DM is a major global health issue characterized by persistent hyperglycemia due to insufficient insulin secretion or action (Ill-Min et al., 2020; Constanze et al., 2017; Zhimei et al., 2019). The main symptoms include increased appetite, thirst, urination, and weight loss (Diagnosis and Classification of Diabetes Mellitus, 2013). In 2021, approximately 536.6 million people aged 20–79 were diagnosed with diabetes globally, and this number is projected to reach 783.2 million by 2045 (Hong et al., 2022). Diabetes is triggered by genetic and environmental factors, leading to the autoimmune destruction of pancreatic-β-cells, impairing insulin production, and disrupting metabolic processes, resulting in hyperglycemia (Décio et al., 2020; Aleksey and Peter, 2008; Kathryn et al., 2013). Prolonged hyperglycemia can lead to severe complications such as diabetic nephropathy, neuropathy, retinopathy, and cardiomyopathy (Janika et al., 2022; David et al., 2023) (Figure 1). Cardiovascular diseases are the leading cause of death among diabetic patients, particularly cardiac complications (Justin et al., 2016; Anoop et al., 2015).
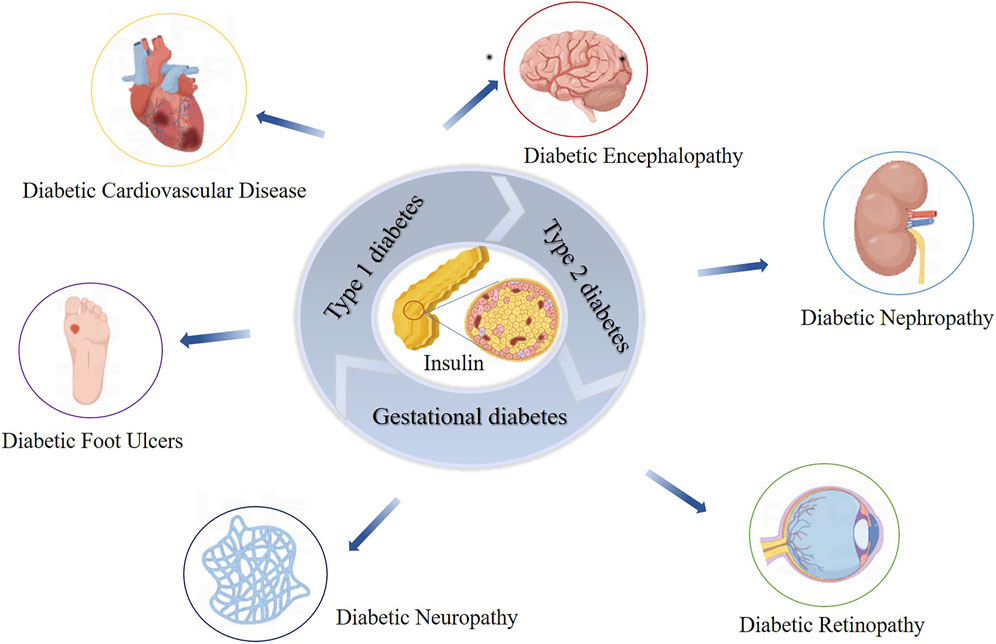
Figure 1. Impact of Insulin Dysfunction on Diabetes Mellitus and Related Complications. This figure illustrates the impact of insulin dysfunction on the development of diabetes mellitus and its associated complications. It shows how impaired insulin action leads to widespread metabolic disturbances, culminating in serious health issues such as nephropathy, retinopathy, neuropathy, cardiovascular disease, and diabetic ulcers.
Over the past few decades, several pharmacological agents have been developed to regulate blood glucose. Metformin primarily reduces hepatic gluconeogenesis through the activation of AMP-activated protein kinase (AMPK), while sulfonylureas (e.g., glimepiride) enhance insulin secretion by closing pancreatic β-cell ATP-sensitive potassium channels (Reuben et al., 2005; Gaochao et al., 2001; Mary, 2004). Thiazolidinediones (e.g., pioglitazone) improve insulin sensitivity via peroxisome proliferator-activated receptor gamma (PPAR-γ) modulation (Aya et al., 2020; Zhiwei et al., 2021). More recent approaches include dipeptidyl peptidase-4 (DPP-4) inhibitors, which prolong the half-life of incretin hormones, and glucagon-like peptide-1 (GLP-1) receptor agonists, which stimulate insulin secretion and reduce glucagon levels (Daniel and Michael, 2006). Additionally, sodium-glucose cotransporter 2 (SGLT2) inhibitors lower blood glucose by promoting urinary glucose excretion (André, 2019). Current antidiabetic drugs mainly control blood glucose levels but cannot prevent or reverse complications (Dandan et al., 2020). Therefore, effective therapeutic strategies are urgently needed to halt or mitigate the progression of diabetes and its complications.
PCs, composed of flavan-3-ol and flavan-3, 4-diol units, include catechins, apocynin, and gallocatechins (Chunhui, 2014; Liwei et al., 2002; Linards et al., 2022; Liang et al., 2016). Their main sources encompass grains, legumes, fruits, chocolate, and drinks like tea and wine (Abdur et al., 2019; Feng et al., 2016) (Figure 2). PCs exhibit diverse bioactivities such as anti-obesity, anti-diabetic, anti-cancer, anti-inflammatory, antioxidant, and cardiovascular protective effects (Eskandar et al., 2023; Antoni et al., 2012; Qian et al., 2023; Chuntang et al., 2022; Van Long et al., 2014; Liang et al., 2012). Studies suggest that PCs can lower blood glucose, improve insulin resistance, regulate insulin secretion, protect pancreatic β-cells in diabetic patients, and effectively alleviate diabetes complications (Javier et al., 2018; Suzanne et al., 2017; Anna et al., 2012). This review summarizes the molecular mechanisms and protective effects of PCs in diabetes treatment, which are shown in Table 1.
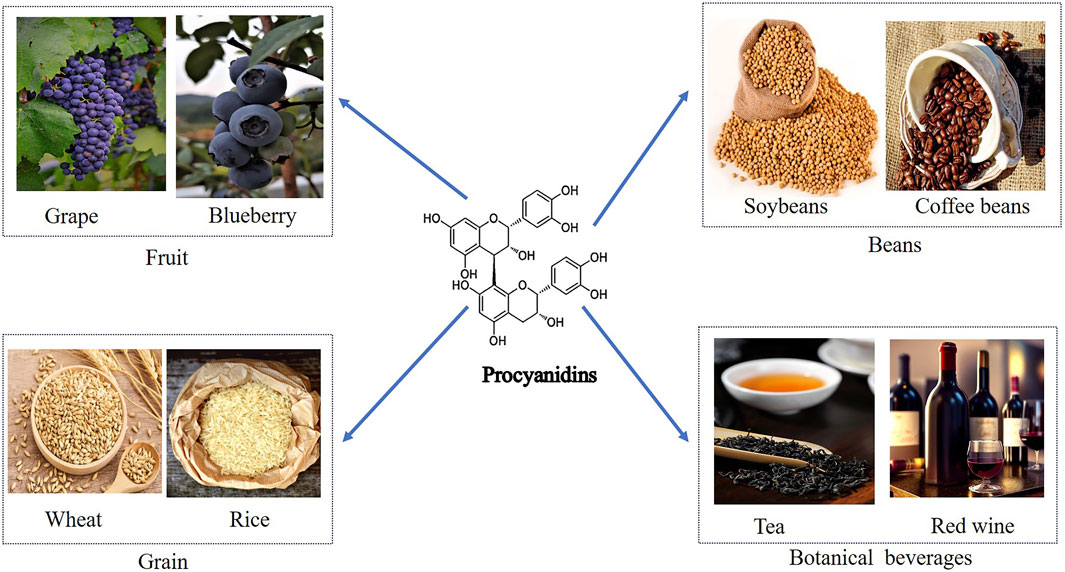
Figure 2. Dietary Sources of Procyanidins and Its Chemical Structure. This figure displays the major dietary sources of PCs and its chemical structure. The sources are grouped into categories including fruits like grapes and blueberries, beans such as soybeans and coffee beans, grains like wheat and rice, and botanical beverages including tea and red wine. The centralfigure illustrates the molecular structure of PCs, emphasizing its presence across diverse food groups.
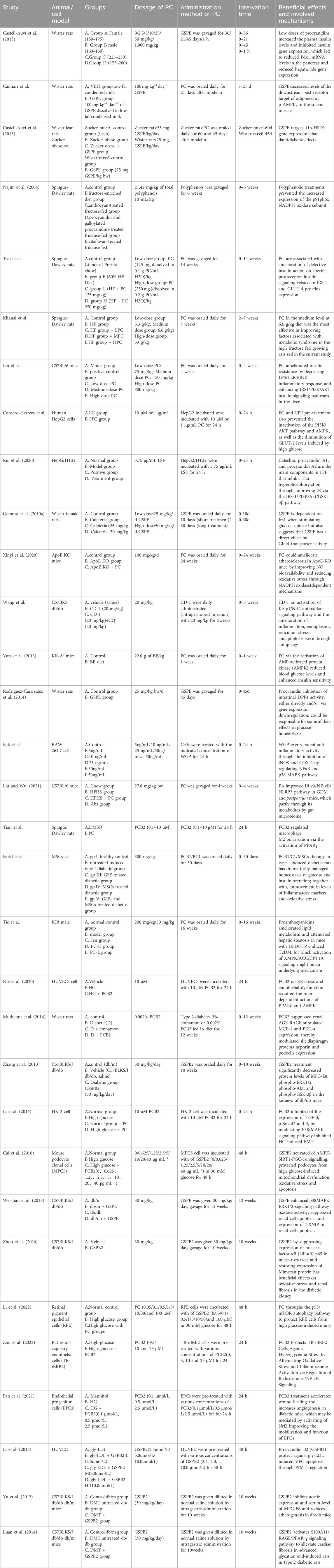
Table 1. Characteristics of the included studies.
2 Pharmacokinetics of procyanidinsPCs are characterized by their double and single bonds, with catechols or epicatechols as fundamental components (Kaul, 1996). The health benefits of PCs are constrained by their low oral bioavailability due to incomplete intestinal absorption and extensive metabolism and excretion (Brigitte et al., 1989; Yue et al., 2021; Claudine et al., 2005; Ying Yng et al., 2014). It is estimated that over 90% of dietary polyphenols remain in the colon, with only a small fraction being indirectly absorbed in the small intestine through interactions with carbohydrates, fibers, and proteins (Michael, 2004; Gary and Claudine, 2005). Multiple factors influence the hydrolytic absorption of PCs, including gastrointestinal pH, dietary composition, and solubility in gastric and intestinal fluids. The acidic environment of the stomach may facilitate initial PCs release from food matrices but can also degrade certain oligomers, reducing their structural integrity and bioavailability. In the small intestine, dietary fibers and proteins can form complexes with PCs, limiting absorption, whereas lipids may enhance PCs solubility through micelle or emulsion formation. Tannins can further decrease bioavailability by competing for binding sites or forming insoluble complexes. Once in the colon, unabsorbed PCs oligomers and polymers interact with enterocyte membrane proteins, potentially modulating metabolic pathways (Liang et al., 2016). Meanwhile, gut microbiota convert these higher-order PCs into smaller phenolic acids (e.g., hydroxybenzoic, hydroxyphenylacetic, and hydroxycinnamic acids) with better solubility and increased absorption (Maaike et al., 2009). Ultimately, these metabolites are excreted in the urine (Figure 3).
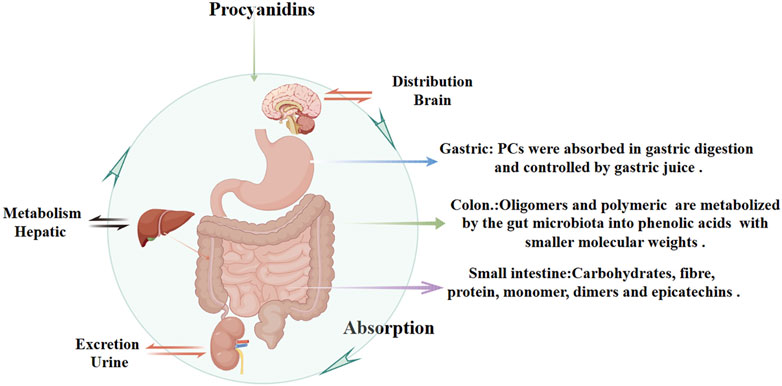
Figure 3. Pharmacokinetics of Procyanidins. This figure illustrates the absorption, distribution, metabolism, and excretion of PCs. PCs are partially absorbed in the stomach and small intestine, with interactions involving dietary components like carbohydrates, fibers, and proteins. In the colon, unabsorbed PCs are metabolized by gut microbiota into phenolic acids with smaller molecular weights, improving solubility and systemic absorption. Hepatic metabolism further processes PCs, and their metabolites are eventually excreted through urine.
Low bioavailability remains a major hurdle for harnessing the therapeutic benefits of PCs, necessitating innovative delivery approaches such as nano-encapsulation, phospholipid complexation, and co-administration with permeability enhancers. These strategies aim to stabilize PCs structure against pH-induced degradation, improve solubility, and promote systemic circulation. Further studies are required to elucidate the optimal conditions, formulations, and dietary adjuncts that maximize PCs efficacy in diabetes management. By advancing our understanding of PCs pharmacokinetics, including absorption, distribution, metabolism, and excretion, and refining targeted formulations, we can better leverage their therapeutic potential in preventing and managing diabetes and its complications.
3 Methods3.1 Literature searchWe conducted a comprehensive search on the PubMed (www.pubmed.com) and Web of Science (www.webofscience.com) databases for articles on procyanidins and diabetes published from 1 January 2000, to 30 December 2024. The research methodology is illustrated in Figure 4.
Figure 4. Literature screening process diagram.
3.2 Search strategyProcyanidins OR Procyanidin B2 OR GSPE OR Procyanidin B3 OR Catechin OR flavan-3-ol) AND (Diabetes mellitus OR diabetic nephropathy OR diabetic retinopathy OR diabetic neuropathy OR diabetic foot ulcer OR diabetic encephalopathy OR diabetic cardiovascular disease OR diabetic cardiomyopathy.
3.3 Inclusion criteria(1): The document type is limited to original research articles or reviews; (2) Relevant to the topic of “procyanidins” and “diabetes”.
3.4 Exclusion criteria(1) Irrelevant to the objective of this review; (2) Duplicated or outdated literature.
4 Mechanism of the protective effect of PCs on diabetes mellitus4.1 Improve the insulin resistanceInsulin, an essential hormone secreted by pancreatic β-cells, regulates glucose metabolism and exerts a profound influence on blood glucose levels (Gary and Patricia, 2021). Typically, insulin binds to receptors on the surfaces of target cells, thereby reducing hepatic glucose output and promoting glucose uptake in skeletal muscle and adipose tissue (Benedetta et al., 2019). When various internal and external factors drive target cells toward insulin resistance, greater amounts of insulin are required to trigger glucose transport and utilization. This phenomenon, referred to as insulin resistance, features elevated glucose and insulin levels in the bloodstream (Rosalyn and Solomon, 1960; Christian et al., 2013).
Located in the cell membrane, the insulin receptor exhibits a tetrameric structure with two α-chains and two β-chains, serving as the primary hub for insulin action and orchestrating the insulin signaling cascade. Upon binding to the α-chain, insulin governs glucose transport and adiposity and modulates cell proliferation and differentiation. This interaction enhances gluconeogenesis while suppressing adipose tissue catabolism (Marinko et al., 2021). When insulin engages the β-chain, it triggers the insulin receptor substrate (IRS1/2), thereby boosting glucose metabolism (via the PI3K/PKB/AKT pathway), protein synthesis (via the TSC1/2-mTOR pathway), and cell proliferation and differentiation (via the RAS-MAPK-ERK1/2 pathway) (Rebecca et al., 2017; Barbara Di et al., 2016; Teri et al., 1991). Interruptions throughout the insulin signaling pathway can ultimately lead to insulin resistance. Often, diverse kinases and phosphatases—for instance, phosphorylated tyrosine—amplify serine/threonine phosphorylation, inactivating IRS proteins and worsening insulin resistance (Kyle and Morris, 2012). Additionally, processes involving kappa kinase β (IKK-β), c-Jun N-terminal kinase (JNK-1), protein kinase C (PKC), and mammalian target of rapamycin (mTOR)-driven serine modification of IRS1 also contribute to insulin resistance, further intensified by free fatty acids, lipotoxicity, oxidative stress, and inflammation (Stergios et al., 2009). PCs are frequently cited for their ability to improve insulin sensitivity in experimentally induced diabetic rats (Antoni et al., 2017; Anna et al., 2013). Previous studies underscore the marked antihyperglycemic effects of PCs in rats maintained on a high-fructose diet (Najim et al., 2004; Takako et al., 2008; Hsiang-Jung et al., 2008). Ramesh CK and colleagues found that PCs extracts from cranberries and blueberries, administered prophylactically, significantly mitigated insulin resistance, boosted glucose sensitivity, and lowered blood glucose in diabetic rats fed a high-fructose diet, reinforcing the therapeutic value of PCs (Ramesh et al., 2010; Ramesh et al., 2012). M Liu et al. provided PCs to T2D mice (75, 150, and 300 mg/kg) via continuous gavage for 4 weeks, documenting improvements in intestinal barrier integrity by restoring morphology and raising the expression of tight junction proteins, including Zonula occludens-1 (ZO-1), Claudin-1, and occludin. This approach substantially reduced insulin resistance, promoted glucose uptake, and lowered blood glucose levels by activating the IRS1/PI3K/AKT signaling pathway (Min et al., 2022). Further in vitro research corroborated these results. I Cordero-Herrera et al. cultured insulin-sensitive human HepG2 cells under high-glucose conditions and administered cocoa-derived polyphenols and epicatechin, which blocked tyrosine phosphorylation and lowered total IR, IRS-1, and IRS-2 levels through PI3K/AKT and AMPK pathway inhibition, thereby mitigating high-glucose-induced insulin signaling impairment that alters gluconeogenesis (Isabel et al., 2014). The activation and subsequent engagement of the insulin receptor (IR) and its downstream mediators, IRS-1 and IRS-2, remain critical for regulating insulin signaling (Mary et al., 2002). In hepatic insulin resistance, normal tyrosine phosphorylation of IR and IRS is compromised, diminishing PI3K activity associated with IRS. Conversely, the oligomeric form of grape seed procyanidin (GSPE) extract induces tyrosine phosphorylation, thereby activating the IR and decreasing AKT and GSK-3β phosphorylation via PI3K/AKT pathway stimulation. This process augments GS phosphorylation, thereby improving hepatic insulin sensitivity (Aurèle et al., 2019; Rui et al., 2020; Gemma et al., 2010a) (Figure 5). Meanwhile, a glucose-lowering formulation containing hawthorn polyphenols, D-chiro-inositol, and epigallocatechin gallate exhibited synergistic hypoglycemic activity, markedly improving insulin resistance in mice, decreasing fasting blood glucose and hepatic gluconeogenesis, and enhancing hepatic glycogen synthesis and storage (Chao et al., 2021). Additionally, a randomized controlled trial revealed that a combination of flavan-3-ols and isoflavones enhanced insulin sensitivity and lipoprotein profiles compared to placebo, further lowering the estimated 10-year CVD risk in women with type 2 diabetes (Peter et al., 2012).
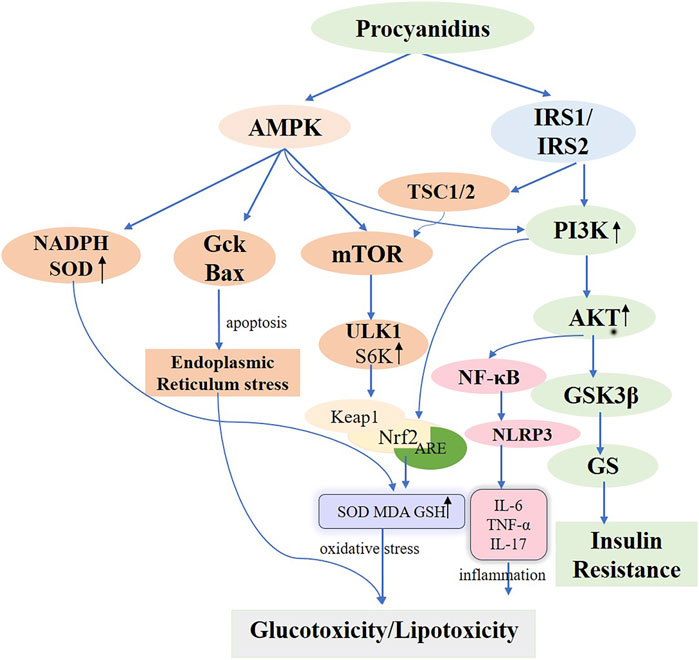
Figure 5. Molecular Mechanisms of Procyanidins in Modulating Diabetes Mellitus. This figure delineates the comprehensive molecular pathways impacted by PCs in the management of DM. PCs enhance insulin sensitivity by activating PI3K/AKT signaling via IRS1/IRS2 and reduce insulin resistance through GSK3β inhibition. PCs also alleviate oxidative stress by activating AMPK and Nrf2 pathways, increasing antioxidants like SOD and GSH, while reducing inflammatory responses mediated by NF-κB and NLRP3. Additionally, PCs modulate cellular stress and autophagy, mitigating endoplasmic reticulum stress and promoting metabolic homeostasis. These mechanisms collectively address glucotoxicity, lipotoxicity, and insulin resistance in diabetes.
In summary, under insulin-resistant conditions, polyphenolic PCs can effectively bolster cellular insulin sensitivity. However, prior investigations have yet to establish the ideal PCs concentration and dose for mitigating insulin resistance or to clarify the therapeutic distinctions among active metabolites from diverse sources. Future studies should center on identifying effective concentrations and characterizing botanical metabolites derived from multiple sources.
4.2 Reduced glucotoxicity and lipotoxicityGlucotoxicity is characterized by irreversible tissue and cell damage resulting from insulin resistance and persistently elevated glucose levels stemming from excessive carbohydrate intake (Mota et al., 2016). The primary cause of glucotoxicity is insulin resistance, which arises from defective islet cell function and damage caused by reduced insulin mRNA expression, associated with diminished transcription or activity of transcription factors involved in insulin production (Daniël and Michaëla, 2011). Insulin resistance significantly contributes to hyperglycemia-induced glucotoxicity. Excess carbohydrates are metabolized into free fatty acids and triglycerides, promoting adipogenesis and lipoatrophy through the activation of enzymes like acetyl coenzyme A carboxylase (ACC), fatty acid synthase (FAS), and SCD-1 (Mota et al., 2016). These processes are influenced by endoplasmic reticulum (ER) stress, oxidative stress, mitochondrial dysfunction, and islet inflammation (Maria et al., 2020; Wenqian et al., 2018; Jiwon and Kun-Ho, 2011).
Prolonged exposure to elevated fatty acid levels triggers lipotoxicity, compromising pancreatic β-cell functionality and viability. This condition activates multiple stress pathways, resulting in β-cell impairment and mortality. ER stress and oxidative stress are especially well-documented contributors to this effect (Kerry et al., 2005; Eloisa Aparecida et al., 2021; Andrei et al., 2007). Cellular lipotoxicity, also known as glycolipotoxicity, is exacerbated by elevated glucose levels (Yiqi et al., 2022). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), a producer of reactive oxygen species (ROS), acts as a key generator of oxidative stress within pancreatic islet β-cells, significantly affecting glucose-induced insulin release and β-cell viability in Type 1 and Type 2 diabetes (Eloisa Aparecida et al., 2021). Animal studies demonstrate that palmitate impairs insulin secretion by upregulating the p47phox NOX subunit protein within rat pancreatic islets (Marta et al., 2010). Shuang et al. (2017) demonstrated that PCs from lychee pericarp decrease mRNA levels of cellular NADPH oxidase subunits (p47phox, p67phox, NOXJ2/gp91phox, and NOXJ4) and enhance superoxide dismutase (SOD) activity, thereby providing a strong antioxidant effect. Additionally, Xinyi et al. (2020) found that cinnamon tannin D1, an A-type PCs oligomer derived from Cinnamomum cinnamomi, protects pancreatic β-cells against glucotoxicity induced by high glucose and palmitic acid, both in vitro and in vivo. The key process entails CD-1 initiating autophagy in pancreatic β-cells via the AMPK/mTOR/UNC-52-like kinase 1 (ULK1) pathway. This autophagic response reduces cell damage by activating the Keap1/Nrf2 antioxidant pathway, which is associated with Kelch-like ECH protein 1 and nuclear factor erythroid 2-related factor 2, thereby mitigating inflammation, endoplasmic reticulum stress, and apoptosis. Consequently, this lowers hyperglycemia and reduces glycotoxicity in diabetic db/db mice (Figure 5). A clinical study found (Hadi et al., 2020) that supplementation with epigallocatechin-3-gallate significantly increased serum total antioxidant capacity (TAC) levels in patients with type 2 diabetes, thereby enhancing the body’s overall antioxidant capacity.
In summary, this section systematically elucidates the mechanisms through which glucotoxicity and lipotoxicity impact pancreatic β-cell function and survival, leveraging both literature and experimental evidence to underscore the potential application of PCs in mitigating these pathological processes. In particular, PCs extracted from lychee pericarp and A-type PCs oligomers (CD-1) derived from cinnamon exhibit promising effects on autophagy activation, mitigation of β-cell damage, and enhancement of glucose metabolism. However, a notable gap remains between these findings and their clinical application. First, many uncertainties exist regarding the absorption, distribution, and metabolism of PCs in humans, including their bioavailability and potential side effects. Moreover, given the trend towards precision medicine, which accounts for individual differences, factors such as a patient’s disease stage, comorbidities, and genetic background must also be considered to fully assess the clinical translational potential of these active compounds in diabetes management. Only by bridging laboratory research with evidence-based medicine can we fully harness the potential of these active components in suppressing glucolipotoxicity and reducing the risk of diabetic complications.
4.3 Maintains glucose homeostasisMaintaining glucose homeostasis is crucial for providing energy to vital organs and sustaining overall health. The liver is key in this dynamic, participating in processes such as glucose synthesis, glycogenolysis, glycolysis, gluconeogenesis, and glucose metabolism. Insulin, primarily stimulated by glucose, is secreted by beta cells under normal physiological conditions. During fasting, the liver generates glucose to fuel the brain, muscles, and adipose tissues, collectively regulating glucose uptake (Xinyi et al., 2020; Wei et al., 2017). PCs regulate glucose uptake, effectively lowering glucose levels through this mechanism (Montserrat et al., 2012). Recent research has underscored the importance of AMPK in managing metabolic conditions, including diabetes, obesity, and fatty liver disease (Sébastien and Reuben, 2017). Furthermore, AMPK boosts glucose absorption in insulin-responsive tissues such as adipocytes, muscle cells, and hepatocytes, proving effective in enhancing glucose uptake in vitro (Zhang et al., 2009; Yuta et al., 2013; Montserrat et al., 2004; Isabel et al., 2015). Elevated phosphorylation levels of AMPK were noted in the liver, muscle tissue, and adipocytes of mice subjected to insulin resistance from high-fat diets and streptozotocin (STZ)-induced diabetes models (Masahito et al., 2010; Peiling et al., 2013). Yuta et al. (2013), Ken-yu et al. (2022) utilized anthocyanins extracted from black beans to treat type 2 diabetic mice, activating AMPK in skeletal muscle cells to improve glucose utilization and decrease gluconeogenesis, while concurrently down-regulating phosphoenolpyruvate carboxykinase (PEPCK) and glucokinase (GK) activities—crucial enzymes in glycolysis that boost intracellular glucose catabolism.
Glucose Transporter Type 4 (GLUT4), a crucial glucose transport regulator, is predominantly found in adipose, skeletal, and heart muscles. Significantly, skeletal muscle serves as a crucial target for managing hyperglycemia, accounting for roughly 80% of postprandial insulin-mediated glucose absorption and is essential for maintaining glucose equilibrium (Nia et al., 2002; Alan and Kahn, 2001). PCs enhance glucose absorption, facilitate GLUT4 translocation in mouse L6 myotubes, and promote glucose uptake within skeletal muscle via insulin and AMPK-dependent pathways (Miki et al., 2011). G Montagut et al. and HH Lee et al. showed that enhanced GLUT4 translocation increases Akt phosphorylation in adipocytes, further boosting a critical pathway in the insulin signaling cascade (Gemma et al., 2010b; Hee-Hyun et al., 2010). Additionally, PCs affect glucose synthesis and metabolism by boosting the functions of enzymes including glucokinase, hexokinase, and glycogen synthase, and reducing the functions of liver enzymes like glucose-6-phosphatase, phosphoenolpyruvate carboxykinase, and fructose-1,6-bisphosphatase (Zhang et al., 2009; Gopalsamy Rajiv et al., 2011) (Figure 5).
GLP-1, a crucial hormone linked to intestinal insulin, is secreted by L-cells within the intestinal lining and remains vital for human glucose metabolism. Studies suggest that polyphenols can stimulate GLP-1 secretion, helping to manage insulin resistance, hyperglycemia, and type 2 diabetes (Dao et al., 2011). Recent studies have demonstrated that botanical extracts emulate the actions of gut insulinotropic hormones. For example, PCs inhibit DPP-4 (the catabolic enzyme of GLP-1) expression, and tetrameric PCs cinnamon tannin A2 enhances GLP-1 secretion within 1 hour of ingestion (Noemi et al., 2012; Eun-Jung et al., 2011). Meanwhile, researchers noted that oligomers and polymers of PCs enhance GLP-1 activity and insulin secretion independently of glucose loading. This finding indicates that PCs may target L cells in the intestines as a crucial regulator of blood glucose, though the exact mechanism is yet to be elucidated and warrants further investigation (Yoko et al., 2019).
In summary, PCs regulate the function of glucose-targeting organs, modulate glucose metabolism and synthesis, and maintain glucose homeostasis through multiple pathways. However, the above studies have not clarified the differences in efficacy between PCs structures and total extracts, nor the specific targets of individual metabolites. Therefore, future research should focus on these aspects to provide more targeted options for clinical applications.
4.4 Regulation of pancreatic beta cell functionInsulin secretion by pancreatic β-cells is crucial for preserving glucose metabolic homeostasis. Research indicates that PCs modulate islet fibrosis, enhance pancreatic β-cell function and morphology, and promote insulin release in Wistar rats with high-fat-diet-induced diabetes (Anna et al., 2012). The amount of insulin secreted by β-cells hinges on the number of insulin-producing cells. Under insulin-resistant conditions, a direct relationship emerges between β-cell count and insulin demand. Often, a persistent imbalance between β-cell proliferation and cell death sets the stage for type 2 diabetes (Laura et al., 2007). The influence of PCs on apoptosis varies among different cell types. Research has shown that PCs display chemopreventive properties in cancer cells by enhancing apoptosis and suppressing proliferation (Giovanna et al., 2024). Similarly, PCs demonstrate parallel effects on pancreatic β-cell lines. Lídia et al. (2013) established an in vitro model of insulin resistance and diabetes using the pancreatic INS-1E β-cell line. This approach involved subjecting INS-1E β-cells to high glucose and fatty acid conditions to induce dysfunction. The researchers investigated how elevated glucose, fatty acids, and PCs affect glucose- and palmitate-induced apoptosis in INS-1E cells. They found that PCs amplified glucose-induced apoptosis yet did not alter palmitate-induced β-cell apoptosis. This discrepancy likely arises because these two triggers engage different mechanisms underlying INS-1E cell dysfunction. Research indicates that elevated glucose levels diminish glucokinase (Gck) protein expression in pancreatic islet cells, limiting its interaction with voltage-dependent anion channels (VDAC) in the mitochondrial outer membrane. Conversely, palmitic acid triggers apoptosis via LC-CoA or other metabolites, in conjunction with endoplasmic reticulum stress and mitochondrial dysfunction. The results suggest that PCs augment high glucose-induced apoptosis in pancreatic β-cells by increasing glucose uptake under high glucose conditions, potentially via a mechanism that downregulates Gck and promotes Bax protein translocation and oligomerization at the mitochondrial membrane. Furthermore, the study showed that PCs curtail cell proliferation induced by glucose, insulin, and palmitate, although the precise mechanism remains unclear. Meanwhile, epigallocatechin gallate (EGCG), used alone or together with GLP-1 agonist exendin-4, was shown to enlarge islet area and number, expand β-cell area, and boost pancreatic insulin content in diabetic and obese mouse models, thus demonstrating favorable therapeutic outcomes (Nupur et al., 2018).
The onset of diabetes and its associated complications are tightly linked to oxidative stress and inflammation. Elevated amounts of oxidative stressors and inflammatory cytokines are closely associated with pancreatic harm in diabetic laboratory rats. Therefore, reducing oxidative stress and cytokines could represent one pathway through which PCs safeguard pancreatic islet cells. Nrf2 is pivotal in the regulation of intracellular antioxidant defense mechanisms. Mahmoud MF and his team (Mona et al., 2021) noted significant elevations in Nrf2 and HO-1 levels within the pancreatic tissues of rats suffering from STZ-induced diabetes. Studies have demonstrated that catechin-rich PCs improve the functionality of isolated pancreatic β-cells from Wistar rats and primary rat islet cells. This improvement is realized by enhancing insulin secretion through a non-oxidative stress pathway involving the activation of MAPK/PI3K to phosphorylate Nrf2, facilitating its nuclear translocation, binding to the ARE promoter, and ultimately boosting antioxidant gene expression (Thomas et al., 2017). Ji-Hyun et al. (2009) showed that cytokines intensify inflammatory damage in isolated pancreatic islet cells, leading to disrupted insulin secretion. This result is attained by increasing nitric oxide (NO) production, enhancing NF-κB DNA binding, boosting inducible nitric oxide synthase (iNOS) expression, and activating the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) inflammatory pathway, leading to DNA damage and elevated levels of FAS and IL-1β. Wild PCs has successfully inhibited the phosphorylation of MAPKs and PI3K/Akt, thereby preventing lipopolysaccharide (LPS)-induced NFκB nuclear translocation. As a result, this leads to diminished production of NO, prostaglandin E2 (PGE2), and ROS, along with decreased levels of inducible iNOS, cyclooxygenase-2 (COX-2), and cytokines (Min-Ji et al., 2013).
The NOD-like receptor family pyrin domain containing 3 (NLRP3) comprises a large molecular weight protein assembly, incorporating NLRP3, ASC, and caspase-1, which forms an inflammatory vesicle. This complex is responsible for releasing proinflammatory cytokines Interleukin-1β (IL-1β) and Interleukin-18 (IL-18), fulfilling a critical function in regulating insulin resistance (Bo-Zong et al., 2015). Research has shown that silencing NLRP3 inhibits obesity-induced activation of inflammatory vesicles in adipose and hepatic tissues, thereby improving insulin sensitivity—a significant finding in this area (Bolormaa et al., 2011). Initiation of the NF-κB pathway is crucial for regulating the NLRP3 inflammasome, a vital element of the innate immune system. NF-κB is acknowledged as the primary mediator responsible for transcribing NLRP3 and generating precursors of IL-1β and IL-18 (Yuan et al., 2016). Yao et al. (2022) demonstrated that PCs treatment effectively reduces NF-κBp65 nuclear translocation and NLRP3 inflammasome activation, thereby inhibiting excessive NF-κB pathway activation due to high in sugar and fat. The intervention led to a marked decrease in the concentrations of inflammatory markers like IL-6, tumor necrosis factor-α, (TNF-α), IL-17, and CRP, and notably improved insulin sensitivity in gestational diabetic mice.
In summary, PCs can regulate pancreatic β-cell function and influence insulin secretion through apoptosis, oxidative stress, and inflammatory pathways. However, the retrieved literature mainly focuses on pancreatic cell lines, with limited studies on other cell lines. Future research could establish diverse cell models to investigate whether PCs exhibit similar effects on other cell lines (Figure 5).
5 Protective effects of PCs on diabetes mellitus and complications5.1 PCs and type 1 diabetes mellitusType 1 diabetes originates as an autoimmune disease involving the targeted destruction of pancreatic islet β-cells due to specific immune reactions. The β-cell primarily serves as the organ responsible for insulin secretion. Besides producing insulin, β-cells sense glucose levels and release insulin to maintain these levels within the normal physiological range (Jeffrey et al., 2010). Consequently, β-cells function not only as insulin producers but also as regulators—or “thermostats”—of glucose levels. Research indicates that key factors behind pancreatic islet β-cell dysfunction and structural damage include the presence of infiltrating CD4+ and CD8+ T cells, along with macrophages (Alan et al., 1991). When β-cells are depleted, individuals with type 1 diabetes lose glycemic control, resulting in acute conditions like ketoacidosis and severe hypoglycemia, as well as chronic complications such as heart disease, blindness, and kidney failure (Maahs and Rewers, 2006). Hence, targeting macrophages may represent a promising strategy for managing type 1 diabetes.
Macrophages, key components of the innate immune system, maintain immune homeostasis and contribute to overall health. This regulatory function occurs via macrophage polarization in response to both internal and external environmental or pathological stimuli (Guillermo Arango and Albert, 2014). Ying et al. (2019) applied different concentrations of PCs to macrophages from db/db diabetic mice. Their findings indicated that PCs reduced M1 macrophage counts while elevating M2 macrophage markers such as Arginase 1, Ym1, and Fizz1. The proposed mechanism involves PCs activating peroxisome proliferator-activated receptor γ (PPARγ) in macrophages, upregulating PPARγ target genes (CD36 and ABCG1) and driving the transition from M1 to M2 phenotypes. Recently, bone marrow mesenchymal stem cells (MSCs) have been recognized as a promising treatment alternative for diabetes. However, challenges such as oxidative stress and inflammation significantly limit the efficacy of bone marrow MSC transplantation therapy. The antioxidant and anti-inflammatory properties of PCs were validated through ex vivo experiments (Shuang et al., 2017; Min-Ji et al., 2013). Alyaa et al. (2022) administered a daily 300 mg/kg dose of PCs combined with bone marrow MSC transplantation to STZ-induced type 1 diabetic rats over a 30-day period, resulting in successful blood glucose management and enhanced insulin secretion. Furthermore, PCs mitigated oxidative stress and lowered the concentrations of inflammatory markers such as IL-1β, TNF-α, IL-12, and TLR-4, which were elevated by MSCs, while upregulating critical antioxidant enzymes, notably glutathione (GSH). This finding demonstrates that PCs provide substantial protection against oxidative stress and inflammatory responses triggered by diabetes and MSC differentiation (Figure 6).
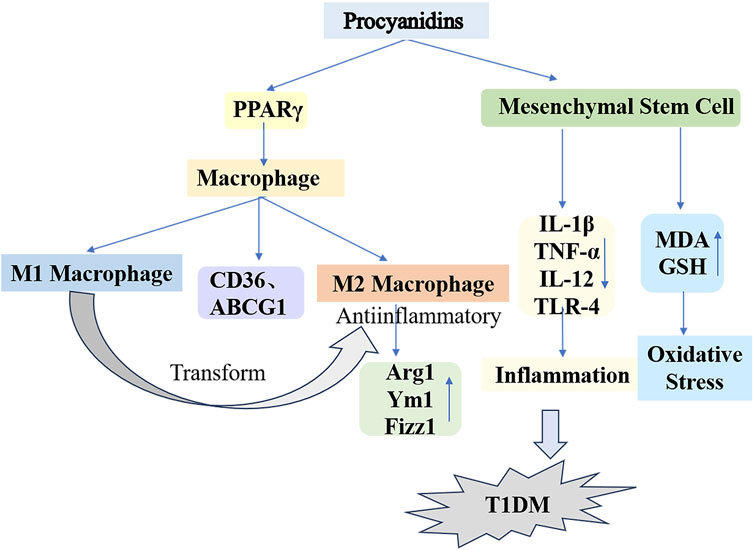
Figure 6. Mechanisms of Procyanidins in Counteracting Type 1 Diabetes Mellitus. This figure illustrates how PCs reduce T1DM progression by modulating macrophage polarization and mesenchymal stem cell activity. PCs promote the shift from pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages, marked by increased Arg1, Ym1, and Fizz1 expression. Additionally, PCs reduce oxidative stress and inflammation by regulating cytokines (e.g., IL-1β, TNF-α, IL-12) and enhancing antioxidant defenses (e.g., GSH, MDA).
In conclusion, the pathogenesis of type 1 diabetes involves the immune-mediated destruction of β-cells and abnormal activation of immune cells, with macrophage polarization playing a critical role in immune regulation, making it a promising therapeutic target. PCs, with their antioxidant and anti-inflammatory properties, can amplify the therapeutic effects of bone marrow mesenchymal stem cell combination therapy, alleviating oxidative stress and inflammatory responses. Although existing studies support its therapeutic potential, further investigation is necessary to clarify its mechanisms, optimize bioavailability, and identify specific targets, while large-scale clinical trials remain imperative to validate its efficacy.
5.2 PCs and type 2 diabetes mellitusType 2 diabetes mellitus (T2DM) is recognized as a metabolic syndrome, primarily marked by insufficient insulin caused by pancreatic β-cell dysfunction and insulin resistance in target organs (NCD Risk Factor Collaboration NCD-RisC, 2016; Sudesna et al., 2017). Multiple genes govern β-cell differentiation and function—for instance, GLP-1, which enhances insulin secretion while reducing glucagon secretion; PAX4, essential for islet development; and PDX1, vital for β-cell differentiation, growth, and the maintenance of mature function. Furthermore, elements including NF-κB, Toll-like receptors, and bone bridging proteins significantly affect insulin resistance, all of which are regulated by epigenetic mechanisms (Karina et al., 2021). Yoko et al. (2019) administered various doses of cocoa-derived proanthocyanidin extracts to ICR mice, observing that PCs with distinct structures enhanced GLP-1 secretion and reduced postprandial hyperglycemia. The probable pathway likely involves PCs augmenting GLUT4 translocation and glucose uptake in skeletal muscle through an AMPK-dependent mechanism, subsequently enhancing GLP-1–activated insulin signaling and lowering postprandial hyperglycemia. In vitro studies show that a 10 µM concentration of PCs optimally attenuates bisphenol-induced islet cell damage, suppresses apoptosis, and improves insulin secretion. In bisphenol-induced T2DM mice, PCs reduced lipid peroxidation, boosted antioxidant enzymes (SOD and Gpx), upregulated mRNA levels of PDX1 and GUT2, and preserved glucose homeostasis. Furthermore, combined catechin and quercetin therapy demonstrated a stronger impact on elevating key enzymatic activities in hepatic glucose metabolism in diabetic rats relative to monotherapy (Byoung Rai and Pyoung Sim, 2022). Additionally, a double-blind clinical trial reported that continuous intake of a PCs-rich beverage, largely composed of catechin, stimulated insulin secretion and enhanced blood glucose control in individuals with type 2 diabetes (Tomonori et al., 2009). Collectively, these findings prevented BPA-induced islet cell apoptosis, hyperglycemia, and diabetic onset (Ahangarpour et al., 2016).
Insulin resistance is a major contributor to the development of type 2 diabetes. Furthermore, mitochondrial dysfunction plays a pivotal role in the progression of insulin resistance. Essential for maintaining normal mitochondrial function, mitochondrial biosynthesis is regulated by key factors such as Peroxisome receptor gamma coactivator 1-alpha (PGC-1α) and silent information regulator factor 2-related enzyme1 (SirT1). PGC-1α acts as a coactivator for transcription factors that regulate mitochondrial genes, including nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM). Conversely, SirT1-mediated deacetylation activates PGC-1α (Helena et al., 2009; Bor Luen, 2016). In a dexamethasone-induced insulin resistance model using 3T3-L1 adipocytes, flavan-3-ols from PCs enhanced the expression of genes involved in mitochondrial biosynthesis (PGC-1α, SirT1, NRF1) and fusion proteins (Mfn1 and Mfn2). Furthermore, PCs reduced the expression of the fission protein Drp1, thereby improving mitochondrial defects in 3T3-L1 adipocytes by enhancing mitochondrial biosynthesis, dynamics, membrane potential, and antioxidant capacities, ultimately mitigating insulin resistance (Fangfang et al., 2020).
Hyperlipidemia substantially contributes to type 2 diabetes susceptibility. This condition arises from disrupted hepatic lipid metabolism, characterized by elevated triglyceride synthesis, increased cholesterol production, and reduced fatty acid oxidation. These processes drive excessive hepatic fat accumulation, culminating in steatosis (Lewis et al., 2002; David et al., 2007). Thus, optimizing lipid metabolism represents a viable strategy for both preventing and managing type 2 diabetes. In their research, Fangfang et al. (2020) used PCs to treat a T2DM mouse model induced by a high-fat diet (HFD) and STZ. PCs treatment significantly decreased hyperglycemia and hyperinsulinemia, as well as serum triglycerides, total cholesterol, LDL cholesterol, and AST-associated lipokines in T2DM mice compared to controls. PCs treatment enhanced diabetic hyperlipidemia and liver function in mice. Additionally, PCs suppressed adipogenic proteins (FAS, ACC) while upregulating lipolytic proteins (ATGL, HSL, CPT1A). The results indicate that PCs may suppress lipogenesis while enhancing lipid hydrolysis and β-oxidation processes for fatty acids. This effect likely occurs via AMPK/ACC/CPT1A pathway activation, enhancing hepatic lipid metabolism in HFD/STZ-induced diabetic mice. The degree of polymerization in PCs correlates with their biological potency. Zhao et al. (2024) administered equivalent doses of PCs exhibiting varying polymerization levels to HFD/STZ-induced diabetic mice. These PCs significantly lowered fasting blood glucose, improved glucose/insulin tolerance, and optimized lipid profiles (total cholesterol, triglycerides, LDL cholesterol), while reducing oil-red staining in hepatic and serum tissues. They significantly downregulated CHOP and GRP78 expression in diabetic mouse livers, implying a link between hepatic lipid dysregulation and endoplasmic reticulum stress. Furthermore, in vitro experiments showed that administering PCs of different polymerization degrees to palmitic acid–induced HepG2 cells markedly diminished intracellular GRP78 expression. Additionally, this approach activated the protein kinase R-like endoplasmic reticulum kinase (PERK)/active transcription factor 4 (ATF4) signaling pathway, crucial for endoplasmic reticulum stress regulation, and partially mitigated hepatic lipid accumulation. These findings align with earlier investigations in high glucose–induced C57BL/6 mice treated with PB2, indicating that PCs regulate lipid metabolism in diabetes by targeting endoplasmic reticulum sensors such as PERK, IRE1, and ATF6 (Liu Z. et al., 2023; Nie et al., 2020). GSPE with varying polymerization degrees exhibit protective activity on blood glucose, lipid levels, and hepatic oxidative stress in diabetic rats (Zhaoxia et al., 2014). Notably, oligomers outperformed polymers, implying that the oligomeric form of GSPE might provide superior benefits over other forms. In conclusion, PCs and their derivatives, spanning different polymerization degrees, may influence the initiation and progression of T2DM via multiple pathways. Hence, these plant metabolites show promise as active agents for type 2 diabetes management (Figure 7).
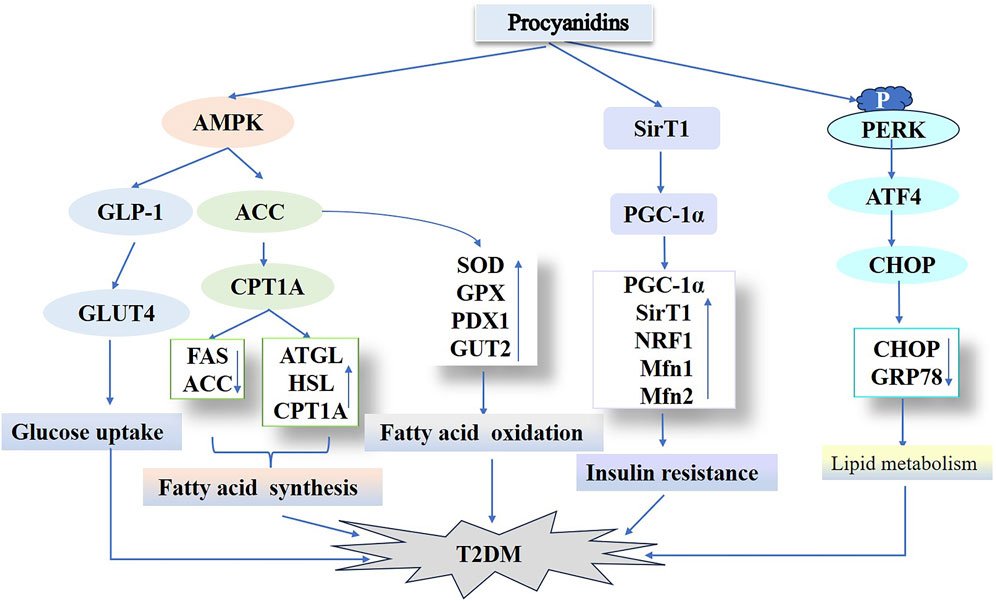
Figure 7. Procyanidins’ Role in Regulating Type 2 Diabetes Mellitus Pathways. This figure highlights how PCs improve T2DM by enhancing glucose uptake through AMPK activation and GLUT4 translocation, while modulating lipid metabolism via ACC, ATGL, and CPT1A. PCs also improve insulin sensitivity and reduce insulin resistance by regulating SIRT1, PGC-1α, and NRF1. Additionally, PCs mitigate endoplasmic reticulum stress and associated T2DM progression through pathways involving PERK and CHOP.
In summary, PCs participate in T2DM pathophysiology via multiple mechanisms, such as enhancing insulin secretion, safeguarding pancreatic β-cells, optimizing mitochondrial function and insulin resistance, and regulating hepatic lipid metabolism. Their polyphenolic metabolites display significant potential in regulating endoplasmic reticulum stress and lipid metabolism. As natural plant metabolites, PCs hold considerable promise as a therapeutic option for T2DM, but their specific mechanisms and clinical applications demand further research.
5.3 PCs and gestational diabetes mellitusGestational diabetes mellitus (GDM) emerges exclusively during pregnancy or is diagnosed when abnormal glucose tolerance is first identified (Sweeting et al., 2022). The pathogenesis of GDM is intricate, primarily involving impaired insulin secretion and reduced functionality. Insulin resistance gradually intensifies during pregnancy, ultimately leading to hyperglycemia (Jasmine et al., 2018; Kimber-Trojnar et al., 2018). Glucose, which traverses the placenta, serves as the primary energy source for the developing fetus (Ruszała et al., 2022). Furthermore, the overactivation of adipose tissue, which produces monocytes and macrophages that secrete inflammatory factors, contributes to the pathogenesis of GDM by initiating inflammatory responses. Growing evidence indicates that abnormal inflammatory responses are major contributors to the development of insulin resistance (Skórzyńska-Dziduszko et al., 2016; Akash et al., 2018; Gao et al., 2016). In a study utilizing a GDM mouse model induced by a high-fat diet and sugar, PCs (27.8 mg/kg/d) were administered from 4 weeks before pregnancy until delivery to evaluate their impact on insulin resistance, including during the postnatal period and in offspring. PCs effectively managed weight gain during pregnancy, decreased serum fasting blood glucose (FBG), fasting insulin, oral glucose tolerance test area under the curve, and insulin tolerance test area under the curve levels, and enhanced the Homeostatic Model Assessment (HOMA) insulin sensitivity index. These interventions positively affected fasting glucose and insulin levels. Additionally, PCs improved glucose metabolism and reduced concentrations of inflammatory mediators such as IL-6, TNF-α, IL-17, and CRP, thereby diminishing inflammation and hepatic inflammatory infiltration in mice 4 weeks postnatally. However, these treatments did not significantly impact the offspring. In a related observation, PCs were used in a GDM mouse model with gut flora defects induced by a combination of broad-spectrum antibiotics and a high-fat, high-sugar diet. This treatment enhanced insulin resistance by inhibiting the NF-κB/NLRP3 activation pathway, thereby altering the levels of gut flora metabolites 4-hydroxyphenylacetic acid and 3- (4-hydroxyphenyl) propionic acid (Yao et al., 2022). In a double-blind, randomized, controlled trial on gestational diabetes (Zhang et al., 2017), it was observed that mothers in the epigallocatechin 3-gallate group showed significant improvements in diabetes parameters, with fewer cases of neonatal complications compared to the placebo group.
In conclusion, PCs exert significant protective effects on the pathogenesis of GDM by enhancing insulin sensitivity, lowering levels of inflammatory factors, and alleviating liver inflammation, while effectively regulating glucose metabolism during pregnancy and postpartum stages. Despite limited effects on offspring mice, PCs continue to show potential as a treatment for GDM, but their long-term safety and mechanisms require further research and verification.
5.4 Effect of PCs on diabetic complications5.4.1 PCs and diabetic nephropathyDiabetic nephropathy (DN) is a primary microvascular complication of diabetes mellitus, characterized by proteinuria, glomerular enlargement, reduced tubular filtration, renal fibrosis, and renal dysfunction, ultimately leading to end-stage renal disease (Samsu, 2021). The progression of diabetic nephropathy is multifaceted, influenced by factors such as renal hemodynamic abnormalities, dyslipidemia, oxidative stress, and hormone synthesis, including Angiotensin II (Ang II). Additionally, its progression is influenced by inflammation, the renin-angiotensin system (RAAS), advanced glycation end-product (AGE) formation, and signaling molecules, including transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF), PKC, MAPK, and ROS. Collectively, these factors accelerate the progression of diabetic nephropathy, making it a leading cause of end-stage renal disease (Javier et al., 2020; Su Woong and Jae-Young, 2021; Majid, 2013; Wei-Jian et al., 2015; Yashpal et al., 2011; Mandeep Kumar and Umesh Kumar, 2013). In a rat model induced by STZ, a 12-week regimen of PCs treatment resulted in improvements in proteinuria and podocyte injury. Its probable mechanism includes activation of renal AGE-RAGE signaling pathways, decreasing levels of monocyte chemotactic protein-1 (MCP-1) and PKC-A, which consequently reduces the production of slit diaphragm proteins such as nephrin and podocin, thereby mitigating the impact of AGE-mediated DN pathogenesis (Muthenna et al., 2014). Oral administration of PCs significantly enhanced kidney function and reduced pathological alterations in db/db mice. Proteomic evaluation revealed that following PCs administration, 53 proteins were downregulated and 60 upregulated, with milk fat globule-epidermal growth factor 8, (MFG-E8) as the most notably upregulated protein in diabetic kidneys. Inhibition of MFG-E8 via shRNA transfection decreased phosphorylation levels of ERK1/2, Akt, and GSK-3β in the kidneys of db/db mice, improving renal histopathology. Conversely, overexpression of MFG-E8 had opposite effects. PCs treatment reduced MFG-E8, phospho-ERK1/2, phospho-Akt, and phospho-GSK-3β levels in db/db mouse kidneys, indicating MFG-E8’s influence on the progression of diabetic nephropathy. PCs appear to mitigate diabetic nephropathy by reducing MFG-E8 levels and modulating the ERK1/2, Akt, and GSK-3β pathways (Zhang et al., 2013).
Diabetic glomerulosclerosis is a defining feature of diabetic nephropathy. A key process in glomerulosclerosis and tubular epithelial fibrosis involves the epithelial-to-mesenchymal transition (EMT) induced by high glucose levels (Xu et al., 2017). PCs mitigate high glucose-triggered EMT within HK-2 cells by activating the TGF-β/Smads and P38/MAPK pathways, upregulating α-SMA, FN, and Waveform proteins, and downregulating E-cadheri
留言 (0)