Bloodstream infection (BSI) is defined as the presence of microorganisms in the bloodstream, as confirmed by a positive blood culture (BC) in a patient exhibiting clinical signs of infection (Laupland and Leal, 2020). Sepsis is a life-threatening condition resulting from a dysregulated immune response to infection that leads to organ dysfunction (Singer et al., 2016; Rhee et al., 2019). In 2020, the World Health Organization (WHO) reported that sepsis affects 49 million people and causes 11 million deaths globally each year, with many of the victims being children. Sepsis also disables millions more. The WHO recognized sepsis as a major public health issue and urged all United Nations (UN) member states to enhance sepsis prevention, recognition, and management (Singer et al., 2016; Rudd et al., 2020; World Health Organization, 2020). In recent years, the incidence of bloodstream infections (BSI) has been increasing, particularly among the young and elderly populations (Kontula et al., 2021). Approximately one out of three episodes of bacteremia are associated with organ dysfunction, according to a recent large population-based study. BSI-induced severe sepsis is a common cause of death and frequently results in septic shock and multiple organ dysfunction syndrome (MODS) (Weiss et al., 2020; Agyeman et al., 2017). BSI or septic shock remain an important cause of morbidity and mortality (Weiss et al., 2015; Martinón-Torres et al., 2018), with mortality rates reported as high as 40% (Weiss et al., 2015; Wang et al., 2014; Dou et al., 2024; Allel et al., 2023). Multiple analyses indicate that BSIs in the ICU significantly increase both the length of stay (LOS) and healthcare costs (Goudie et al., 2014; Timsit et al., 2020; Tabah et al., 2022). Bloodstream infection (BSI) presents a major challenge in the critically ill (Munro et al., 2024). Infection prevention and control efforts should prioritize preventing BSIs in the youngest age groups, particularly in neonatal and pediatric intensive care units (Zingg et al., 2017).
Bloodstream infections (BSI) can be caused by a variety of pathogens, and the microbiology of ICU BSIs varies globally (Munro et al., 2024). Understanding the clinical features and distribution of pathogenic bacteria in BSI-induced severe sepsis in local ICUs is crucial for treating severely infected children. In this study, we conducted a retrospective analysis to identify the risk factors for mortality and to characterize the pathogens associated with BSI-induced severe sepsis in the pediatric intensive care unit (PICU).
2 Material and methods2.1 Study designThis retrospective study was conducted using clinical data of children with BSI−induced severe sepsis in the pediatric intensive care unit (PICU) at a tertiary pediatric hospital between January 2015 and December 2023. This study was approved by the Institutional Review Board of the hospital. Patient data were anonymized and de-identified prior to analysis. Due to the retrospective nature of the study, the requirement for informed CoNSent was waived. The inclusion criteria were as follows: 1) patients aged 1 month to 18 years admitted to the PICU, 2) positive blood culture during hospitalization, and 3) meeting the diagnostic criteria for severe sepsis in children. The exclusion criteria were: 1) negative blood culture during hospitalization, and 2) isolated organisms identified as contaminants or fixed values.
2.2 Microbiologic methodsBlood samples were collected from peripheral veins of the central venous catheter. Each 1-5 mL blood sample was immediately inoculated into a culture bottle and transported to the laboratory. Blood cultures were processed BD BACTEC FX 400 automatic bacteria culture system. Drug susceptibility tests were performed using the conventional minimum inhibitory concentration (MIC) test with BD phoenix TM 100 automated microbiology system. This study was to examine the prevalence of four important drug-resistance phenotypes: difficult-to-treat resistance (DTR), fluoroquinolone resistance (FQR), carbapenem resistance (CR), and extended-spectrum cephalosporin resistance (ECR).
2.3 Data collectionWe collected the laboratory indicators and scores within 24 hours of the first positive blood culture sample sent for testing. The following demographic and laboratory data were collected: age, body weight, gender, white blood cell (WBC), C-reactive protein (CRP), Procalcitoninalanine (PCT), albumin (ALB), and serum creatinine (SCr). The P/F value (PaO2/FiO2), pediatric sequential organ failure assessment (pSOFA) scores (Mohamed El-Mashad et al., 2020), International Society on Thrombosis and Hemostasis (ISTH) disseminated intravascular coagulation (DIC) scores, sepsis-induced coagulopathy (SIC) scores, underlying conditions, and Nosocomial infection were obtained. Comorbidities such as septic shock, respiratory failure, liver function impairment, and acute kidney injury (AKI) were also evaluated. Furthermore, data on the need for continuous renal replacement therapy (CRRT) and mechanical ventilation (MV), duration of CRRT and MV, as well as length of hospital stay and stay in the PICU were recorded.
2.4 DefinitionsSepsis: SIRS in the presence of or as a result of suspected or proven infection. Severe sepsis: sepsis plus one of the following: cardiovascular organ dysfunction or acute respiratory distress syndrome or two or more other organ dysfunctions. Septic shock: sepsis and cardiovascular organ dysfunction, cardiovascular dysfunction: despite administration of isotonic intravenous fluid bolus 40 mL/kg in 1 hr., decrease in BP (hypotension) 5th percentile for age or systolic BP 2 SD below normal for age, or need for vasoactive drug to maintain BP in normal range (dopamine 5 g/kg/min or dobutamine, epinephrine, or norepinephrine at any dose), or two of the following: 1. Unexplained metabolic acidosis: base deficit 5.0 mEq/L.2. Increased arterial lactate 2 times upper limit of normal; 3. Oliguria: urine output 0.5 mL/kg/hr. 4. Prolonged capillary refill: 5 secs. 5. Core to peripheral temperature gap 3°C (Goldstein et al., 2005). Nosocomial infection was defined as the first positive blood culture was drawn after day 2 of the hospital admission, with no primary diagnosis of infectious disease present on admission (as defined with International Classification of Disease (ICD) diagnosis codes and Major Diagnostic Categories). Respiratory failure is the inability of the respiratory system to maintain oxygenation or eliminate carbon dioxide, resulting in hypoxemia (PaO2 < 60 mmHg) with or without hypercapnia (PaCO2 > 50 mmHg), or the need for noninvasive or invasive ventilator support. Liver function impairment was defined as transaminase levels three times above the upper limit of normal. Acute kidney injury is defined as an increase in serum creatinine levels by at least 0.3 mg/dl within 48 hours or 1.5−fold the baseline, which is known or presumed to have occurred within the preceding 7 days, or-according to the urine output criterion-urine volume less than 0.5 ml/kg/hour for at least 6 hours. Contaminants: The most frequently identified contaminants in cultures were Staphylococcus epidermidis and Staphylococcus hominis. Critical value reports from blood cultures were systematically monitored, and clinicians assessed whether these organisms were true pathogens or contaminants based on the patient’s clinical presentation and relevant laboratory parameters. Difficult-to-treat resistance (DTR) was defined as resistance or intermediate resistance in vitro to all β-lactam categories, including carbapenems and fluoroquinolones. Carbapenem resistance (CR) was defined as resistance in vitro to imipenem or meropenem. Extended-spectrum cephalosporin resistance (ECR) was defined as resistance in vitro to ceftriaxone or cefepime (excluding natural drug resistance). Fluoroquinolone resistance (FQR) was defined as resistance in vitro to ciprofloxacin or levofloxacin.
2.5 Outcomes analysisThe primary outcome was 28-day in-hospital mortality, and the secondary outcome was the distribution of pathogens in bloodstream infection-induced severe sepsis.
2.6 Statistical analysisRaw data was firstly processed by Whonet 5.6 software. Data processing was performed using Graphpad Prism 8.4.2 for Windows (GraphPad Software, San Diego, California, USA) and SPSS 26.0 software packages (IBM Corp., Armonk, NY, USA). We used the median (interquartile range or IQR) and proportion (percentage) to describe continuous and categorical data, respectively. The Manne-Whitney U test was used to compare two medians, and the chi-square test was used to compare proportions. Multiple logistic regression was employed to identify independent risk factors, controlling for confounding variables. Statistical significance was defined as P < 0.05 (two-tailed).
3 Result3.1 Study populationDuring the study period, a total of 9032 blood cultures were collected from patients suspected of bloodstream infections (BSI). Among these, 382 cultures tested positive. After excluding possible contaminants and duplicates, we analyzed 200 episodes confirmed as bloodstream infections leading to severe sepsis. The annual positive rates in the 9 years from 2015 to 2023 were 3.09%, 3.96%, 3.05%, 2.83%, 1.51%, 1.38%, 1.93%, 2.18%, and 1.43%, respectively (Figure 1).
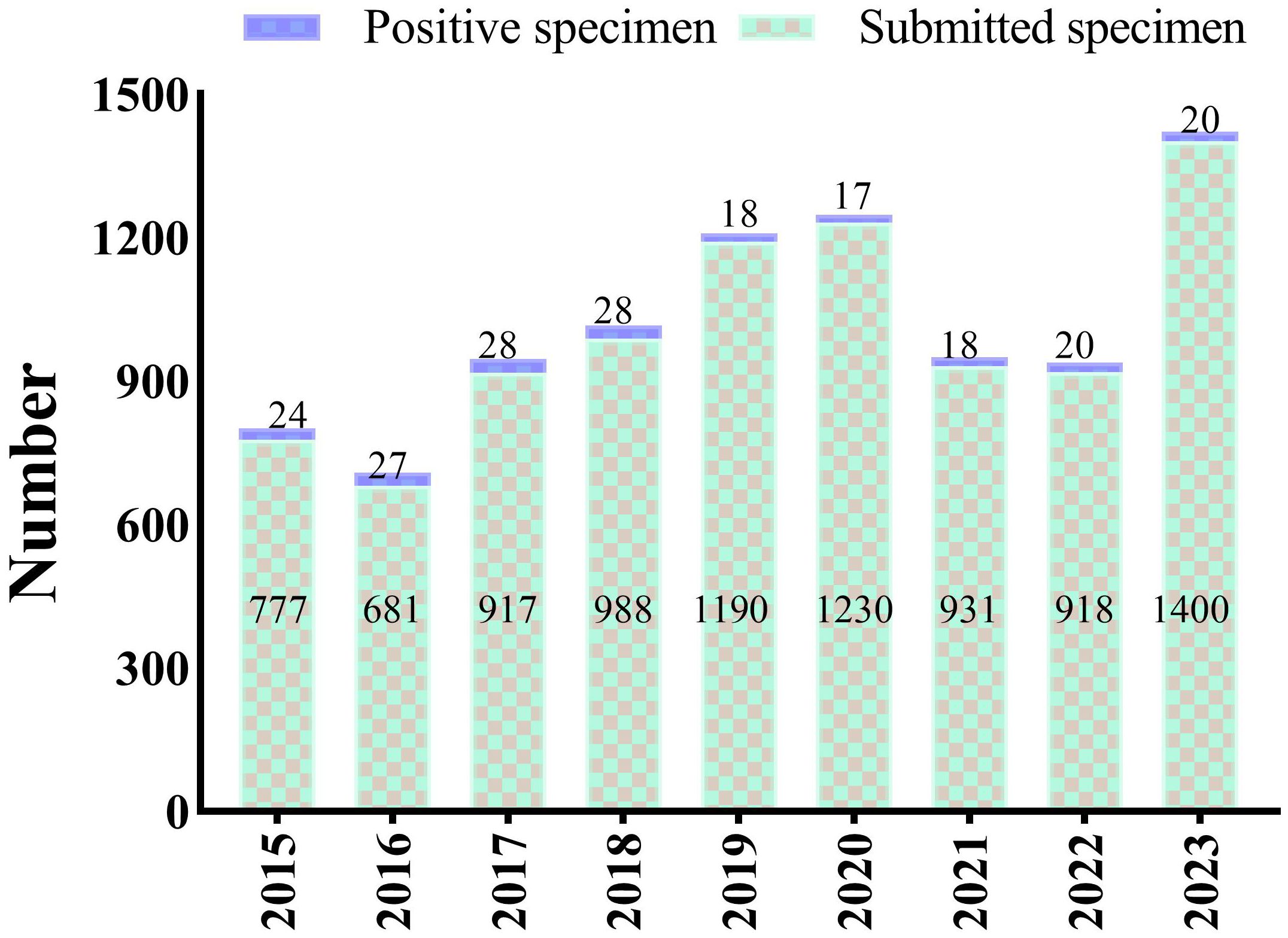
Figure 1. Annual number of blood cultures including the submitted specimens and positive specimens, from 2015 to 2023.
3.2 Characteristics of patients diagnosed with BSI-induced severe sepsisOne hundred and ninety-two cases of bloodstream infection-induced severe sepsis were identified. The clinical characteristics of these patients are summarized in Table 1. We categorized the children into survival and non-survival groups, and their clinical characteristics are presented in Table 1. The 28-day in-hospital mortality rate was 36.98% (71/192).
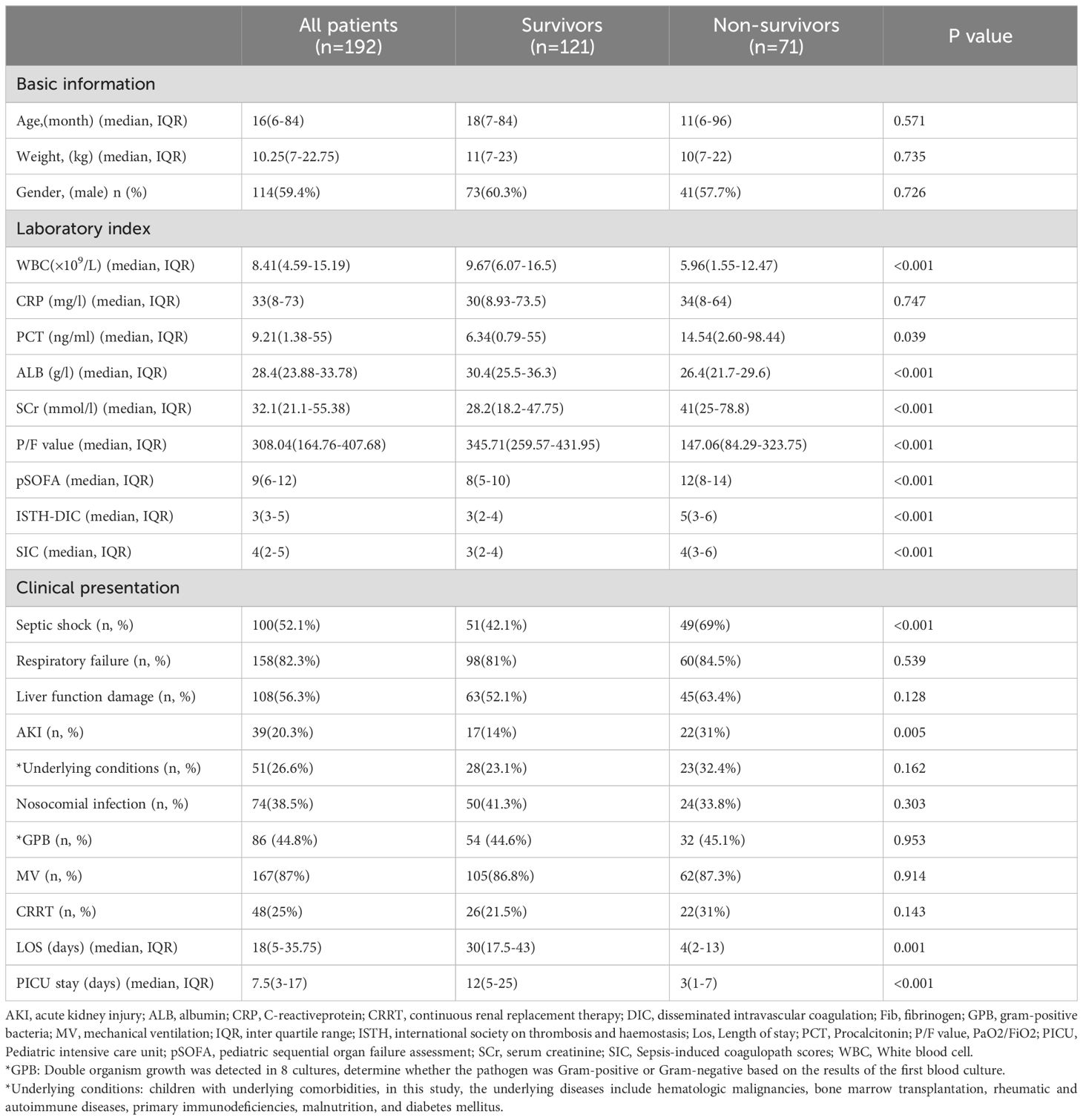
Table 1. Clinical characteristics and outcomes associated with blood culture-proven bacterial sepsis in 192 children.
The median age of these patients, of whom 114 (59.4%) were male, was 16 (6-84) months and the median weight was 10.25 (7-22.75) kg. Septic shock was present in 100 cases (52.1%), respiratory failure in 158 cases (82.3%), liver function impairment in 108 cases (56.3%), acute kidney injury in 39 cases (20.3%), and underlying conditions were present in 51 cases (26.6%) at presentation.
During the 28-day hospital stay, 71 patients (36.98%) died. The endpoint of the study was the 28-day in-hospital mortality (all-cause). Patients discharged from the hospital within 28 days were deemed alive unless proven otherwise. When comparing the survival and non-survival groups, in terms of laboratory tests, there was no significant difference in CRP. There were also no significant differences in respiratory failure, liver function damage, underlying conditions, gram-positive bacteria and nosocomial infection. Additionally, the requirements of MV and CRRT were similar between the two groups.
The levels of WBC (9.67 vs. 5.96 × 10^9/L, P < 0.001), ALB (30.4 vs. 26.4 g/l, P < 0.001), and P/F value (345.71 vs.147.06, P < 0.001) in the survival group were significantly higher than in the no-survival group, while the opposite was true for PCT (6.34 vs. 14.54 ng/ml, P = 0.039), SCr (28.2 vs. 41 mmol/l, P < 0.001), pSOFA (8 vs. 12, P < 0.001), ISTH-DIC (3 vs. 5, P < 0.001), and SIC (3 vs. 4, P < 0.001). The prevalence of septic shock (42.1% vs. 69%, P < 0.001) and acute kidney injury (14% vs. 31%, P = 0.005) in the survival group were significantly lower than in the non-survival group. Compared with the two groups, LOS (30 vs. 4 days, P = 0.001) and PICU stay (12 vs. 3 days, P < 0.001) in survival the group were significantly higher than in the no- survival group.
3.3 Analyses of risk factors for 28-day in-hospital mortality of BSIUnivariate analysis revealed that ALB, pSOFA, P/F value, Septic shock, and AKI were associated with 28-day in-hospital mortality (Table 2). Multivariate analysis further showed that pSOFA (OR 1.176; 95% CI: 1.046-1.321, p = 0.007) and P/F value (OR 0.994; 95% CI: 0.991-0.997, P < 0.001) were independently correlated with 28-day in-hospital mortality (Table 2). Conversely, septic shock and AKI were not identified as independent risk factors for 28-day in-hospital mortality.
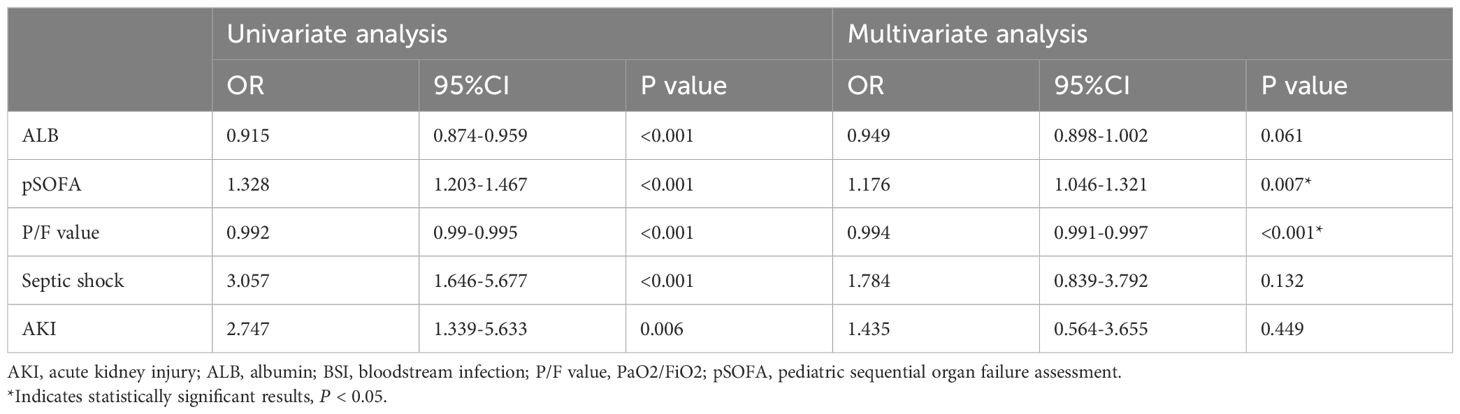
Table 2. Logistic regression analysis of risk factors for 28-day mortality in 192 children with BSI.
3.4 Microbial findingsDuring the study period, a total of 200 pathogenic bacteria were isolated from blood cultures in PICU. Among these, 110 strains (55.0%) were identified as Gram-negative bacteria, 88 strains (44.0%) were identified as gram-positive bacteria, and 2 strains (1%) were identified as Candida albicans (Table 3, Figure 2). Double organism growth was detected in 8 cultures. The most commonly isolated pathogens were Staphylococcus aureus, Coagulase-negative Staphylococcus, and Escherichia coli. Among Gram-negative bacteria, Escherichia coli (21 strains, 10.5%), Pseudomonas aeuroginosa (19 strains, 9.5%), Klebsiella pneumoniae (18 strains, 9.0%), and Acinetobacter baumannii (18 strains, 9.0%) were the most prevalent. Among gram-positive bacteria, Staphylococcus aureus (25 strains, 12.5%), Coagulase-negative Staphylococcus (25 strains, 12.5%), Streptococcus pneumoniae (15 strains, 7.5%), and Enterococcus (10 strains, 5.0%) were the most common.
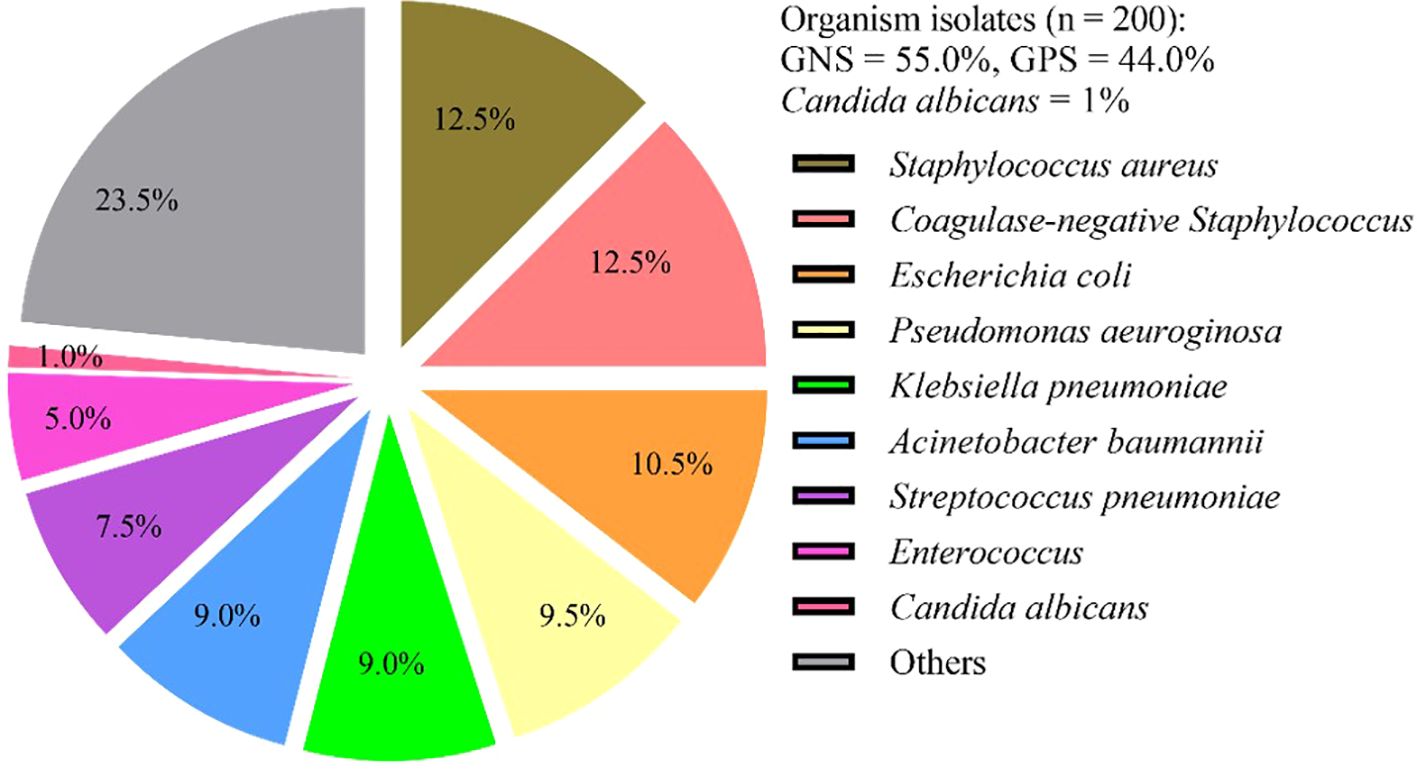
Figure 2. Frequency and distribution of bacteria isolated from patients investigated for BSI in PICU. BSI, Bloodstream infection; GNB, gram-negative bacteria; GPB, gram-positive bacteria; Others included (n = 47): Salmonella (n = 6), Streptococcus paldyne (n = 5), Serratia marcescens (n = 5), Enterobacter aerogenes (n = 3), Haemophilus influenzae (n = 4), Streptococcus agalactiae (n = 3), Enterobacter cloacae (n = 3), Streptococcus pyogenes (n = 2), Streptococcus salivarius (n = 2), Burkholderia cepacia (n = 2), Streptococcus bovis (n = 1), Acinetobacter jeonii (n = 1), Bacillus pallanus (n = 1), Stenotrophomonas maltophila (n = 1), Bordetella bronchitis (n = 1), Tsukamura genus (n = 1), Burkholderia (n = 1), Acid Klebber (n = 1), Citrobacterium festoni (n = 1), Acinetobacter lofe (n = 1), Xylose-oxidizing Chromobacterium (n = 1), Clostridium septicaemic meningitis (n = 1).
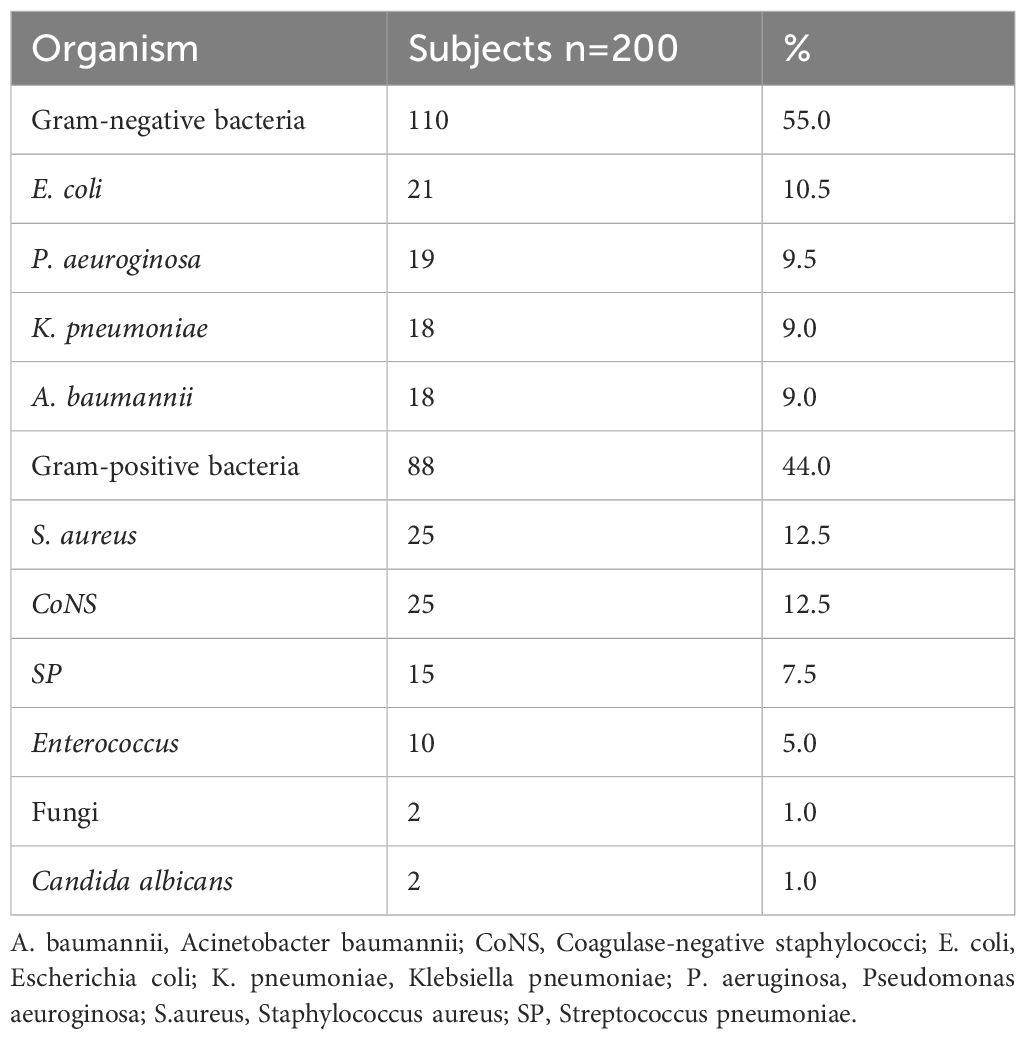
Table 3. Microbiology of BSI children admitted in the PICU.
3.5 Antimicrobial resistance pattern of main bacterial isolatesAs depicted in Table 4, Staphylococcus aureus (S. aureus) showed a resistance of 95.0%, 23.1%, 4.8% and 65.0% to penicillin, tetracycline, trimethoprim-sulfamethoxazole and erythromycin, respectively. The detection rate of methicillin resistant Staphylococcus aureus (MRSA) was 65.0%. Coagulase-negative staphylococci (CoNS) showed resistance rates ≥ 80% to penicillin and erythromycin. Streptococcus pneumoniae (SP) exhibited the highest resistance rates to tetracycline (100%) and erythromycin (91.7%). Enterococcus showed 50.0% resistance to penicillin. Vancomycin or linezolid-resistant strains were not isolated in gram-positive bacteria.
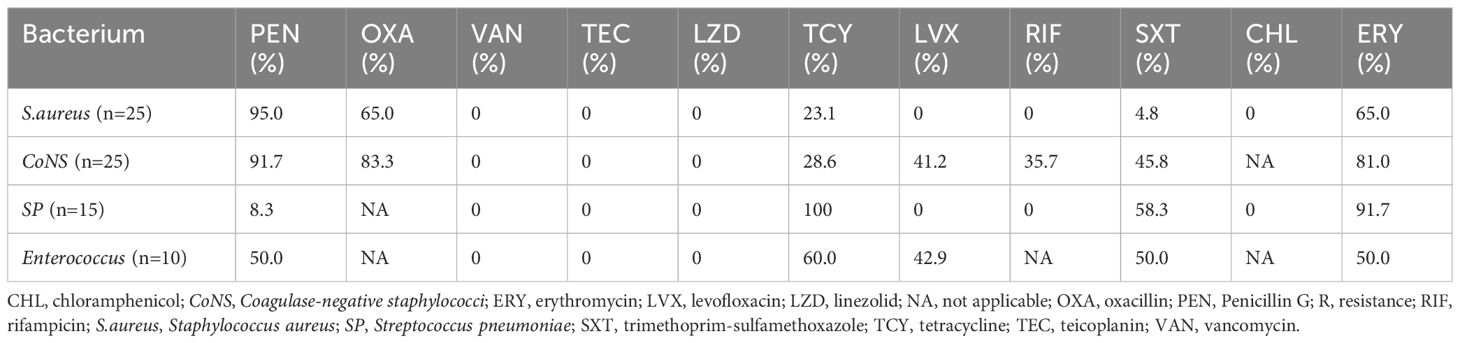
Table 4. Antimicrobial resistance rates of main gram-positive bacteria isolated from patients investigated for BSI in PICU.
As shown in Table 5, high frequencies of resistance to ampicillin-sulbactam (55.6%), aztreonam (52.9%), ceftriaxone (85.7%), and trimethoprim-sulfamethoxazole (72.2%) were observed among Escherichia coli (E. coli). Minimal resistance frequency of E. coli was detected to amikacin (0%), meropenem (17.1%), and piperacillin/tazobactam (16.7%). Klebsiella pneumoniae (K. pneumoniae) demonstrated a resistance of 11.1%, 38.5%, 38.5%, and 23.1% to amoxicillin/clavulanate, aztreonam, cefepime, and imipenem, respectively. Pseudomonas aeuroginosa (P. aeuroginosa) of the resistance rates was 14.3%, 7.7%, 7.7%, and 15.0% to amikacin, cefepime, levofloxacin, and imipenem, respectively. Acinetobacter baumannii (A. baumannii) showed resistance rates exceeding 50% against all tested antibiotics.
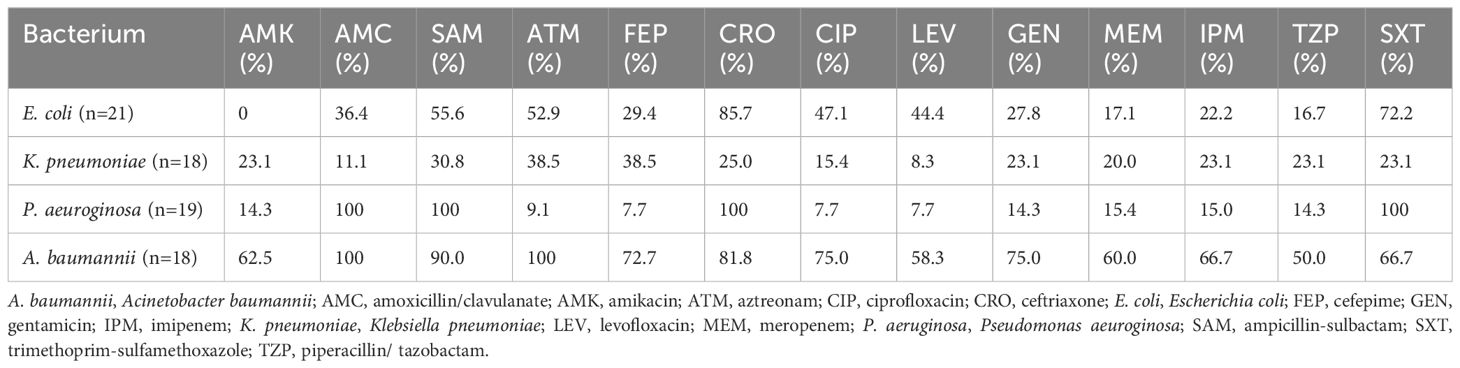
Table 5. Antimicrobial resistance rates of main gram- negative bacteria isolated from patients investigated for BSI in PICU.
3.6 The distribution of special antimicrobial resistance phonotypesAs shown in Table 6 and Figure 3, the detection rates of CR, ECR, and FQR were 22.2%, 85.7%, and 47.1% in E. coli; 23.1%, 38.5%, and 15.4% in K. pneumoniae; 15.4%, 7.7%, and 7.7% in P. aeruginosa; and 66.7%, 81.8%, and 75.0% in A. baumannii, respectively. DTR strains were not isolated in main gram- negative bacteria. The detection rate of CR in A baumannii (66.7%) was higher than that in P aeruginosa (15.4%). The detection rates of ECR and FQR in E. coli were higher than those in K. pneumoniae. A. baumannii exhibited the highest levels of CR and FQR, while E coli demonstrated the highest level of ECR.
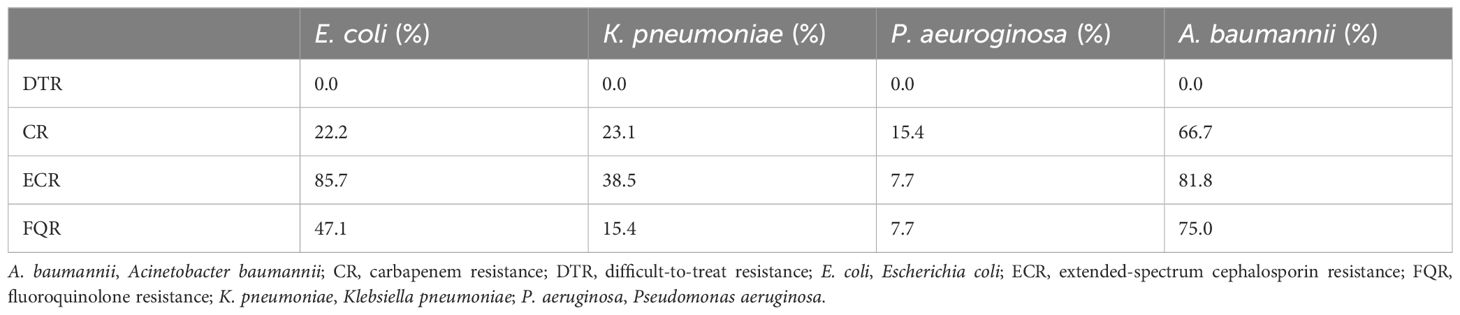
Table 6. The special antimicrobial resistance phonotypes of main gram- negative bacteria isolated from patients investigated for BSI in PICU. .
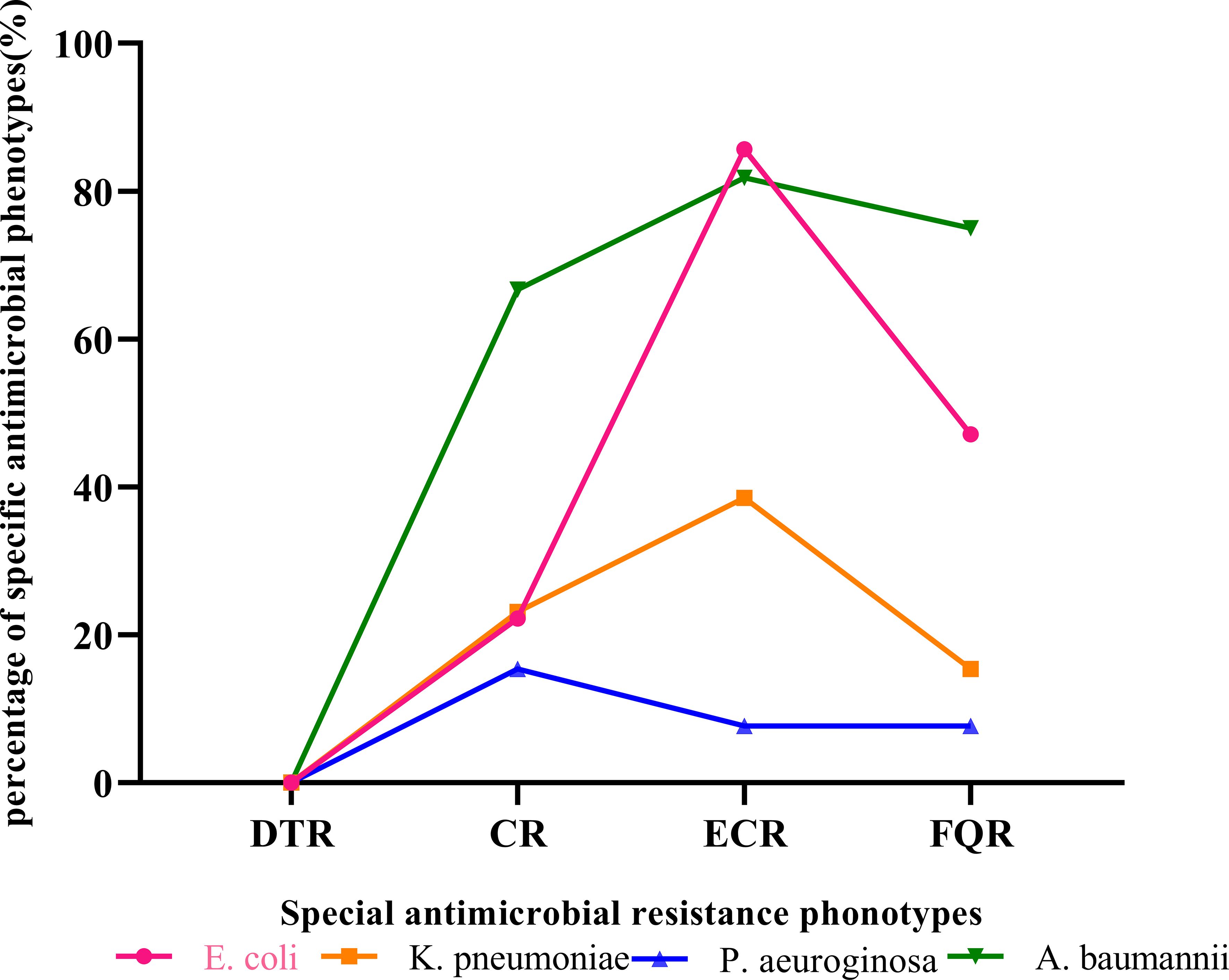
Figure 3. The special antimicrobial resistance phonotypes of main gram- negative bacteria isolated from patients investigated for BSI in PICU. A. baumannii, Acinetobacter baumannii; CR, carbapenem resistance; DTR, difficult-to-treat resistance; E. coli, Escherichia coli; ECR, extended-spectrum cephalosporin resistance; FQR, fluoroquinolone resistance; K. pneumoniae, Klebsiella pneumoniae; P. aeruginosa, Pseudomonas aeuroginosa.
4 DiscussionIn this study, the overall positive rate of blood cultures from 2015 to 2023 was 4.23%, which is consistent with findings from a previous study (Limmathurotsakul, 2017). After excluding contaminants and duplicates, the adjusted average positive rate of blood cultures was 2.21%. Sepsis can be induced by a wide range of pathogens, with bacterial infections accounting for the majority of cases. However, up to 42% of sepsis episodes are culture-negative (Lin et al., 2018), indicating the potential involvement of non-bacterial etiologies (Phua et al., 2013). Additionally, some studies have identified viruses as another significant cause of sepsis, particularly in pediatric populations (Lin et al., 2018), which may help explain the relatively low positive rate of blood cultures observed in our study. we conducted a retrospective analysis of clinical data from a single-center PICU over a 9-year period to investigate BSI-induced severe sepsis. The 28-day in-hospital mortality rate was 36.98%. Among the patients, 26.6% had underlying conditions, 82.3% experienced respiratory failure, 56.3% exhibited liver function impairment, 52.1% developed septic shock, and 20.3% suffered from acute kidney injury. In children with BSI-induced severe sepsis, mortality was higher in those with high pSOFA and low P/F values. The mortality rates for pediatric severe sepsis or septic shock have been reported as high as 40% (Weiss et al., 2015; Wang et al., 2014). A retrospective study analyzed 132 children with BSI-induced severe sepsis, revealing a mortality rate of 28.8% (Dou et al., 2024). Taddei et al. (Allel et al., 2023) recently noted crude mortality rates for ICU-acquired bloodstream infections exceeding 40%. In our study, the 28-day mortality rate was 36.98%. The SOFA score has been recommended by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine for predicting mortality in sepsis. Specifically, a 2-point increase in the SOFA score is associated with a 10% increase in mortality (Shankar-Hari et al., 2016). A systematic review and meta-analysis noted that SOFA showed the highest sensitivity and specificity in predicting in-hospital mortality in sepsis (Qiu et al., 2023). Our multivariate analysis revealed that both pSOFA (OR 1.176; 95% CI: 1.046-1.321, p = 0.007) and P/F value (OR 0.994; 95% CI: 0.991-0.997, P < 0.001) were independently correlated with 28-day in-hospital mortality.
Organ dysfunction syndrome commonly developed in severe sepsis and was associated with higher morbidity and mortality compared to severe sepsis without organ dysfunction syndrome (Lin et al., 2017). Agyeman et al. (2017) demonstrated a strong association between organ dysfunction and mortality, reporting a notable increase to 17% with at least one organ dysfunction and 29% with two or more organ dysfunctions. In our study, although not statistically significant, the survival group eb3xhibited lower rates of respiratory failure and liver function impairment compared to the non-survival group. Septic shock (42.1% vs. 69%, P < 0.001) and acute kidney injury (14% vs. 31%, P = 0.005) were significantly less frequent in the survival group than in the non-survival group. These findings collectively indicate higher disease severity in the non-survival group than in the survival group. Hence, continuous assessment of SOFA score, P/F value index, and preservation of organ function may enhance the prognosis of children with BSI-induced severe sepsis.
In this study, a total of 200 pathogenic bacteria were isolated from blood cultures in the PICU. Among these, 110 strains (55.0%) were identified as gram-negative bacteria, 88 strains (44.0%) as gram-positive bacteria, and 2 strains (1%) as Candida albicans. The most frequently isolated pathogens were Staphylococcus aureus (S.aureus) (12.5%), Coagulase-negative Staphylococcus (CoNS) (12.5%), and Escherichia coli (E. coli) (10.5%), consistent with findings from previous studies (Kontula et al., 2021; Dou et al., 2024; Fu et al., 2021). According to the Infectious Disease Surveillance of Pediatrics (ISPED) program in China from 2016 to 2020, a total of 288,377 clinical strains were isolated from 11 member units, with Gram-positive and Gram-negative bacteria accounting for 42.1% and 57.9%, respectively. Among these, the top two pathogenic bacteria derived from blood specimens were CoNS (46.3%), and E coli (6.0%) (Fu et al., 2021). A study conducted in PICUs in China on bloodstream infection pathogens reported 31.8% gram-positive bacteria, 65.9% gram-negative bacteria, and 2.27% fungi (Dou et al., 2024). In a population-based study of bloodstream infection, Kontula et al. (Kontula et al., 2021) showed among all BSIs, gram-positive bacteria caused 46% of infections, gram-negative bacteria 46%, fungi 1.5%. E. coli was the most common causative pathogen (29%), followed by S. aureus (13%), CoNS (8%).
There has been an increase in bacteremia caused by S aureus, CoNS and gram-negative pathogens across all age groups in children (Pai et al., 2015). Several surveys had demonstrated a rising trend in multidrug-resistant bloodstream infections (MDR BSIs) over time (Kontula et al., 2021; de Kraker et al., 2013). In our study, we analyzed the antimicrobial resistance pattern of the main bacterial isolates. In resistance patterns, all the main gram-positive bacteria were susceptible to vancomycin and linezolid, similar to the study of ZY L et al (Lyu et al., 2023). S. aureus exhibited frequent resistance to Penicillin and erythromycin, while showing high susceptibility to glycopeptides. Some studies have noted inferior outcomes were reported in methicillin-susceptible S aureus bacteremia (MSSA-B) treated with glycopeptides compared with β-lactams (McMullan et al., 2020; 2016; McDanel et al., 2015). In clinical practice, glycopeptides are preferred for methicillin-resistant S aureus bacteremia (MRSA-B), whereas for MSSA-B, β-lactams should be preferred over glycopeptides.
Infections caused by resistant Gram-negative bacteria are increasingly concerning in both developing and developed countries (Pai et al., 2015). In present study, E. coli showed high resistance to aztreonam, ceftriaxone, cefuroxime and trimethoprim-sulfamethoxazole. Minimal resistance was observed in E. coli against amikacin, piperacillin/tazobactam, and meropenem, consistent with findings from other studies (Dou et al., 2024; Legese et al., 2022). We observed an alarming resistance of K. pneumoniae to meropenem (20.0%), even higher than reported in another study (Legese et al., 2022). A baumannii exhibited resistance rates exceeding 50% against all tested drugs, notably high resistance to meropenem (60.0%), consistent with previous studies (Dou et al., 2024; Legese et al., 2022).
The pathogens of bloodstream infection vary across regions, and multidrug resistance (MDR) poses a threat to public health. Our study showed that the validity rate of the carbapenem-resistant E. coli (CR-ECO) was 22.2%, which was lower than that of the carbapenem-resistant K. pneumoniae (CR-KPN) (23.1%). Compared to the study by ZY L et al (Lyu et al., 2023), our detection rates of CRECO were higher, while the detection rates of CR-KPN were significantly lower. In our study, among the non-fermentative gram-negative bacilli isolates, 15.4% and 15% of the P. aeruginosa, were resistant to meropenem and imipenem. Furthermore, the resistance rates of A. baumannii to meropenem and imipenem were 60.0% and 66.7%. The detection rates of ECR and FQR in E. coli were higher than those in K. pneumoniae. A. baumannii exhibited the highest levels of CR and FQR, while E coli demonstrated the highest level of ECR. In the study of ZY L et al (Lyu et al., 2023), K. pneumoniae demonstrated the highest level of ECR, E. coli the highest level of FQR, and A. baumannii the highest level of CR. There were significant differences in medication habits and drug resistance among different regions. Monitoring local multidrug resistance (MDR) and selecting antibiotics reasonably based on susceptibility testing were crucial.
5 ConclusionIn conclusion, among children with BSI-induced severe sepsis, higher mortality was observed in those with elevated pSOFA scores and lower P/F values. The common pathogen of BSI−induced severe sepsis in PICU is Gram−negative bacteria. The three most frequently isolated pathogens were Staphylococcus aureus, Coagulase-negative Staphylococcus, and Escherichia coli. The pathogens of bloodstream infection vary across regions, monitoring local multidrug resistance (MDR) and selecting antibiotics reasonably based on susceptibility testing were crucial.
6 LimitationThis study has several limitations. Firstly, being retrospective, it was confined to cases and data from a single center. Consequently, these findings may not generalize to centers with different patient populations or antibiotic profiles. Secondly, the study focused exclusively on patients with documented bloodstream infections, potentially introducing selection bias since not all bacteremia cases were confirmed by positive blood cultures. Thirdly, retrospective studies could not further explore the mechanism of MDR resistance, and we did not analyze the molecular mechanisms of the drug-resistant strains.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThis study was approved by Ethics Committee of Children’s Hospital, Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsJC: Writing – original draft. HH: Data curation, Writing – review & editing. RZ: Writing – review & editing, Data curation. YF: Writing – review & editing. CJ: Writing – review & editing, Conceptualization, Resources.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GlossaryA. baumannii: Acinetobacter baumannii
AKI: acute kidney injury
ALB: albumin
AMK: amikacin
AMC: amoxicillin/clavulanate
ATM: aztreonam
BSI: bloodstream infection
CHL: chloramphenicol
CIP: ciprofloxacin
CoNS: Coagulase-negative staphylococci
CR-ECO: carbapenem-resistant E. coli
CR-KPN: carbapenem-resistant K. pneumoniae
CRO: ceftriaxone
CRP: C-reactive protein
CRRT: continuous renal replacement therapy
CXM: cefuroxime
E. coli: Escherichia coli
DTR: difficult-to-treat resistance
ECR: extended-spectrum cephalosporin resistance
ERY: erythromycin
FEP: cefepime
Fib: fibrinogen
FOX: cefoxitin
FQR: fluoroquinolone resistance
GEN: gentamicin
GNB: gram-negative bacteria
GPB: gram-positive bacteria
I: intermediate
IQR: inter quartile range
ISTH-DIC: international society on thrombosis and hemostasis
K. pneumoniae: Klebsiella pneumoniae
Los: Length of stay
P/F value: oxygenation index (PaO2/FiO2)
LVX: levofloxacin
MEM: meropenem
MV: mechanical ventilation
NA: not applicable
OXA: oxacillin
P. aeruginosa: Pseudomonas aeuroginosa
PCT: Procalcitonin
PEN: Penicillin
PICU: Pediatric intensive care unit
pSOFA: pediatric sequential organ failure assessment
R: resistance
RIF: rifampicin
S.aureus: Staphylococcus aureus
SAM: ampicillin-sulbactam
SCr: serum creatinine
SIC: Sepsis-induced coagulopath scores
SP: Streptococcus pneumoniae
SXT: trimethoprim-sulfamethoxazole
TCY: tetracycline
TZP: piperacillin/tazobactam
VAN: vancomycin
IPM: imipenem
WBC: White blood cell
ReferencesAgyeman, P. K. A., Schlapbach, L. J., Giannoni, E., Stocker, M., Posfay-Barbe, K. M., Heininger, U., et al. (2017). Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc. Health 1, 124–133. doi: 10.1016/s2352-4642(17)30010-x
PubMed Abstract | Crossref Full Text | Google Scholar
Allel, K., Stone, J., Undurraga, E. A., Day, L., Moore, C. E., Lin, L., et al. (2023). The impact of inpatient bloodstream infections caused by antibiotic-resistant bacteria in low- and middle-income countries: A systematic review and meta-analysis. PloS Med. 20, e1004199. doi: 10.1371/journal.pmed.1004199
PubMed Abstract | Crossref Full Text | Google Scholar
de Kraker, M. E., Jarlier, V., Monen, J. C., Heuer, O. E., van de Sande, N., Grundmann, H. (2013). The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 19, 860–868. doi: 10.1111/1469-0691.12028
PubMed Abstract | Crossref Full Text | Google Scholar
Dou, J. Y., Zhou, Y. P., Cui, Y., Sun, T., Shi, J. Y., Xiong, X., et al. (2024). Pathogenic characteristics and influence factors of bloodstream infection-induced severe sepsis in pediatric intensive care unit. Zhonghua. Yi. Xue. Za. Zhi. 104, 198–204. doi: 10.3760/cma.j.cn112137-20230729-00115
PubMed Abstract | Crossref Full Text | Google Scholar
Fu, P., Xu, H., Jing, C., Deng, J., Wang, H., Hua, C., et al. (2021). Bacterial epidemiology and antimicrobial resistance profiles in children reported by the ISPED program in China 2016 to 2020. Microbiol. Spectr. 9, e0028321. doi: 10.1128/Spectrum.00283-21
PubMed Abstract | Crossref Full Text | Google Scholar
Goldstein, B., Giroir, B., Randolph, A. (2005). International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8. doi: 10.1097/01.Pcc.0000149131.72248.E6
PubMed Abstract | Crossref Full Text | Google Scholar
Goudie, A., Dynan, L., Brady, P. W., Rettiganti, M. (2014). Attributable cost and length of stay for central line-associated bloodstream infections. Pediatrics 133, e1525–e1532. doi: 10.1542/peds.2013-3795
PubMed Abstract | Crossref Full Text | Google Scholar
Kontula, K. S. K., Skogberg, K., Ollgren, J., Järvinen, A., Lyytikäinen, O. (2021). Population-based study of bloodstream infection incidence and mortality rates, Finland 2004-2018. Emerg. Infect. Dis. 27, 2560–2569. doi: 10.3201/eid2710.204826
PubMed Abstract | Crossref Full Text | Google Scholar
Legese, M. H., Asrat, D., Swedberg, G., Hasan, B., Mekasha, A., Getahun, T., et al. (2022). Sepsis: emerging pathogens and antimicrobial resistance in Ethiopian referral hospitals. Antimicrobial. Resistance. Infect. Control. 11, 83. doi: 10.1186/s13756-022-01122-x
PubMed Abstract | Crossref Full Text | Google Scholar
Limmathurotsakul, D. (2017). Causes and outcomes of sepsis in southeast Asia: a multinational multicentre cross-sectional study. Lancet Global Health 5, e157–e167. doi: 10.1016/s2214-109x(17)30007-4
PubMed Abstract | Crossref Full Text | Google Scholar
Lin, J. C., Spinella, P. C., Fitzgerald, J. C., Tucci, M., Bush, J. L., Nadkarni, V. M., et al. (2017). New or progressive multiple organ dysfunction syndrome in pediatric severe sepsis: A sepsis phenotype with higher morbidity and mortality. Pediatr. Crit. Care Med. 18, 8–16. doi: 10.1097/pcc.0000000000000978
PubMed Abstract | Crossref Full Text | Google Scholar
Lyu, Z. Y., Zhen, J. H., Meng, Q. Y., Zhou, W., An, J. Y., Dong, F. (2023). Bacterial etiology and antimicrobial resistance pattern of pediatric bloodstream infections in Beijing 2015-2019. Infect. Drug Resistance. 16, 6297–6308. doi: 10.2147/idr.S426000
PubMed Abstract | Crossref Full Text | Google Scholar
Martinón-Torres, F., Salas, A., Rivero-Calle, I., Cebey-López, M., Pardo-Seco, J., Herberg, J. A., et al. (2018). Life-threatening infections in children in Europe (the EUCLIDS Project): a prospective cohort study. Lancet Child Adolesc. Health 2, 404–414. doi: 10.1016/s2352-4642(18)30113-5
PubMed Abstract | Crossref Full Text | Google Scholar
McDanel, J. S., Perencevich, E. N., Diekema, D. J., Herwaldt, L. A., Smith, T. C., Chrischilles, E. A., et al. (2015). Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 61, 361–367. doi: 10.1093/cid/civ308
PubMed Abstract | Crossref Full Text | Google Scholar
McMullan, B. J., Bowen, A., Blyth, C. C., Van Hal, S., Korman, T. M., Buttery, J., et al. (2016). Epidemiology and mortality of staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatr. 170, 979–986. doi: 10.1001/jamapediatrics.2016.1477
PubMed Abstract | Crossref Full Text | Google Scholar
McMullan, B. J., Campbell, A. J., Blyth, C. C., McNeil, J. C., Montgomery, C. P., Tong, S. Y. C., et al. (2020). Clinical management of staphylococcus aureus bacteremia in neonates, children, and adolescents. Pediatrics 146, e20200134. doi: 10.1542/peds.2020-0134
PubMed Abstract | Crossref Full Text | Google Scholar
Mohamed El-Mashad, G., Said El-Mekkawy, M., Helmy Zayan, M. (2020). Paediatric sequential org
留言 (0)