Melanoma is one of the deadliest skin cancer types (1). With the introduction of modern therapy strategies such as immune checkpoint inhibition (ICI) and BRAF/MEK-directed targeted therapy (TT), the prognosis of patients with advanced melanoma has improved significantly with response rates up to 70% and 5-year overall survival rates up to 50% (2–5). After the introduction of these modern systemic therapies, the initial focus of research was on factors that determine a good or poor treatment outcome.
For both, ICI and TT, there is now an increasing amount of long-term survival data available from randomized clinical trials (RCT). Analysis of these data demonstrate that patient survival curves plateau after only 3-5 years after initiation of therapy (6, 7). Therefore, there is an increasing interest in identifying the factors that are determinants of a patient’s long-term survival or even cure (8). Especially from RCT study results, we know that at treatment baseline, an overall low tumor burden with low serum lactate dehydrogenase (LDH), as well as the absence of brain metastasis correlates positively with long-term patient survival upon the respective first-line therapy (9). In contrast, studies on real-world data on factors affecting long-term survival of melanoma patients with distant metastases are rare.
The aim of the present study was to identify factors predictive for long-term versus short-term survival of a real-world cohort of stage IV melanoma patients after onset of a modern first-line therapy with PD-1-based ICI or BRAF/MEK-directed TT.
Patients and methodsStudy designPatients with histologically confirmed melanoma of the skin or of unknown primary (MUP) diagnosed with stage IV by AJCCv8 and started a first-line systemic treatment between May 2010 and October 2021. From the prospective multicenter skin cancer registry ADOREG of the German Dermatological Cooperative Oncology Group (DeCOG), only patients who were alive either ≤1 year or ≥5 years after stage IV diagnosis were included. The study endpoint was overall survival (OS), defined as time after start of first-line therapy in stage IV and death of any cause. Patient and tumor characteristics at baseline of first-line therapy in stage IV, sex (m vs f), age (≤ vs > 65 years), M stage by AJCCv8 (M1a/b vs M1c/d), serum LDH, numbers of organs involved with metastasis (<3 vs ≥3), and presence of specific organ metastasis (lung, liver and brain) were analyzed for distribution and association to OS. This analysis was performed on the total patient cohort, as well as on patient subgroups subdivided by type of first-line therapy (PD-1-based ICI, and BRAF/MEK-directed TT) and tumor BRAF mutation status. All patients gave written informed consent before documentation of their data in the ADOREG registry. The ADOREG registry was approved by the ethics committee of the University Duisburg-Essen (15-6566-BO).
Statistical analysisThe chi-square test, student’s t-test, receiver operating curves (ROC) analysis, and multinomial logistic regression analysis were performed to investigate the effects of baseline patient and tumor characteristics, as well as therapy selection, on patient survival (OS). P<0.05 was considered statistically significant. Univariate statistical analysis consisting of chi-square test, student’s t-test, ROC analysis, and multivariate analysis were performed with SPSS (Version 25, IBM, Armonk, NY, USA).
ResultsPatient characteristicsData cut-off was February 2022. Of the patients enrolled into the ADOREG at that time, 3066 were diagnosed with stage IV melanoma. Of these, 395 patients met the selection criteria for the present study (Figure 1). Among them, 237 (60.0%) were men and 158 (40.0%) were women. The mean age of the patients was 64.3 years (range 19-96). 128 patients (32.4%) received BRAF/MEK-directed TT, 174 (44.1%) received PD-1 ICI monotherapy, and 93 (23.5%) received PD-1 plus CTLA-4 dual ICI therapy. Detailed baseline characteristics of the patients and their tumors including tumor subtype, BRAF mutation status, primary tumor thickness (Breslow), and primary tumor ulceration are listed in Table 1.
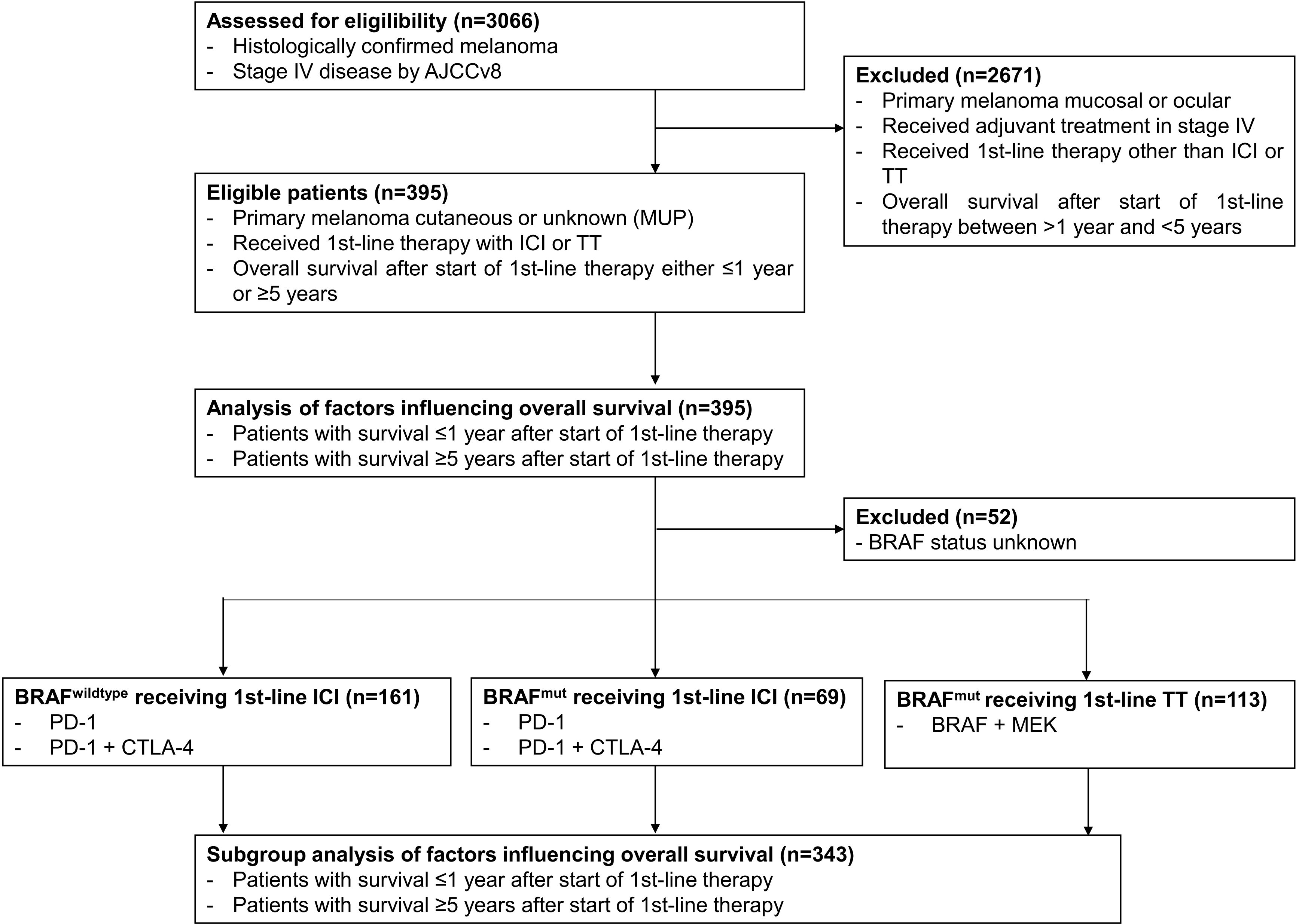
Figure 1. Schematic presentation of the study flow.
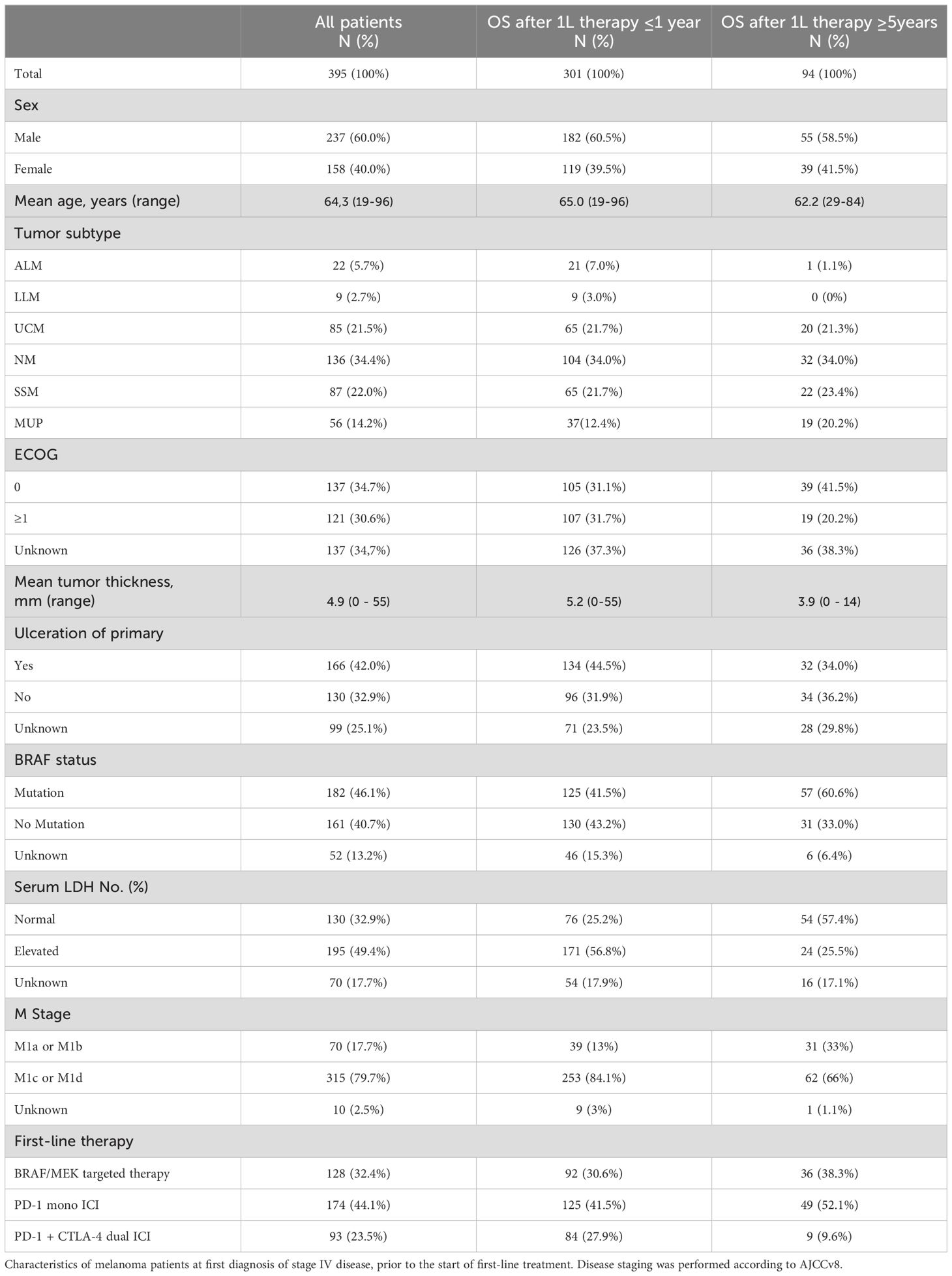
Table 1. Patient characteristics.
Patients with elevated serum LDH are less likely to show long-term survivalIn order to determine which baseline factors affect long-term survival in stage IV melanoma patients we first performed multinomial regressions. Due to the high number of missing values, ECOG, tumor thickness and tumor ulceration status were excluded from the analysis. First, the entire patient cohort was analyzed regardless of the type of therapy received. Among the included factors, a statistically significant negative association was only found between a high serum LDH level (HR=4.619 CI=2.550 - 8.368; P<0.001) and long-term survival ≥5 years. The other included factors such as therapy (ICI vs. TT) (HR=0.816 CI=0.433 – 1.539; P=0.530), brain metastases (no vs. yes) (HR=1.928 CI=0.975 – 3.184; P=0.059), liver metastases (no vs. yes) (HR=1.587 CI=0.782 – 3.222; P=0.201), lung metastases (no vs. yes) (HR=1.302 CI=0.711 – 2.386; P=0.393), number of organs involved (<3 vs. ≥3) (HR=0.337 CI=0.337 – 1.367; P=0.275), M category (M1a or b vs. c or d) (HR=2.012 CI=0.824 – 4.913; P=0.125), age (<65 vs. ≥65) (HR=0.985 CI=0.541 – 1.794; P=0.961) and gender (male vs. female) (HR=0.948 CI=0.523 – 1.717; P=0.859) showed no statistically significant effect on long-term survival (OS ≥5 years) (Figure 2A, Supplementary Table 1).
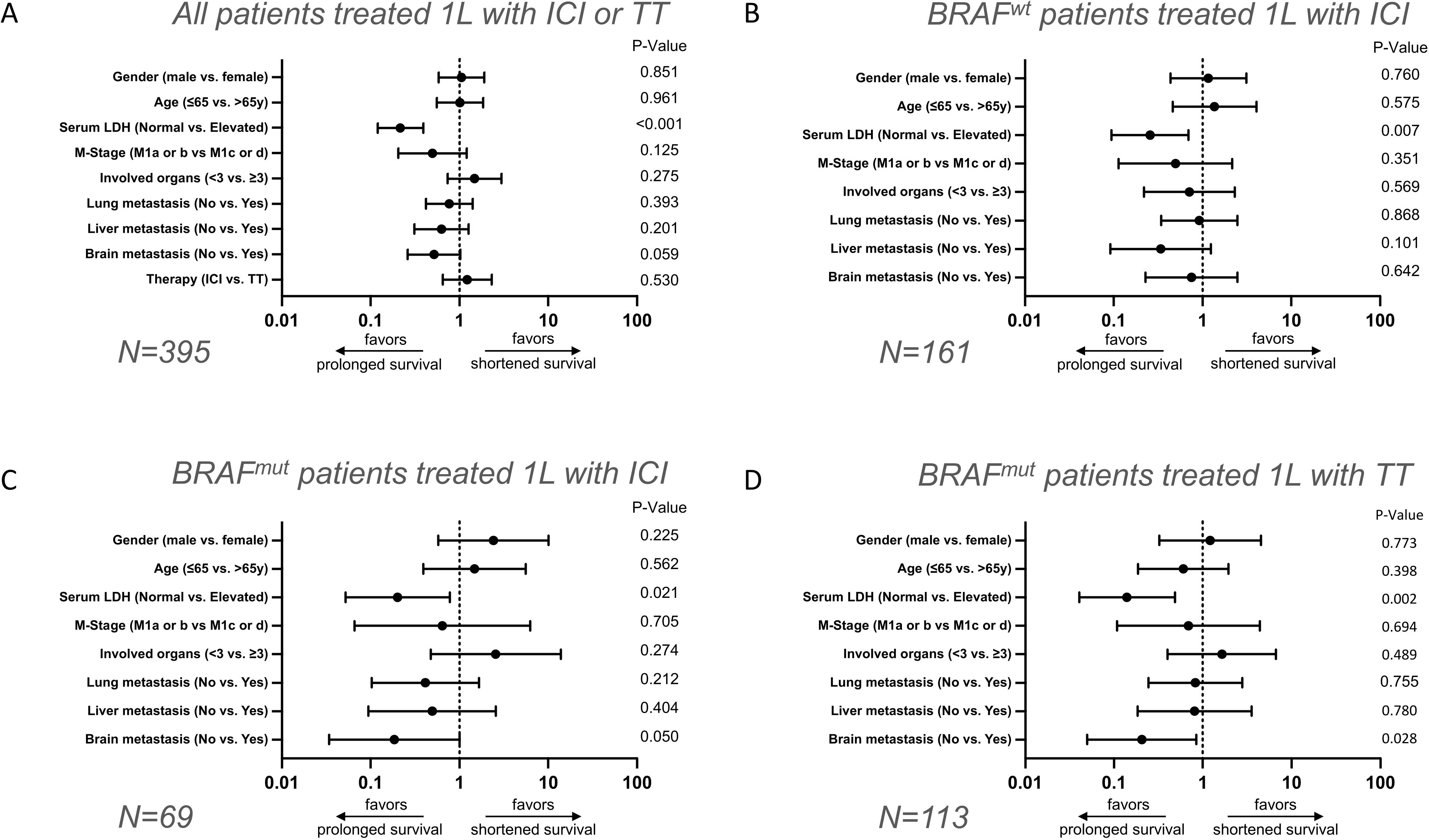
Figure 2. Multivariate analysis of baseline clinical parameters on long-term versus short-term survival after 1L therapy in stage IV melanoma patients. Forest plots show multivariate evaluation of clinical parameters associated with long-term OS ≥5 years versus short-term OS ≤1 year in melanoma patients who received ICI or TT (A), BRAF-wildtype patients who received ICI (B), BRAF-mutant patients who received ICI (C), and BRAF-mutant patients who received TT (D).
To investigate the extent to which factors influence long-term survival in relation to the type of first-line therapy and BRAF status, the total patient cohort was subdivided into three subgroups. The first subgroup included BRAF-wildtype patients who received first-line ICI. Again, only elevated serum LDH (HR=3.887; CI=1.440–10.490; P=0.007) had a statistically significant negative impact on long-term survival (OS ≥5 years). All other factors investigated, such as brain metastases (no vs. yes; HR=1.326; CI=0.403–4.360; P=0.642), liver metastases (no vs. yes; HR=2.959; CI=0.808–10.841; P=0.101), lung metastases (no vs. yes; HR=1.088; CI=0.403–2.936; P=0.868), number of organs involved with metastasis (<3 vs. ≥3; HR=1.409; CI=0.434–4.575; P=0.569), M category (M1a/b vs. c/d; HR=2.014; CI=0.462–8.776; P=0.351), age (<65 vs. ≥65 years; HR=0.733; CI=0.247-2.173; P=0.575) and sex (male vs. female; HR=0.858; CI=0.322–2.288; P=0.760) indicated no statistically significant effect on the relative chance of long-term survival (Figure 2B, Supplementary Table 2).
In contrast, in the subgroup of BRAF-mutant patients receiving ICI a negative impact of the presence of brain metastasis (HR=5.391; CI=0.998–29.118; P=0.05) was found on long-term survival (OS ≥5 years), in addition to a negative impact of elevated serum LDH (HR=4.973; CI=1.279–19.341; P=0.021); Figures 2C, 3. The other factors included such as liver metastases (no vs. yes; HR=2.021; CI=0.387–10.541; P=0.404), lung metastases (no vs. yes; HR=2.422; CI=0.603–9.721; P=0.212), number of organs affected (<3 vs. ≥3; HR=0.390; CI=0.072–2.103; P=0.274), M category (M1a/B vs. c/d; HR=1.533; CI=0.159–15.140; P=0.705), age (<65 vs. ≥65; HR=0.676; CI=0.180-2.542; P=0.562), sex (male vs. female; HR=0.413; CI=0.099–1.723; P=0.225) showed no statistically significant effect on long-term survival (Figure 2C, Supplementary Table 3).
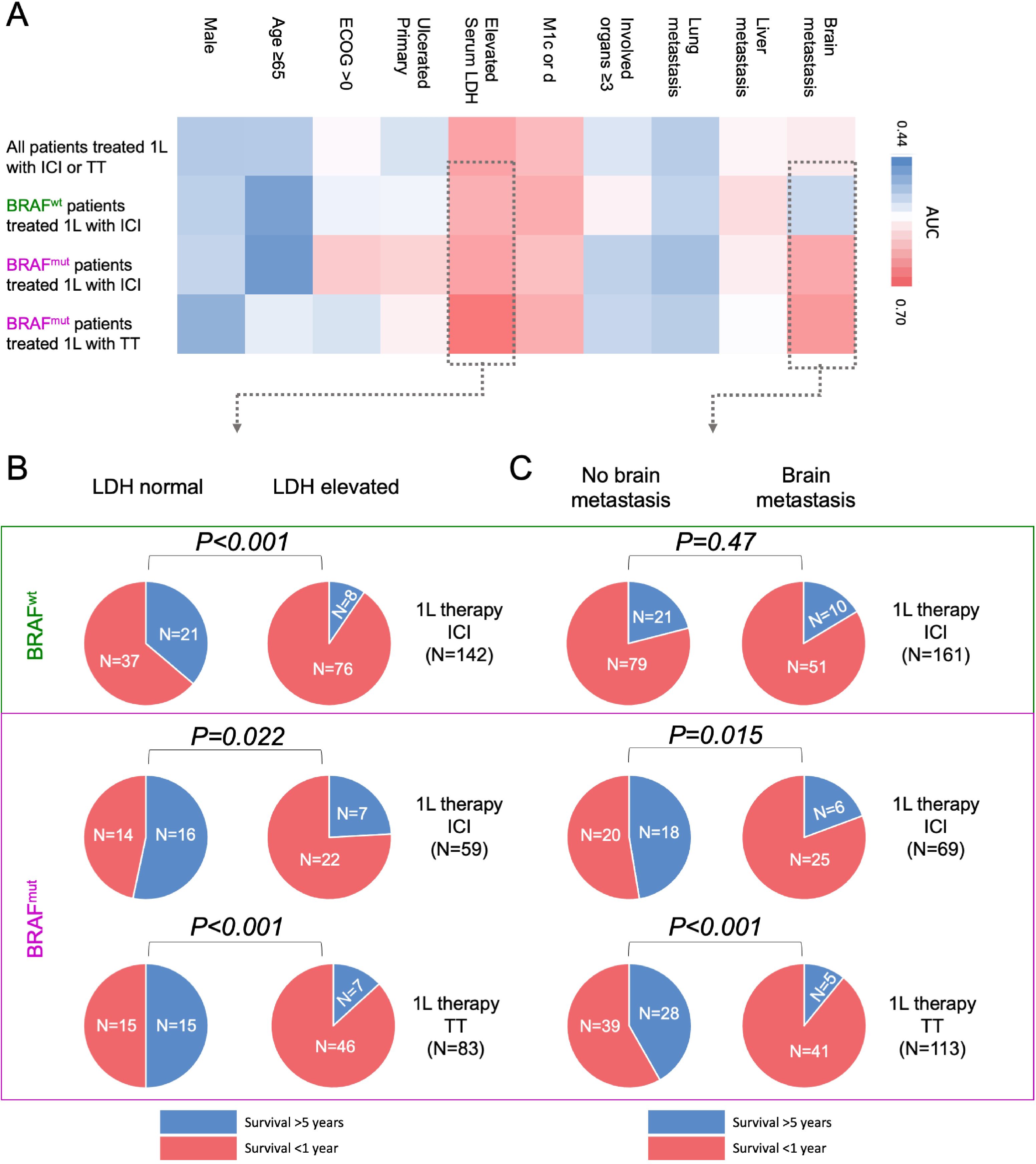
Figure 3. Impact of clinical parameters on long-term versus short-term survival after 1L therapy in stage IV melanoma patients by univariate analysis. (A) The heatmap shows the univariate evaluation of clinical parameters associated with long-term OS ≥5 years versus short-term OS ≤1 year depending on therapy type and BRAF status. Pie charts show the impact of (B) serum LDH, and (C) presence of brain metastasis on long-term OS ≥5 years versus short-term OS ≤1 year in BRAF-wildtype patients (top, green) who received ICI versus BRAF-mutant patients who received ICI or TT (bottom, purple).
Last, the group of BRAF-mutant patients under TT was considered. Here again, an elevated serum LDH level was associated with poor long-term survival (HR=7.124; CI=2.066–24.572; P=0.002). Similarly, the presence of brain metastases was associated with poor OS (HR=4.854; CI=1.186–19.869; P=0.028). The other factors included such as liver metastases (no vs. yes; HR=1.235; CI=0.281–5.439; P=0.780), lung metastases (no vs. yes; HR=1.214; CI=0.360–4.095; P=0.755), number of organs affected (<3 vs. ≥3; HR=0.609; CI=0.149–2.484; P=0.489), M category (M1a/b vs. c/d; HR=1.447; CI=0.229–9.157; P=0.694), age (<65 vs. ≥65 years; HR=1.659; CI=0.513–5.372; P=0.398), sex (male vs. female; HR=0.824; CI=0.220-3.078; P=0.773) had no statistically significant effect on the relative probability of long-term survival (Figure 2D, Supplementary Table 4).
Presence of brain metastases is differentially associated with long-term survival dependent on BRAF mutation statusMultiple receiver operating characteristic (ROC) curve analyses were conducted on the four distinct patient subgroups to corroborate the findings derived from the multivariate analysis. Compared with the other factors tested, increased serum LDH was again negatively associated with long-term survival (OS ≥5 years) in all subgroups; Figure 3A, Supplementary Table 5). Similary to the results from the multinomial regression, ROC analyses found a different survival impact of the presence of brain metastases between BRAF-mutant and BRAF-wildtype melanoma patients. In BRAF-wildtype patients who received ICI, ROC analysis showed no effect of brain metastasis on long-term survival (AUC=0.523, P=0.624). In contrast, for BRAF-mutant patients a negative association between the presence of brain metastasis and long-term survival could be detected, regardless of whether patients received ICI (AUC=0.640; P=0.047) or TT (AUC=0.655; P=0.005) (Figure 3A, Supplementary Table 5).
In a next step, the absolute distribution of patients with normal vs. elevated serum LDH, and absent vs. present brain metastases was considered in terms of long-term vs short-term OS after 1st-line therapy in the total patient cohort as well as in the subgroups. Looking at the distribution of normal versus elevated serum LDH, we found that in BRAF-wildtype patients treated with ICI, patients with normal serum LDH were significantly more likely to survive ≥5 years (P<0.001; Figure 3B). This association could be detected in a similar extent in BRAF-mutant patients, independent of their type of treatment. Thus, BRAF-mutant patients with normal serum LDH at baseline were significantly more likely to survive ≥5 years when treated with ICI (P=0.022). BRAF-mutant patients with normal serum LDH treated with TT also showed significantly higher probability to survive ≥5 years (P<0.001; Figure 3B). In contrast, associations were differentially distributed when considering the presence or absence of brain metastasis. In BRAF-wildtype patients treated with ICI, there was no statistically significant difference between patients with or without brain metastases in terms of OS ≥5 years after therapy start (P=0.472; Figure 3B). However, in BRAF-mutant patients, the presence of brain metastasis had a significant impact on long-term survival independent of treatment type. Thus, in BRAF-mutant patients treated with ICI, patients without brain metastases were significantly more likely to survive ≥5 years (P=0.015). Similarly, BRAF-mutant patients treated with TT had a significantly higher probability of survival ≥5 years if they started treatment without brain metastases (P<0.001; Figure 3C).
DiscussionThe introduction of modern systemic therapies such as BRAF/MEK-directed TT and PD-1-based ICI has led to a significant improvement in overall survival of stage IV melanoma patients with distant metastases (6, 10). In the past, numerous influencing clinical baseline parameters such as ECOG, elevated serum LDH, brain and liver metastases, among others, have been identified to be associated with poorer treatment response, and a shortened progression-free and overall survival (11–14). The data on long-term overall survival of stage IV melanoma patients is currently limited, with published data mostly restricted to analyses of data from clinical trials (RCT). For example, a study published in 2019 examined the long-term survival of BRAF-mutant patients treated with the BRAF/MEK inhibitors dabrafenib and trametinib, and identified performance status, age, sex, number of organ sites with metastasis, and LDH serum level as predictors of survival ≥5 years (6).
In the present study, we investigated real-world patient data from stage IV melanoma patients for predictors of particular survival groups, long-term (≥5 years) versus short-term (≤1 year) OS, and examined differences in special patient subgroups by type of first-line therapy and tumor BRAF mutation status. Regardless of the presence of a BRAF mutation, an elevated serum LDH at baseline was the most significant predictor for a particularly early death, defined as an OS less than one year. An increase in serum LDH level indicates higher metabolic activity of tumor cells and correlates with a higher tumor burden. It is well established that elevated serum LDH levels are associated with poorer patient outcomes, as demonstrated in almost every registration study (4, 6, 10–12). Further subdividing the investigated patients into subgroups by type of first-line therapy, the presence of brain metastases was an independent predictor for survival ≤1 year in BRAF-mutated patients regardless if they received BRAF/MEK-directed TT or PD-1-based ICI. Surprisingly, this association could not be detected in BRAF-wildtype melanoma patients who received ICI.
Interestingly, we found that the presence of a BRAF mutation significantly impacts the long-term survival of melanoma patients with brain metastasis. Specifically, a notable decrease in the probability of achieving long-term survival of more than 5 years was observed in patients with brain metastases harboring a BRAF mutation compared to their wildtype counterparts. One explanation could be a fundamentally higher aggressiveness of BRAF-mutant melanomas, which has been described in several studies conducted prior to the introduction of targeted tumor therapy (15, 16). The data of this investigation correlate with a study from 2023 in which the authors observed that patients with BRAF–mutated melanoma had a low mutational burden in tumor tissue from brain metastases. On a molecular level the study showed a lower infiltration of immune cells in brain metastases of BRAF-mutant patients compared to BRAF-wildtype patients and a resulting significantly shorter survival time in BRAF-mutant patients with brain metastases compared to BRAF-wildtype patients with brain metastases (17).
Our present study and its statistical analysis have some limitations. First, the study is based on real-world patient data, which means that the compared treatment groups are not stratified or balanced. Second, both statistical analysis procedures using chi-square tests and ROC analyses are univariate analyses. With these, there is a risk of bias due to other clinical parameters. Therefore, an additional multivariate analysis was performed. Another limitation is the partial lack of data for some clinical parameters such as ECOG and histopathology. A further limitation of this study is the inclusion of patients treated between May 2010 and October 2021, with a data cutoff in February 2022, which prevents a true evaluation of five-year survival for the most recently treated patients. This is of particular interest, as the approved treatment modalities have changed dramatically over this period.
Taken together, our study results show that serum LDH predicts long-term survival of stage IV melanoma patients independently of treatment type and BRAF mutation status. Brain metastasis has a relevant impact on long-term survival in BRAF-mutated, but not in BRAF-wildtype patients. Investigations correlating additional clinical factors, such as the location, size, and number of brain metastases, with the intracranial therapeutic response in BRAF-mutated and BRAF wild-type patients are urgently needed. Investigation of molecular features of brain metastases in BRAF-mutated vs. BRAF-wildtype melanomas may lead to new insights in tumor biology and may yield new therapeutic approaches. In the future, there may be potential to identify new drug targets for therapy or prevention of brain metastases in melanoma patients by conducting protein expression studies using transcriptomic or proteomic approaches.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Ethics committee of the University Duisburg-Essen. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsJ-MP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Software, Writing – review & editing. LR: Writing – review & editing, Data curation, Investigation. RH: Data curation, Investigation, Writing – review & editing. PT: Data curation, Investigation, Writing – review & editing. JU: Data curation, Investigation, Writing – review & editing. CP: Data curation, Investigation, Writing – review & editing. AK: Data curation, Investigation, Writing – review & editing. PM: Data curation, Investigation, Writing – review & editing. RG: Data curation, Investigation, Writing – review & editing. MW: Data curation, Investigation, Writing – review & editing. FM: Data curation, Investigation, Writing – review & editing. CB: Data curation, Investigation, Writing – review & editing. UL: Data curation, Investigation, Writing – review & editing. JS: Data curation, Investigation, Writing – review & editing. FK: Data curation, Investigation, Writing – review & editing. AT: Data curation, Writing – review & editing. GL: Data curation, Investigation, Writing – review & editing. EL: Data curation, Investigation, Writing – review & editing. LZ: Data curation, Investigation, Writing – review & editing. AR: Data curation, Investigation, Writing – review & editing. KG: Data curation, Investigation, Writing – review & editing. DS: Data curation, Funding acquisition, Investigation, Resources, Writing – review & editing. SU: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. J-MP was supported by the DFG in the framework of the DFG Clinician Scientist Programme UMEA, FU 356/12-1. SU and DS received funding from the Else-Kröner-Fresenius Stiftung (EKFS; University Medicine Essen Medical Scientist Academy UMESciA). SU received funding from the German Cancer Consortium (DKTK; JF 2023 TRICKY).
AcknowledgmentsWe thank the patients who participated in this study, their families, and the staff members at the study sites who cared for them. We acknowledge support by the Open Access Publication Fund of the University of DuisburgEssen.
Conflict of interestJ-MP served as consultant and/or has received honoraria from Bristol-Myers Squibb, Novartis and received travel support from Bristol-Myers Squibb, Pierre Fabre, Novartis and Therakos. RH is employee of Helios Kliniken GmbH. PT has received honoraria from Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, Pierre Fabre, CureVac, Merck Serono, Sanofi, Roche, Kyowa Kirin and Biofrontera; and travel support from Bristol-Myers Squibb and Pierre Fabre. JU received honoraria speaker honoraria or honoraria as a consultant and travel support from Bristol-Myers Squibb, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi/Regeneron, Sunpharma outside the submitted work. CP received honoraria speaker honoraria or honoraria as a consultant and travel support from Novartis, BMS, MSD, Merck Serono, MSD, Celgene, AbbVie, Sunpharma, Pierre Fabre, UCB, Nutricia Milupa, Janssen and LEO, outside the submitted work. AK served as a speaker and/or consultant and/or advisory board for MSD, AbbVie, Boehringer Ingelheim, Janssen, and Sanofi. PR declares research support from Bristol Myers Squibb, Merck Sharp & Dohme and Novartis; speakers and advisory board honoraria from Bristol Myers Squibb, Beiersdorf, Merck Sharp & Dohme, Pierre Fabre, Sun Pharma, Immunocore, Sanofi and Novartis, and travel support from Bristol Myers Squibb, Merck Sharp & Dohme, Sanofi, Sun Pharma, and Pierre Fabre, outside the submitted work. RG received honoraria as speaker from BMS, MSD, Novartis, Amgen, Merck Serono, Almirall Hermal, SUN, Sanofi/Regeneron, Pierre-Fabre, as advisory board member from BMS, Novartis, Almirall Hermal, MSD, Amgen, SUN, Sanofi/Regeneron, Pierre-Fabre, 4SC, MerckSerono, Pfizer, Immunocore, Delcath, for meeting support from SUN, Pierre-Fabre, Boehringer Ingelheim and for research projects to institution from Amgen, Merck-Serono, SUN Pharma, Sanofi/Regeneron, Kyowa-Kirin, Almirall-Hermal. MW received grants from Bristol-Myers Squibb and Merck Sharp & Dohme, consulting fees from Merck Sharp & Dohme, Immunocore and Novartis, lecture honoraria from Bristol-Myers Squibb and Merck Sharp & Dohme and Pierre-Fabre, and advisory board honoraria from Merck Sharp & Dohme. CB has received speaker’s fees or/and advisor’s honoraria by Almirall-Hermal, Novartis, Roche, BMS, MSD, Delcath, Pierre Fabre, Regeneron, Sanofi, and Immunocore. FM has received travel support or/and speaker’s fees or/and advisor’s honoraria by Novartis, Roche, BMS, MSD, Pierre Fabre, Sanofi and Immunocore and research funding from Novartis and Roche. UL reports relevant financial activities research support from Merck Sharp and Dohme; speakers and advisory board honoraria from Merck Sharp and Dohme, Novartis and Roche, Sanofi Aventis and travel support from Sun Pharma. GL received travel support from Sun Pharma, Pierre Fabre, research funding from Novartis. FK received travel support for participation in congresses and/or speaker honoraria from Novartis, Lilly, Bristol-Myers Squibb, Janssen, Pierre Fabre, Almirall, and Boehringer Ingelheim outside of the present publication. EL served as consultant and/or has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre-Fabre, Sanofi, Sunpharma, Takeda and travel support from Bristol-Myers Squibb, Pierre- Fabre, Sunpharma and Novartis, outside the submitted work. LS served as consultant and has received honoraria from BMS, MSD, Novartis, Pierre Fabre, Sanofi, and Sunpharma and travel support from MSD, BMS, Pierre Fabre, Sanofi, Sunpharma and Novartis, outside the submitted work. AR reports grants from Novartis, Bristol Myers Squibb and Adtec; personal fees from Merck Sharp & Dohme; and nonfinancial support from Amgen, Roche, Merck Sharp & Dohme, Novartis, Bristol Myers Squibb and Teva. DS reports partial financial support from Bristol Myers Squibb for the conduct of this study and drug supply nivolumab and ipilimumab support; grants or contracts from Amgen, Array/Pfizer, Bristol-Myers Squibb, MSD, Novartis and Roche; consulting fees from 4SC, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Haystick, Immunocore, InFlarX, Innocent, LabCorp, Merck Serono, MSD, Nektar, NeraCare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron, Sun Pharma; honoraria from Bristol-Myers Squibb, MSD/Merck, Merck Serono, Novartis, Roche, Sanofi and Sun Pharma; support for attendings meetings or travel support from Bristol-Myers Squibb, MSD, Merck Serono, Novartis, Pierre Fabre and Sanofi; participation on drug safety monitoring or advisory boards for 4SC, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Immunocore, InFlarX, Merck Serono, MSD, Nektar, NeraCare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron and SunPharma; leadership roles for DeCOG, German Cancer Society, Hiege-Stiftung, Deutsche Hautkrebsstiftung, NVKH e.V. and EuMelaReg. SU declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Novartis and Roche, and travel support from Bristol Myers Squibb, and Merck Sharp & Dohme.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that Generative AI was used in the creation of this manuscript. The translation platform DeepL was used to help expand vocabulary and improve the stylistics of written English in the manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1536642/full#supplementary-material
Supplementary Table 1 | Multivariate analysis on baseline characteristics with regard to longterm overall survival. Multinomial regression analysis examining the impact of baseline characteristics on OS ≥5 years after start of 1L ICI or TT therapy in n=280 stage IV melanoma patients.
Supplementary Table 2 | Multivariate analysis on baseline characteristics with regard to longterm overall survival (BRAF wildtype). Multinomial regression analysis examining the impact of baseline characteristics on OS ≥5 years after start of 1L ICI therapy in n=139 stage IV BRAF-wildtype melanoma patients.
Supplementary Table 3 | Multivariate analysis on baseline characteristics with regard to longterm overall survival (BRAF mutant). Multinomial regression analysis examining the impact of baseline characteristics on OS ≥5 years after start of 1L ICI therapy in n=59 stage IV BRAF-mutnat melanoma patients.
Supplementary Table 4 | Multinomial regression analysis examining the impact of baseline characteristics on OS ≥5 years after start of 1L TT therapy in n=82 stage IV BRAF-mutant melanoma patients.
Supplementary Table 5 | ROC analysis for OS ≥5 years in total patients and subgroups.
References2. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
PubMed Abstract | Crossref Full Text | Google Scholar
3. Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. New Engl J Med. (2014) 372:30–9. doi: 10.1056/NEJMoa1412690
PubMed Abstract | Crossref Full Text | Google Scholar
4. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. New Engl J Med. (2014) 372:320–30. doi: 10.1056/NEJMoa1412082
PubMed Abstract | Crossref Full Text | Google Scholar
5. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl J Med. (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
PubMed Abstract | Crossref Full Text | Google Scholar
6. Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. (2019) 381:626–36. doi: 10.1056/NEJMoa1904059
PubMed Abstract | Crossref Full Text | Google Scholar
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2022) 40:127–37. doi: 10.1200/JCO.21.02229
PubMed Abstract | Crossref Full Text | Google Scholar
8. Michielin O, Atkins MB, Koon HB, Dummer R, Ascierto PA. Evolving impact of long-term survival results on metastatic melanoma treatment. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-000948
PubMed Abstract | Crossref Full Text | Google Scholar
10. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
PubMed Abstract | Crossref Full Text | Google Scholar
11. Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. (2016) 114:256–61. doi: 10.1038/bjc.2015.467
PubMed Abstract | Crossref Full Text | Google Scholar
12. Hauschild A, Larkin J, Ribas A, Dréno B, Flaherty KT, Ascierto PA, et al. Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600–mutated metastatic melanoma: pooled analysis of 4 randomized clinical trials. JAMA Oncol. (2018) 4:1382–8. doi: 10.1001/jamaoncol.2018.2668
PubMed Abstract | Crossref Full Text | Google Scholar
13. Pires da Silva I, Ahmed T, McQuade JL, Nebhan CA, Park JJ, Versluis JM, et al. Clinical models to define response and survival with anti–PD-1 antibodies alone or combined with ipilimumab in metastatic melanoma. J Clin Oncol. (2022) 40:1068–80. doi: 10.1200/JCO.21.01701
PubMed Abstract | Crossref Full Text | Google Scholar
14. Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. (2017) 82:45–55. doi: 10.1016/j.ejca.2017.05.033
PubMed Abstract | Crossref Full Text | Google Scholar
15. Shinozaki M, O'Day SJ, Kitago M, Amersi F, Kuo C, Kim J, et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. (2007) 13:2068–74. doi: 10.1158/1078-0432.CCR-06-2120
PubMed Abstract | Crossref Full Text | Google Scholar
16. Ugurel S, Thirumaran RK, Bloethner S, Gast A, Sucker A, Mueller-Berghaus J, et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PloS One. (2007) 2:e236. doi: 10.1371/journal.pone.0000236
PubMed Abstract | Crossref Full Text | Google Scholar
17. Vasudevan HN, Delley C, Chen WC, Mirchia K, Pan S, Shukla P, et al. Molecular features of resected melanoma brain metastases, clinical outcomes, and responses to immunotherapy. JAMA Netw Open. (2023) 6:e2329186. doi: 10.1001/jamanetworkopen.2023.29186
留言 (0)