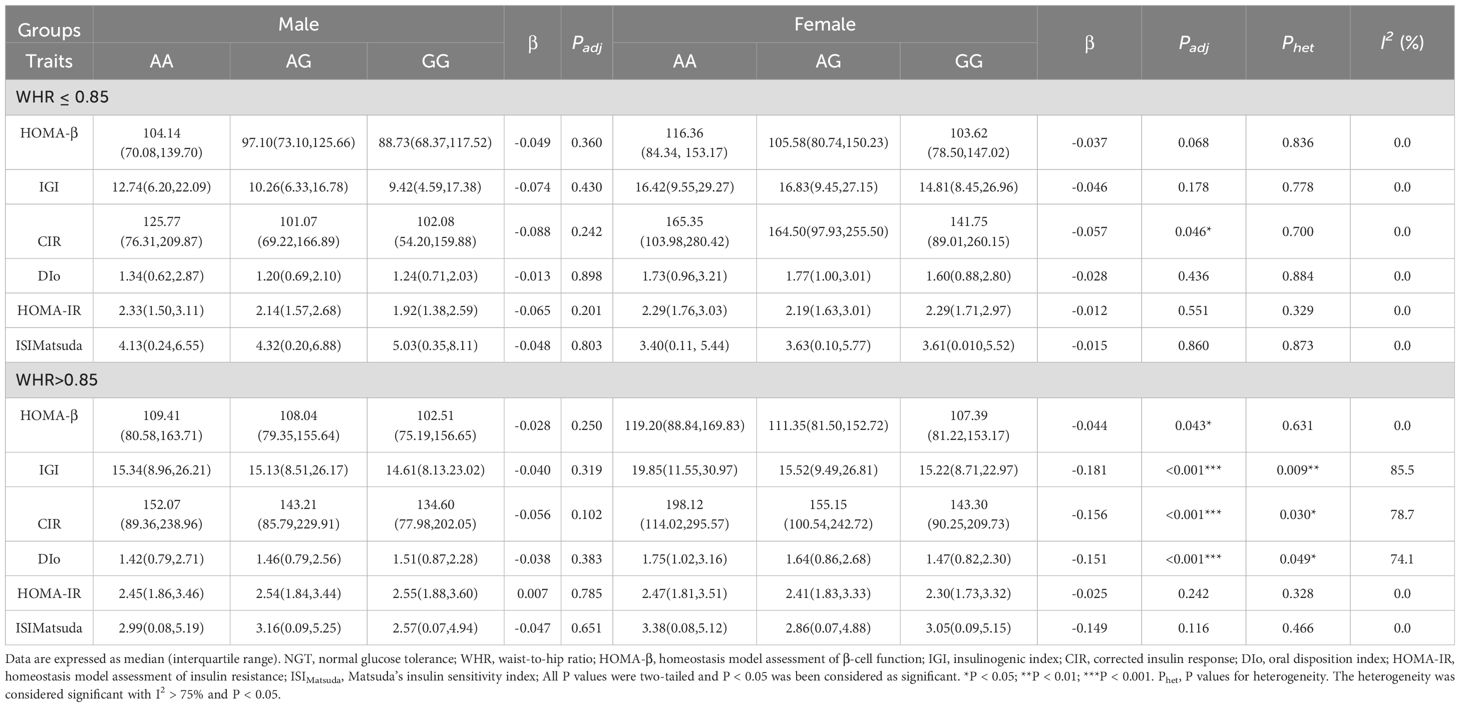
Table 2. The associations between the MTNR1B rs724030 variant and islet function/insulin resistance in the NGT individuals stratified by WHR.
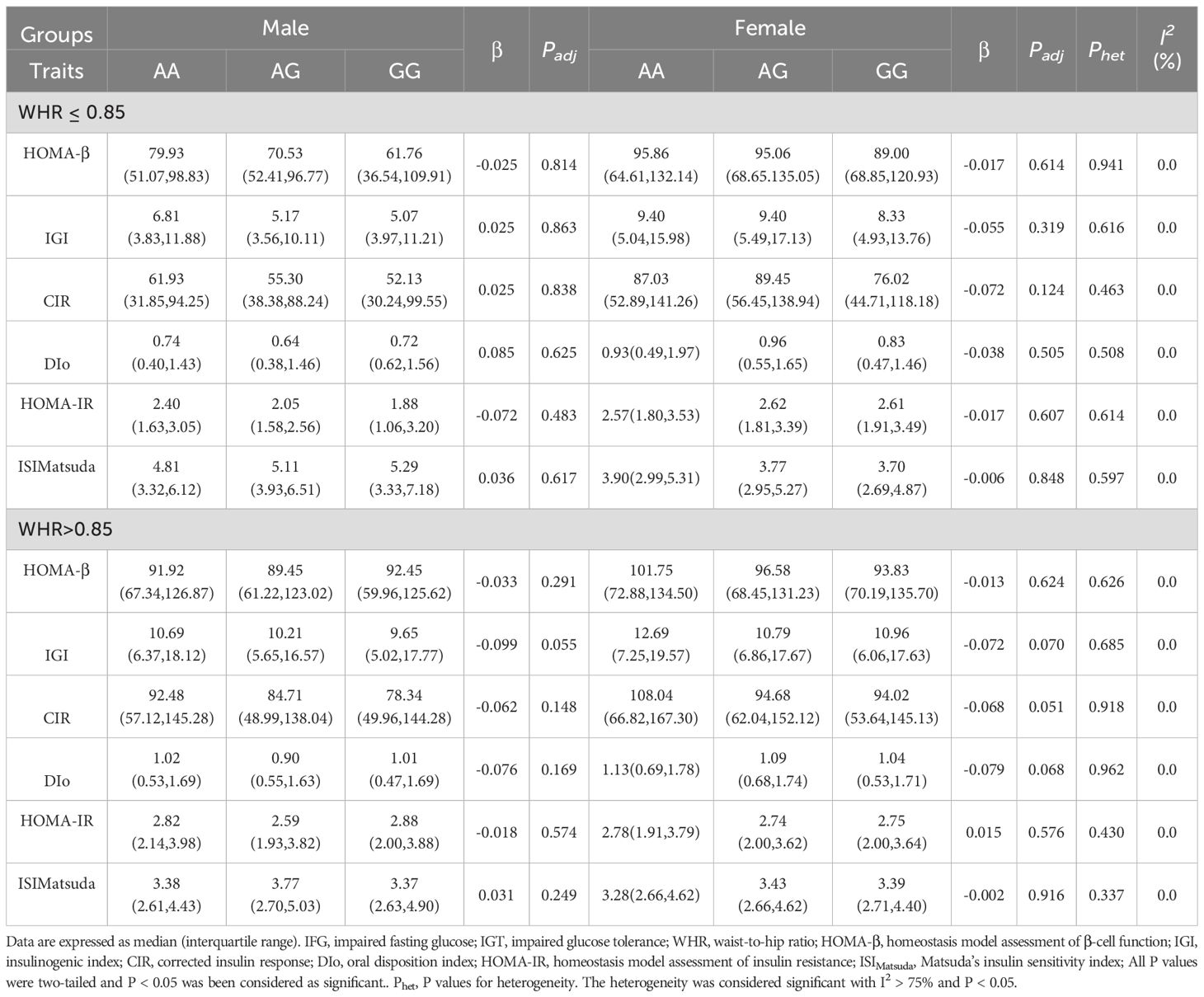
Table 3. The associations between the MTNR1B rs724030 variant and islet function/insulin resistance in the IFG/IGT individuals stratified by WHR.
MTNR1B Rs724030 A>G variant is not associated with insulin clearance in the NGT individualsWe calculated the insulin clearance rate according to the method of a previously published article (25). Endogenous insulin clearance during the OGTT was assessed by CpAUC120/InsAUC120 (25). We found that the MTNR1B rs724030 variant was not significantly associated with insulin clearance(All P > 0.05), as shown in Supplementary Table S3.
DiscussionDuring the last ten years, several studies have demonstrated that different variants in MTNR1B are associated with an increased risk of T2DM. For instance, Chambers, J.C. et al. found that the G allele of rs2166706 in MTNR1B is associated with an increased risk of T2DM in individuals of Asian Indian ancestry (17). Later, B. F. et al. found that the T allele of rs1387153 in MTNR1B can increase the risk of T2DM in individuals of European ancestry (26). Over the next few years, the researchers successively identified the association between the G allele of rs10830963 in MTNR1B and the increased risk of T2DM among people of different ethnicities (27, 28). Our study found no association between the MTNR1B rs724030 variant and the risk of predibetes, at least in the Chinese population. The increased risk of prediabetes likely arises from the combined influence of genetic and environmental factors. A single genetic variation may be difficult to fully explain the risk, highlighting the need to consider gene-environment interactions.
MTNR1B is a glycemic-related gene. Genetic variants in MTNR1B associated with fasting plasma glucose levels are increasingly known across ethnic groups (29). Previous studies have established that some SNPs in MTNR1B are associated with higher fasting plasma glucose levels, such as the rs1387153 T allele (30), the rs10830963 G allele (31), the rs1447352 G allele (18), rs2166706 G allele (17). In our study, after stratifying people by BMI status, we also found that the minor (G) allele of the variant in MTNR1B rs724030 was associated with a per-allele increase of 0.029 (95%CI 0.007–0.050) mmol/L and 0.153 (95%CI 0.067–0.240) mmol/L in fasting plasma glucose levels (P = 0.009) and 30-minute plasma glucose levels (P = 0.001), respectively, in the NGT individuals with normal BMI levels. It demonstrated that studies of continuous glycemic traits in the NGT individuals can improve the understanding of the mechanisms involved in β-cell function and glucose homeostasis. However, we found no association between this variant and 120-minute plasma glucose levels in all participants. Among individuals who are overweight and obese in the NGT individuals, we found no association between this variant and glycemic-related traits. At the same time, no associations were found in the IFG/IGT individuals. Therefore, we suggest that the G allele of rs724030 in MTNR1B may have a possible association with higher plasma glucose levels in populations with normal glucose tolerance and with normal BMI levels but not in populations in the prediabetic state. It may be a potential risk factor for abnormal glucose metabolism in healthy individuals in the future but needed to be further verified. HbA1c reflects the average plasma glucose levels over the past three months and indicates long-term glycemic status, unlike fasting plasma glucose (32). As previously shown, rs1387153 (33) and rs10830963 (34) in MTNR1B are associated with increased HbA1c levels. However, we found no association between rs724030 and HbA1c levels in all subgroups, the individals we studied were elderly subjects based on OGTT or ethnic heterogeneity may contribute to the inconsistent results.
Melatonin, a neurohormone, regulates the circadian rhythm by transmitting photoperiodic information from the eyes to the brain. Melatonin signaling is mainly mediated by two high-affinity receptors, MT1 and MT2, encoded by the MTNR1A and MTNR1B genes, respectively (35). MT2-mediated melatonin signaling could indirectly regulate glucose levels and insulin secretion through the brain control center of the circadian clock (30). In rodent and human islets, MTNR1B is expressed and co-localizes with insulin. Researchers have demonstrated that treating the pancreatic beta-cells with melatonin can worsen insulin secretion and that exogenously administered melatonin inhibits insulin secretion in rodents (36). MTNR1B is identified to inhibit glucose-stimulated insulin secretion through binding with melatonin and decreasing cAMP levels (10). Consistent with previous studies, herein, in the NGT individuals, we observed that subjects carrying the minor (G) allele of the variant in MTNR1B rs724030 tend to have lower IGI and DIo levels. These two indicators can measure β-cell function adjusted for insulin sensitivity and predict the development of diabetes over ten years (37). Previous studies have also demonstrated that the rs10830963 G allele in MTNR1B is associated with decreased insulin secretion measured as CIR, AUCIns/AUCGluc (38), peak insulin response, acute insulin response (AIR), disposition index (DI) (16). Similarly, Palmer, N.D. et al. also found that rs1387153 T allele in MTNR1B affects insulin secretion pathways reflected by AIR and disposition index (DI) (39). It demonstrated that variants in MTNR1B can influence β-cell function. In our study, we found that the G allele of rs724030 in MTNR1B was associated with decreased IGI (P = 0.001) and DIo (P = 0.007) in the NGT individuals, especially in those NGT participants with normal BMI levels. It indicates that this indicator can reflect the deterioration of β-cell function and predict the possibility of impaired plasma glucose regulation. However, similar association wasn’t found in obese subgroups in the NGT individuals, and no associations in the IFG/IGT individuals.
It is well known that obesity has continued to increase for three decades worldwide (40) and has become a tremendous public health concern globally. Obesity can be categorized as general and abdominal obesity, while abdominal obesity has been established to be associated with T2DM (41). Among the conventional indicators of obesity, waist circumference and WHR are used to reflect abdominal obesity, and BMI is used to reflect general obesity. Previous studies have identified that increased waist circumference and WHR is associated with higher T2DM risk (42). Probably because the distribution of abdominal fat correlates with increased insulin resistance. Consistent with previous observations, in the NGT individuals, we found that the minor (G) allele of the variant in MTNR1B rs724030 is associated with decreased IGI, CIR, and DIo, especially in female subjects with WHR greater than 0.85. Moreover, this correlation was specific due to the significant heterogeneity in the corresponding male subgroup. Possible explanations for this finding include the following: Differences in sex hormone levels, such as estrogen and testosterone, may influence fat distribution, with women tending to accumulate fat in the abdomen, hips, and thighs. Additionally, men and women differ in insulin secretion, sensitivity, and glucose metabolism, which could result in sex-specific effects of the same SNP. Lastly, variations in diet, exercise, and lifestyle habits between men and women may amplify the SNP’s sex-specific effects through gene-environment interactions. At the same time, we did not find any association between this variant and islet function/islet resistance indices in the IFG/IGT individuals after stratifying by WHR. We hypothesize that abnormal glucose regulation may obscure the effects of other factors on islet function, such as genetic variants.
The strengths of this study included that OGTT was used to recognize the status of glucose regulation in every group population, which allowed us to identify the association between the variant and postprandial indices. More importantly, this is the first study to investigate the associations of this variant with clinical features and islet function/resistance in the Chinese Han population. The present study also has some inevitable limitations. For example, findings of this study are unlikely to be explained by a single genetic variant alone. A limitation of this study is the lack of consideration for potential confounding environmental factors, such as diet, physical activity, and other lifestyle behaviors, which are known to significantly influence glucose levels and insulin resistance. While we focused on genetic associations, the absence of detailed data on these variables limits our ability to fully disentangle genetic effects from environmental influences. Future studies should integrate comprehensive assessments of lifestyle factors to provide a more holistic understanding of the interplay between genetics and environment in determining metabolic outcomes. While this study primarily focuses on the analysis of MTNR1B gene variants and their associations with prediabetes risk, we acknowledge the limitation of not including variants from other genes that may also contribute to prediabetes risk. Future studies including a broader range of genetic variants would provide a more comprehensive understanding of the genetic architecture underlying prediabetes susceptibility.
In conclusion, we found that the MTNR1B rs724030 variant is correlated with the increase of fasting and 30-minute plasma glucose levels in the NGT individuals with normal BMI levels. Importantly, we also found that this variant is associated with the impairment of islet function measured as IGI and DIo in the same subgroup. Moreover, the current study provides, for the first time, insights into the sex dimorphisms of the effects of the MTNR1B rs724030 variant on impairing islet function after stratifying by WHR in the NGT individuals. These discoveries provide a basis for accurately assessing islet function in healthy Chinese populations and offer a new perspective on precision prevention. Future studies must further explain the BMI-specific differences and sex dimorphisms in this variant’s impact on islet function.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by The Ethics Committee from the First Affiliated Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsSZ: Formal Analysis, Methodology, Writing – original draft. WB: Formal Analysis, Writing – original draft, Writing – review & editing. YW: Formal Analysis, Writing – original draft. MS: Formal Analysis, Methodology, Writing – review & editing. YQ: Methodology, Writing – review & editing. HD: Project administration, Resources, Writing – review & editing. SZ: Project administration, Resources, Writing – review & editing. QF: Data curation, Methodology, Project administration, Resources, Writing – review & editing. KX: Data curation, Methodology, Project administration, Resources, Writing – review & editing. HJ: Writing – review & editing. TY: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (8240032853, 82230028, 82070803, 82200888 and 82100837), the Natural Science Foundation of Jiangsu Province (BK20241116, BK20220714 and BK20210959), and the Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (No. PY2023014).
AcknowledgmentsWe thank all study participants and the research staff who participated in this work.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1398687/full#supplementary-material
References1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
PubMed Abstract | Crossref Full Text | Google Scholar
6. Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. (2020) 52:680–91. doi: 10.1038/s41588-020-0637-y
PubMed Abstract | Crossref Full Text | Google Scholar
7. Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 east asian individuals. Nature. (2020) 582:240–5. doi: 10.1038/s41586-020-2263-3
PubMed Abstract | Crossref Full Text | Google Scholar
8. Eizirik DL, Pasquali L, Cnop M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. (2020) 16:349–62. doi: 10.1038/s41574-020-0355-7
PubMed Abstract | Crossref Full Text | Google Scholar
9. Weaver JR, Odanga JJ, Breathwaite EK, Treadwell ML, Murchinson AC, Walters G, et al. An increase in inflammation and islet dysfunction is a feature of prediabetes. Diabetes/metabolism Res Rev. (2021) 37:e3405. doi: 10.1002/dmrr.3405
PubMed Abstract | Crossref Full Text | Google Scholar
10. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, et al. Common variant in mtnr1b associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. (2009) 41:82–8. doi: 10.1038/ng.288
PubMed Abstract | Crossref Full Text | Google Scholar
11. Chen J, Spracklen CN, Marenne G, Varshney A, Corbin LJ, Luan J, et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. (2021) 53:840–60. doi: 10.1038/s41588-021-00852-9
PubMed Abstract | Crossref Full Text | Google Scholar
12. Mahajan A, Spracklen CN, Zhang W, Ng MCY, Petty LE, Kitajima H, et al. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat Genet. (2022) 54:560–72. doi: 10.1038/s41588-022-01058-3
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ocak O, Silan F, Sahin EM. Melatonin receptor gene polymorphisms as a risk factor in patients with diabetic peripheral neuropathy. Diabetes Metab Res Rev. (2022) 38:e3573. doi: 10.1002/dmrr.3573
PubMed Abstract | Crossref Full Text | Google Scholar
14. Wei L, Jiang Y, Gao P, Zhang J, Zhou X, Zhu S, et al. Role of melatonin receptor 1b gene polymorphism and its effect on the regulation of glucose transport in gestational diabetes mellitus. J Zhejiang Univ Sci B. (2023) 24:78–88. doi: 10.1631/jzus.B2200136
PubMed Abstract | Crossref Full Text | Google Scholar
15. Jurgens SJ, Pirruccello JP, Choi SH, Morrill VN, Chaffin M, Lubitz SA, et al. Adjusting for common variant polygenic scores improves yield in rare variant association analyses. Nat Genet. (2023) 55:544–8. doi: 10.1038/s41588-023-01342-w
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wood AR, Jonsson A, Jackson AU, Wang N, van Leewen N, Palmer ND, et al. A genome-wide association study of ivgtt-based measures of first-phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes. (2017) 66:2296–309. doi: 10.2337/db16-1452
PubMed Abstract | Crossref Full Text | Google Scholar
17. Chambers JC, Zhang W, Zabaneh D, Sehmi J, Jain P, McCarthy MI, et al. Common Genetic Variation near Melatonin Receptor Mtnr1b Contributes to Raised Plasma Glucose and Increased Risk of Type 2 Diabetes among Indian Asians and European Caucasians. Diabetes. (2009) 58:2703–8. doi: 10.2337/db08-1805
PubMed Abstract | Crossref Full Text | Google Scholar
18. Sabatti C, Service SK, Hartikainen AL, Pouta A, Ripatti S, Brodsky J, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. (2009) 41:35–46. doi: 10.1038/ng.271
PubMed Abstract | Crossref Full Text | Google Scholar
19. Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, Donner KM, et al. Finngen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
PubMed Abstract | Crossref Full Text | Google Scholar
20. Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, et al. Genetics of 35 blood and urine biomarkers in the uk biobank. Nat Genet. (2021) 53:185–94. doi: 10.1038/s41588-020-00757-z
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ning G, Reaction Study G. Risk evaluation of cancers in chinese diabetic individuals: A longitudinal (Reaction) study. J Diabetes. (2012) 4:172–3. doi: 10.1111/j.1753-0407.2012.00182.x
PubMed Abstract | Crossref Full Text | Google Scholar
22. Chen X, Li S, Yang Y, Yang X, Liu Y, Liu Y, et al. Genome-wide association study validation identifies novel loci for atherosclerotic cardiovascular disease. J Thromb Haemost. (2012) 10:1508–14. doi: 10.1111/j.1538-7836.2012.04815.x
PubMed Abstract | Crossref Full Text | Google Scholar
24. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a who consultation. Diabetes Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
PubMed Abstract | Crossref Full Text | Google Scholar
25. Fu Z, Wu Q, Guo W, Gu J, Zheng X, Gong Y, et al. Impaired insulin clearance as the initial regulator of obesity-associated hyperinsulinemia: novel insight into the underlying mechanism based on serum bile acid profiles. Diabetes Care. (2022) 45:425–35. doi: 10.2337/dc21-1023
PubMed Abstract | Crossref Full Text | Google Scholar
26. Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. (2010) 42:579–89. doi: 10.1038/ng.609
PubMed Abstract | Crossref Full Text | Google Scholar
27. Qi Q, Stilp AM, Sofer T, Moon JY, Hidalgo B, Szpiro AA, et al. Genetics of type 2 diabetes in U.S. Hispanic/latino individuals: results from the hispanic community health study/study of latinos (Hchs/sol). Diabetes. (2017) 66:1419–25. doi: 10.2337/db16-1150
PubMed Abstract | Crossref Full Text | Google Scholar
28. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
PubMed Abstract | Crossref Full Text | Google Scholar
29. Rasmussen-Torvik LJ, Guo X, Bowden DW, Bertoni AG, Sale MM, Yao J, et al. Fasting glucose gwas candidate region analysis across ethnic groups in the multiethnic study of atherosclerosis (Mesa). Genet Epidemiol. (2012) 36:384–91. doi: 10.1002/gepi.21632
PubMed Abstract | Crossref Full Text | Google Scholar
30. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, et al. A variant near mtnr1b is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. (2009) 41:89–94. doi: 10.1038/ng.277
PubMed Abstract | Crossref Full Text | Google Scholar
31. Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in mtnr1b influence fasting glucose levels. Nat Genet. (2009) 41:77–81. doi: 10.1038/ng.290
PubMed Abstract | Crossref Full Text | Google Scholar
32. Mortensen HB, Christophersen C. Glucosylation of human haemoglobin a in red blood cells studied in vitro. Kinetics of the formation and dissociation of haemoglobin A1c. Clin Chim Acta. (1983) 134:317–26. doi: 10.1016/0009-8981(83)90370-4
PubMed Abstract | Crossref Full Text | Google Scholar
33. Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common Variants at 10 Genomic Loci Influence Hemoglobin a(1)(C) Levels Via Glycemic and Nonglycemic Pathways. Diabetes. (2010) 59:3229–39. doi: 10.2337/db10-0502
PubMed Abstract | Crossref Full Text | Google Scholar
34. Wheeler E, Leong A, Liu CT, Hivert MF, Strawbridge RJ, Podmore C, et al. Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PloS Med. (2017) 14:e1002383. doi: 10.1371/journal.pmed.1002383
PubMed Abstract | Crossref Full Text | Google Scholar
37. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-H glucose levels. Diabetes Care. (2009) 32:335–41. doi: 10.2337/dc08-1478
PubMed Abstract | Crossref Full Text | Google Scholar
38. Prokopenko I, Poon W, Magi R, Prasad BR, Salehi SA, Almgren P, et al. A central role for grb10 in regulation of islet function in man. PloS Genet. (2014) 10:e1004235. doi: 10.1371/journal.pgen.1004235
PubMed Abstract | Crossref Full Text | Google Scholar
39. Palmer ND, Goodarzi MO, Langefeld CD, Wang N, Guo X, Taylor KD, et al. Genetic variants associated with quantitative glucose homeostasis traits translate to type 2 diabetes in mexican americans: the guardian (Genetics underlying diabetes in hispanics) consortium. Diabetes. (2015) 64:1853–66. doi: 10.2337/db14-0732
PubMed Abstract | Crossref Full Text | Google Scholar
40. Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. (2017) 390:2627–42. doi: 10.1016/S0140-6736(17)32129-3
PubMed Abstract | Crossref Full Text | Google Scholar
41. Lu B, Yang Y, Yang Z, Feng X, Wang X, Zhang Z, et al. Insulin resistance in chinese patients with type 2 diabetes is associated with C-reactive protein independent of abdominal obesity. Cardiovasc Diabetol. (2010) 9:92. doi: 10.1186/1475-2840-9-92
留言 (0)