According to data from the World Health Organization (WHO), neurodegenerative diseases and mental disorders represent a significant public health concern worldwide. It is estimated that more than 55 million people suffer from dementia, with Alzheimer’s disease being the most common form (1). Around 30% of individuals worldwide have encountered a mental disorder at some stage in their lives (2), and neurological conditions were responsible for more than 9 million fatalities in 2016 alone (3). Additionally, the occurrence of Parkinson’s disease has risen substantially in recent years. In 2019, it was estimated that over 8.5 million people were impacted, effectively doubling the figures reported 25 years earlier (4).
Brain-derived neurotrophic factor (BDNF) is vital for the survival, maintenance, and regeneration of specific neuronal populations in the adult central nervous system. Its role is critical in supporting neuronal health and facilitating neuroplasticity. Secreted by neurons and glial cells, brain-derived neurotrophic factor (BDNF) primarily facilitates neuronal survival, supports synaptic plasticity, and encourages neurogenesis. BDNF plays a significant role in various brain functions, such as memory, learning, and emotional regulation. Furthermore, it has been linked to the protection and recovery of the nervous system following injury or in the context of neurodegenerative diseases (5).
The decline in neurotrophic factor levels, particularly brain-derived neurotrophic factor (BDNF) and its receptors, is a well-documented physiological event in neurodegenerative conditions like Alzheimer’s disease (6, 7), Parkinson’s disease (8, 9), multiple sclerosis (10, 11) and amyotrophic lateral sclerosis (12). BDNF plays a neuroprotective role, particularly in the hippocampus, where it helps mitigate the effects of neurodegeneration. Consequently, a reduction in BDNF plasma levels is closely linked to deteriorating brain health (13, 14).
Exercise has been shown to offer considerable advantages for both muscular and metabolic disorders, and there has been a growing focus on its effects on cognitive function. It is crucial to note that these effects necessitate comprehensive research due to their potential positive influence on various conditions, including neurodegenerative diseases and psychiatric disorders. The acknowledgment of these benefits has resulted in the consideration of physical exercise as a supplementary therapeutic approach in both research and clinical settings (3). Thus, investigating the relationship between brain-derived neurotrophic factor and physical activity is vital for preventing the onset of multiple pathologies and enhancing the pathophysiological characteristics of various diseases.
This review examines the impact of physical exercise on the regulation of brain-derived neurotrophic factor (BDNF) in individuals with neurodegenerative diseases, focusing on brain plasticity and neuroprotection. While previous studies have linked exercise to the release of BDNF, aspects such as the influence of different types of exercise (aerobic, resistance, strength) on BDNF modulation in diseases like Alzheimer’s and Parkinson’s have not been explored in depth. A better understanding of how exercise regimens affect BDNF regulation in these patients is needed, as neurodegenerative diseases could alter this response. The novelty of this review lies in its integrated approach, which not only examines how exercise influences BDNF levels but also explores the molecular mechanisms involved in neuroplasticity, neurogenesis, and cognitive improvement. Additionally, the therapeutic implications of increasing BDNF levels through physical exercise in patients with neurodegenerative diseases are highlighted, an area that remains underexplored. This opens new avenues for non-pharmacological therapeutic strategies to complement conventional treatments. In summary, this review aims to establish a clearer connection between the molecular mechanisms underlying BDNF-mediated neuroplasticity and how physical exercise may support these processes in the context of neurodegeneration. It is expected that this analysis will provide new insights into the development of more accessible and effective therapeutic interventions for neurodegenerative disorders.
2 MethodologyThis review synthesized information on the relationship between physical exercise, brain-derived neurotrophic factor (BDNF), and molecular mechanisms linked to neuroplasticity and neurodegenerative diseases. Data collection occurred using databases such as PubMed, Google Scholar, ScienceDirect, and EBSCOhost and MEDLINE. Search terms combined: brain-derived neurotrophic factor (BDNF), Physical exercise, neurodegenerative diseases, dementia, Parkinson’s disease, neuroplasticity. The selection process involved inclusion and exclusion criteria emphasizing experimental and non-experimental studies focusing on exercise, BDNF, and neuroplasticity-related variables. Abstracts were screened for relevance, followed by full-text evaluation to confirm study alignment with the review’s objectives. Inclusion criteria included studies conducted in humans or rodents, published in English or Spanish, and examining exercise as an independent variable affecting BDNF, or long-term potentiation. Exclusion criteria included studies lacking pre-and post-exercise measurements, those offering incentives for cognitive testing, or failing to analyze exercise as a primary factor. Selected articles underwent manual duplication checks, forming the basis for the analysis and sub-sections of the review.
3 Brain-derived neurotrophic factor (BDNF)Brain-Derived Neurotrophic Factor (BDNF), sometimes referred to as abrineurina, is part of the neurotrophic growth factor family (15). BDNF is first synthesized as the pro-BDNF isoform, with a molecular weight ranging from 28 k to 32 kDa, the canonical form being 28 kDa. This pro-BDNF is cleaved both intracellularly and extracellularly to produce the mature BDNF (mBDNF) of 14 kDa and a 17 kDa propeptide. Pro-BDNF interacts with the p75NTR receptor, while mBDNF binds to the TrkB receptor, triggering signaling pathways that support neuronal survival. In contrast, signaling through the p75NTR receptor by pro-BDNF can induce apoptotic or pruning effects on neurons, indicating a nuanced relationship between the two isoforms (16).
BDNF is synthesized presynaptically in the cell bodies of sensory neurons that project to the dorsal horn, whereas in the hippocampus, its primary source is postsynaptic dendrites (17, 18). Peripherally, BDNF is detected in serum and is associated with blood plasma platelets, as well as being present in vascular endothelial cells and peripheral blood mononuclear cells (19, 20). Within the peripheral nervous system, BDNF also contributes to axonal regeneration. The expression of the BDNF gene and its receptor, TrkB, is found in various other tissues throughout the body, including endothelial cells, lungs and the heart (21, 22). BDNF is highly expressed in the hippocampus, a brain region known for its plasticity and critical involvement in learning and memory processes (23, 24) This neurotrophic factor forms a complex with its primary receptor, tropomyosin receptor kinase B (TrkB), which initiates a cascade of signaling pathways vital for neuronal function (14). BDNF has been implicated in various processes, including cognitive modulation, neuroplasticity, angiogenesis, and synaptogenesis (25, 26). Research has demonstrated that levels of BDNF can significantly impact both structural and functional changes in the brain (27), influencing hippocampal neurogenesis (25), long-term potentiation (28), increases in hippocampal volume, and the survival of hippocampal neurons (29, 30). Furthermore, the deterioration of the hippocampus has been strongly associated with multiple neurodegenerative diseases (31–34).
3.1 BDNF geneticsThe Val66Met single nucleotide polymorphism (SNP), also referred to as rs6265, is the most prevalent genetic modifier of BDNF. This specific polymorphism results in the substitution of valine for methionine at codon 66 (35). Research involving various animal models has demonstrated that replacing one or two Val alleles with a Met allele leads to a decrease in exercise-induced BDNF expression within the hippocampus, which subsequently impairs hippocampal plasticity and memory formation (36).
While these findings have not yet been definitively validated in humans, evaluations of hippocampal memory functions in individuals carrying Val and Met alleles suggest that similar effects may occur in humans as well (37). Furthermore, several previous genomic studies have indicated a correlation between BDNF levels and the Val66Met polymorphism; however, not all investigations have consistently supported this relationship (38, 39).
4 Effects of physical exercise on BDNF levels and its relationship with brain healthPhysical exercise is an essential factor for reducing morbidity and mortality, as well as improving quality of life in individuals with chronic diseases. It has been shown to be effective both in the prevention and management of these conditions. However, most patients do not engage in sufficient physical activity, and global healthcare systems offer few supervised programs, limiting the implementation of sustainable strategies for promoting an active lifestyle (40–43). Recognized by the American College of Sports Medicine (ACSM), physical exercise provides significant benefits in the prevention and treatment of diseases, particularly through its effects on the brain and nervous system (44, 45). In older adults (55–80 years), activities such as walking on a treadmill at moderate intensity three times a week have been shown to increase hippocampal volume by 2%, improving spatial memory and strengthening neural networks (26). Additionally, this type of exercise strengthens communication within neural networks, enhancing memory (46). Furthermore, angiogenesis and the proliferation of endothelial cells have been observed in the cerebral cortex and hippocampus (44, 45, 47).
Table 1 summarizes how various modalities, intensities, and durations of physical exercise interact with biological, genetic, and population factors to modulate BDNF levels. Evidence highlights that high-intensity exercises and sustained regimens are the most effective in promoting brain health, cognitive function, and psychological well-being, mediated by interactions between BDNF, its receptor TrkB, and neuronal plasticity.
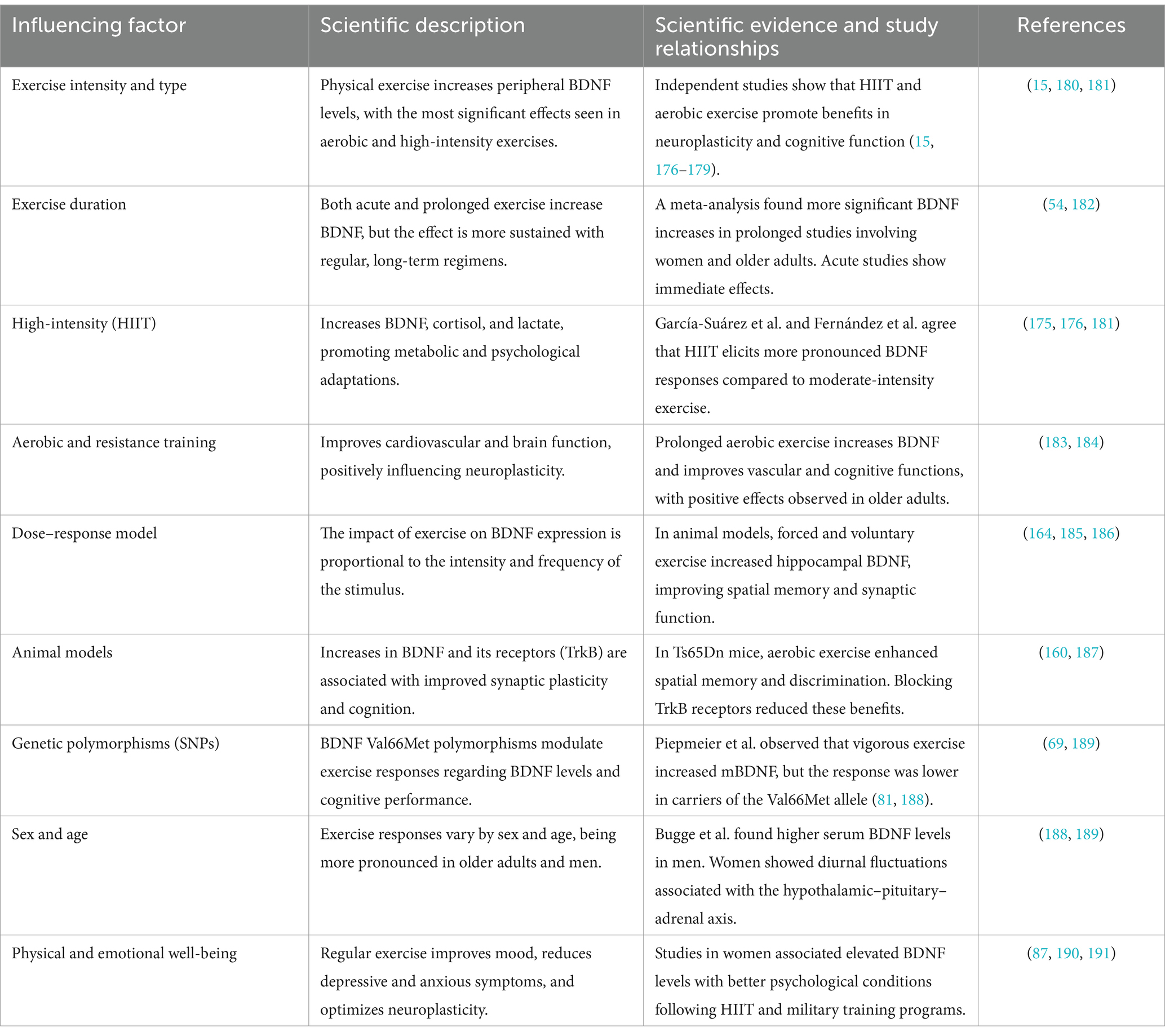
Table 1. Effects of physical exercise on BDNF levels and its relationship with brain health.
Studies in humans and animals demonstrate that the effects of exercise are not only immediate but also cumulative, particularly in vulnerable populations such as older adults or individuals with genetic predispositions. These findings suggest a therapeutic role for exercise in neurodegenerative and mental health conditions, emphasizing the importance of designing personalized interventions based on individual characteristics such as sex, age, and BDNF polymorphisms.
5 BDNF as a mediator of neurogenesis and neuronal plasticityNeuroplasticity refers to the nervous system’s capacity to adjust to environmental changes, which includes modifications in the number of neurons and the enhancement of synaptic connections. This mechanism is essential for learning and memory, enabling organisms to respond appropriately to various environmental stimuli and challenges. At the cellular level, one of the most studied mechanisms related to neuroplasticity and underlying memory formation is long-term potentiation (LTP) (48). Both the performance of exercise and the subsequent increases in BDNF facilitate more efficient and enduring neuronal changes, leading to improvements in long-term potentiation (LTP) (49).
The positive effects of consistent physical activity on neuroplasticity and cognitive functions are clear, particularly through the stimulation of BDNF production. This increase in BDNF fosters improved neuronal development, enhances learning and memory capabilities, and helps prevent cognitive decline. Thus, exercise not only supports general well-being but also significantly contributes to the enhancement of cognitive processes and neuronal plasticity (50). In this regard, BDNF has been recognized as an essential mediator in central synaptic plasticity, a process fundamental for memory formation and learning. It has been observed that BDNF can influence both the facilitation and induction of synaptic changes by stabilizing preexisting synapses and promoting the establishment of new connections (51).
Furthermore, research has shown that BDNF plays a pivotal role in enhancing synaptic transmission, leading to sustained increases like those seen in long-term potentiation (LTP) within brain regions such as the hippocampus and neocortex. This underscores BDNF’s essential function in modulating both synaptic structure and function, positioning it as a significant regulator of cognitive processes (52). By activating its receptor, tropomyosin receptor kinase B (TrkB), BDNF not only promotes neuronal survival but also enhances synaptic plasticity. Conversely, its precursor, proBDNF, has an opposing role; it induces cellular apoptosis through its interaction with the p75 neurotrophin receptor (p75NTR), illustrating their divergent physiological functions (17, 53). Accordingly, the binding of BDNF to TrkB is integral to the mechanisms that facilitate the cognitive enhancements associated with exercise, particularly in learning and memory (54, 55). In contrast, it has been suggested that irisin, a protein released during muscular activity, might increase BDNF expression at the mRNA level and its corresponding protein synthesis. However, the direct connection between BDNF, irisin, and memory in humans is not yet fully established due to the lack of concurrent analyses. Both human studies and animal models have employed various experimental strategies to investigate this association. It has been observed that exercise enhances cognitive processes such as memory through changes in LTP. However, when TrkB receptors, which are essential for BDNF action, are inactivated during exercise, these cognitive effects are not greater than those in control groups (14, 56).
5.1 Neuroplastic changes induced by BDNF expressionPhysical activity can promote neuroplastic changes by modulating the expression of proteins such as BDNF, which is linked to neuronal growth, survival, and synaptic plasticity. Both epidemiological and intervention studies lend support to the notion of utilizing physical exercise to enhance neuroplasticity, especially in pathological contexts. Research has shown that exercise not only leads to structural modifications in the brain but also provides protection against cognitive decline associated with aging (57, 58). Furthermore, more significant cognitive improvements are observed when exercise programs are longer in duration, when participants are older, and when comparing benefits for women to those for men, with women experiencing greater advantages (59). Notably, these exercise-related benefits are also evident in young adult men; a cohort study involving young Swedish men who enlisted in military service at age 18 revealed a substantial positive correlation between cardiovascular fitness and cognitive performance (60).
Several studies explore the differences between free BDNF levels in plasma and serum, with a particular focus on the origin and implications of these differences. According to Radka et al., BDNF levels in human and rat serum are significantly higher than in plasma, with plasma BDNF levels being up to 50 times lower. This BDNF in plasma appears to be derived from platelet degranulation, as it correlates positively with serotonin levels, a marker of platelet release. In contrast, mouse serum did not contain detectable BDNF, reflecting an interspecies difference in the expression of BDNF in specific brain tissues, highlighting its uneven distribution in the nervous system (61) On the other hand, Yoshimura et al. observed that serum BDNF levels are approximately 14 times higher than plasma levels, suggesting that serum, due to its platelet content, contains a higher amount of circulating BDNF. This is also supported by studies showing strong correlations between plasma and serum BDNF levels in healthy volunteers, supporting the idea that differences in BDNF concentration between the two compartments are due to platelet release (62). In a clinical context, Silva et al. reviewed studies on depression and blood BDNF levels, highlighting that BDNF levels in the serum and plasma of depressed patients may reflect alterations in neurotrophic function associated with the pathology, and that these differences may be linked to suicide attempts. Studies also suggest that platelet BDNF content could be a more stable measure of BDNF levels than plasma measurements, due to the shorter half-life of BDNF in plasma (63).
A study examined how physical exercise (PE) and cognitive exercise (CE), both individually and in combination, affect brain function through neurobiological mechanisms that stimulate neuroplasticity. The research included 97 older adults aged 65 to 75, who were randomly divided into four groups: CE followed by rest, PE followed by rest, CE followed by PE, and PE followed by CE. Measurements of plasma BDNF (pBDNF) and serum BDNF (sBDNF) levels, as well as cognitive performance, were conducted both prior to and following the interventions. Results showed that pBDNF levels rose after CE, PE, and rest, while sBDNF levels increased exclusively after PE. Acute PE was found to elevate both pBDNF and sBDNF levels, but prolonged PE did not have the same effect; in contrast, CE only influenced pBDNF levels. No changes in cardiorespiratory fitness were detected in any of the groups. Initial levels of pBDNF and sBDNF were correlated but responded differently to exercise. These results suggest that the biological mechanisms underlying pBDNF and sBDNF responses to exercise require further investigation (64).
In 2020, Muller and his team examined the influence of lactate and BDNF on neuroplasticity resulting from exercise. They discovered that both lactate and BDNF are key mediators in this process. Lactate appears to act as a metabolic signal that triggers the release of BDNF during exercise, thereby promoting synaptic plasticity and neuronal survival (28). The interaction of BDNF with its receptor TrkB is involved in the processes that lead to enhancements in learning and memory associated with exercise (26, 51, 54, 55). In 2019, Lauretta El Hayek and her research team studied the impact of lactate on the influence of exercise on learning and memory using animal models. They studied how lactate activates the SIRT1 signaling pathway in the hippocampus, which in turn activates BDNF and improves learning and memory (65).
The advantages of exercise for BDNF production and neuronal plasticity are significantly associated with improved vascularization in both cerebral and muscular tissues. A recent review indicated that enhanced cardiovascular fitness yields cognitive benefits, which stem from increased cerebral circulation and angiogenesis. This adaptation promotes greater blood flow and an upregulation of neurotrophins within the hippocampus, even following acute exercise sessions (66). Furthermore, several researchers have suggested that the cognitive enhancements resulting from physical exercise may not solely be due to heightened blood perfusion in the brain; rather, they may also arise from the elevated expression of BDNF within the hippocampal structure (67, 68).
In 2019, Piepmeier and colleagues conducted preliminary research examining how varying intensities of acute exercise affect memory and concentrations of BDNF isoforms (proBDNF and mBDNF). The study also considered the influence of the BDNF val66met SNP polymorphism. Participants were divided into three groups: light-intensity exercise, vigorous-intensity exercise, and a control group with no exercise, with evaluations made based on their BDNF allele status. The results showed that exercise intensity and BDNF allele status differentially affect BDNF isoform concentrations and memory performance. Vigorous-intensity exercise increased mBDNF, while carriers of the BDNF allele exhibited lower concentrations of mBDNF. Light-intensity exercise improved memory, but BDNF allele carriers had worse memory performance at 24 h (69).
5.2 Molecular mechanism by which BDNF intervenes in plasticityPhysical exercise triggers a cascade of molecular and cellular mechanisms that foster neuronal plasticity and neurogenesis by promoting the expression of the BDNF-encoding gene (70).
5.2.1 The BDNF/TrkB signaling pathwayAerobic exercise is a form of physical activity that depends on the metabolic utilization of oxygen from the bloodstream for muscle function. BDNF, by interacting with a specific receptor on the cell surface known as tropomyosin receptor kinase B (TrkB), can initiate excitatory or inhibitory signaling pathways, leading to neuronal survival, growth, and differentiation (24, 26). The activation of TrkB triggered by BDNF can initiate distinct signaling pathways that are either excitatory or inhibitory, depending on the target neurons (71). In excitatory neurons, BDNF enhances the expression of molecules like VGluT2, which are critical for glutamatergic neurotransmission, thereby amplifying synaptic excitation (72, 73). Conversely, in inhibitory neurons, BDNF increases the expression of markers associated with GABAergic neurotransmission, such as GABA, GAD65, and GAD67 (74). However, this process is also linked to a reduction in potassium-chloride cotransporter 2 (KCC2) levels, which can disrupt inhibitory balance and, under certain conditions, diminish inhibitory function. The outcome of BDNF–TrkB activation depends on the neurotransmitter profile of the target synapses and the associated molecular changes, including VGluT2 and KCC2 expression (71).
Studies evaluating the cognitive abilities of rats exposed to different experimental conditions (enriched environment with toys and stimulating activities, while others underwent moderate exercise, such as running on a rotating wheel) demonstrate that the combination of moderate exercise and an enriched environment leads to a marked enhancement in learning and memory in rats, along with elevated levels of BDNF and TrkB expression in their brains (75).
Physical activity promotes hippocampal BDNF expression and TrkB signaling via lactate, a metabolite linked to enhanced learning and memory. This process involves FNDC5 (Fibronectin type III domain-containing protein 5), a myokine regulated by PGC1a and ERRa transcriptional activity. Despite uncertainties about how cleaved FNDC5 activates BDNF, these findings highlight a critical mechanism by which exercise benefits the central nervous system, offering potential therapeutic strategies for neurological disorders (65).
The relationship between BDNF, glutamate release, and calcium permeability is critical for synaptic plasticity. BDNF released from the postsynaptic neuron can act on both the same neuron and the presynaptic neuron. In the presynaptic neuron, BDNF increases the number of vesicles containing glutamate, thereby enhancing its release into the synapse (76). In the postsynaptic neuron, BDNF activation of the TrkB receptor boosts the activity of both AMPA and NMDA-type glutamate receptors, which increases their presence on the cell membrane. The activation of NMDA receptors allows a greater influx of Ca2+ into the cell, a process essential for long-term potentiation (LTP). This increase in calcium permeability, facilitated by glutamate release and receptor activity, underpins neuroplasticity in key brain regions, including the hippocampus and cerebral cortex, and supports neuronal survival, growth, and synaptic function (77). Furthermore, BDNF has been demonstrated to increase the density of TrkB receptors on the cell membrane, thereby supporting neuronal survival, growth, and synaptic plasticity in areas such as the hippocampus and cerebral cortex (78). Thus, the interplay between BDNF, glutamate release, and calcium influx is central to the mechanisms of synaptic plasticity.
5.2.2 CREB pathwayAll neurotrophins are first produced as prepro-neurotrophins and subsequently undergo structural modifications via proteolysis. Exercise can activate the CREB pathway, stimulating mRNA synthesis and protein production (79). BDNF, for example, is synthesized as a prepro-neurotrophin and stored in vesicles, where it is converted into pro-BDNF or its mature form, mBDNF, through proteolysis (80). These forms of BDNF have different effects when binding to their receptors, promoting neuroplasticity processes. Pro-BDNF, when binding to the p75NTR receptor, can induce apoptosis and synaptic depression, whereas mBDNF, when binding to TrkB receptors, activates enzymes that release more BDNF, enhancing neuroplasticity (81).
5.2.3 Influence of physical exercise on BDNF and cognitive functionTable 2 summarizes the key factors influencing the relationship between physical exercise, Brain-Derived Neurotrophic Factor (BDNF), and cognitive function. It compiles the scientific descriptions of how exercise impacts BDNF expression and cognitive health, along with evidence from studies that elucidate the molecular, genetic, and aging-related dynamics. The table also explores the variability in exercise responses due to genetic factors, particularly the BDNF gene polymorphism, and highlights the molecular pathways involved in neuroprotection, including the roles of BDNF receptors and intracellular signaling. Additionally, it provides an overview of the findings regarding the effects of exercise on cognitive decline, aging, and neuroplasticity. The references cited are critical studies that support these insights.
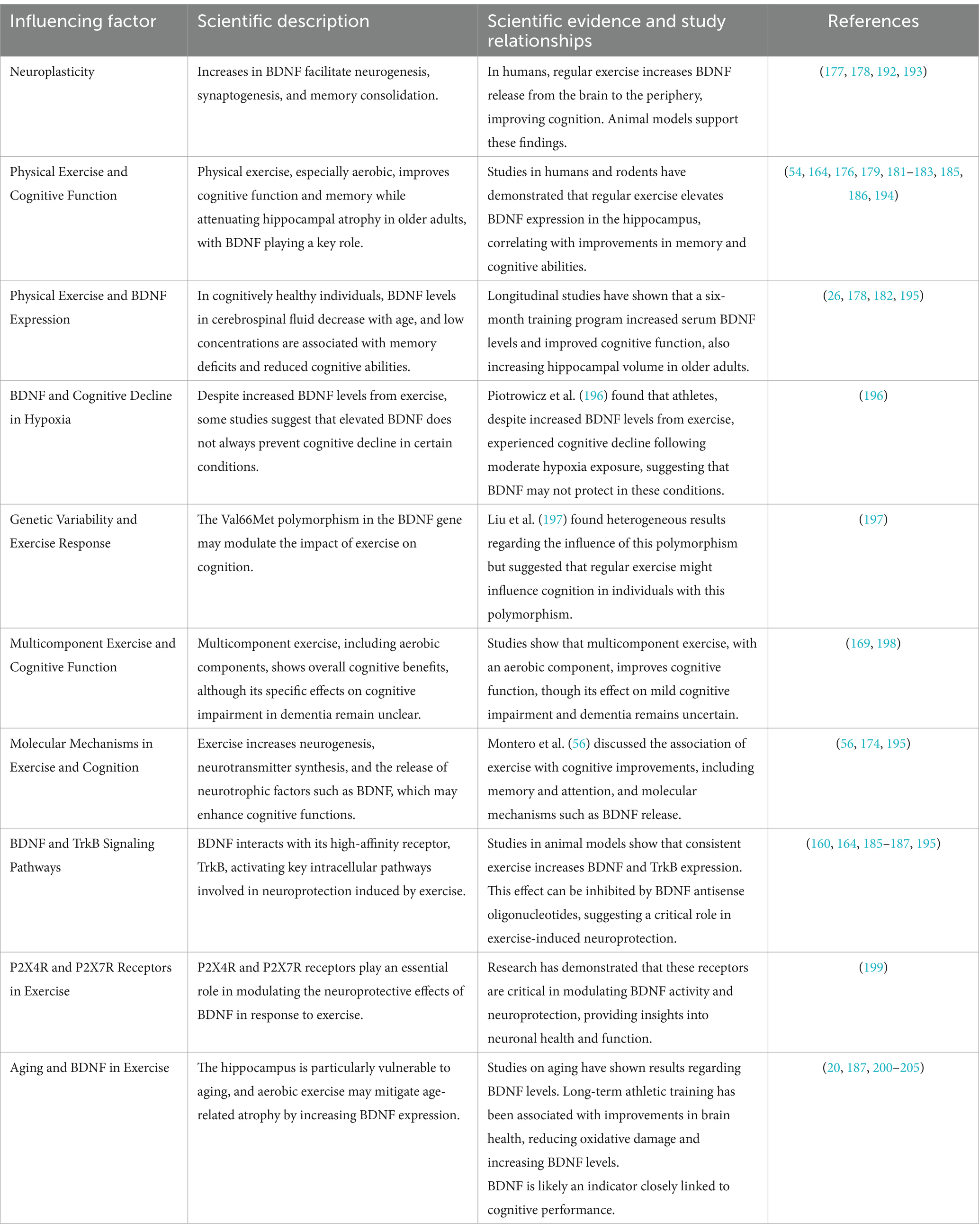
Table 2. Influence of physical exercise on BDNF and cognitive function.
6 BDNF and exercise in neurodegenerative diseases and neuropsychiatric disordersBrain-derived neurotrophic factor (BDNF) is essential in various neuropsychiatric disorders (82). Lower BDNF levels have been associated with neurodegenerative conditions, including Alzheimer’s and Parkinson’s diseases, leading to declines in memory and cognitive function (83). Conversely, higher BDNF levels in the brain correlate with reduced cognitive decline and enhanced memory and recall abilities (84, 85). Furthermore, BDNF is linked to genetic networks related to brain aging and Alzheimer’s disease (38, 86). Research has shown that physical exercise can significantly elevate plasma BDNF levels in individuals with neurodegenerative disorders (87). BDNF exerts its effects on neurons through various intracellular signaling pathways activated by TrkB or p75 receptors. Clinically, BDNF has been implicated in the pathophysiology of multiple brain diseases (30).
In 2022, Campbell and colleagues examined the impact of early-life stress on the regulation of BDNF and explored how exercise interventions may influence this relationship. The study examined the effects of early-life stress in animal models and evaluated how exercise could modulate changes in BDNF expression. The results indicated that early-life stress can negatively impact BDNF regulation, potentially contributing to mental and cognitive health issues. However, exercise interventions demonstrated potential for counteracting these negative effects by increasing BDNF expression and promoting neuronal plasticity in the brain. In summary, the study indicates that physical activity could serve as a beneficial intervention to counteract the negative effects of early-life stress on BDNF regulation, potentially offering important benefits for mental health and emotional well-being across the lifespan (88).
6.1 Genetic interaction of the Val66Met polymorphism with environmental factors in neurodegenerative diseases and neuropsychiatric disordersMultiple studies have established a connection between the Val66Met polymorphism and neuropsychiatric disorders; however, most of these findings have not been replicable, possibly due to interactions among factors such as age, sex, environment, and ethnic origin (5). Evidence indicates that both genetic and environmental factors impact brain development and the risk of neuropsychiatric disorders. Among the environmental factors, including adverse prenatal environments, childhood trauma, climate, and life stress, are crucial in the development of these brain disorders (89, 90).
The BDNF Val66Met polymorphism interacts with multiple environmental and epigenetic factors, heightening the likelihood of developing neuropsychiatric disorders. Specifically, this polymorphism has been linked to amnestic mild cognitive impairment and its advancement to Alzheimer’s disease, attributed to increased methylation of the BDNF promoter (91). For example, in older women with anxiety/depression, increased DNA methylation of BDNF was found, particularly in BDNF Val66Met heterozygotes (92). Conversely, in the context of depression, the BDNF Val66Met polymorphism influences the association between life stress and depression, with a more pronounced effect observed for life stress events compared to childhood adversity (93).
Serum levels of brain-derived neurotrophic factor (BDNF) are influenced by factors such as age, physical activity, and neuropsychiatric disorders, including dementia, depression, anxiety, schizophrenia, and bipolar disorder. A study examining military personnel revealed an increased risk of mental health disorders within this population. This research specifically assessed serum BDNF levels among military samples, encompassing administrative staff and three groups from the Special Operations Forces (SOF). Notably, the SOF-TC group demonstrated higher BDNF levels compared to the control group, which was not subjected to stress. This elevation may be attributed to variations in age or the effects of rigorous physical and mental training, including experiences related to post-traumatic stress disorder (PTSD). These findings indicate that BDNF levels could serve as a valuable biomarker for evaluating mental health in military personnel facing operational stress, thereby offering potential insights for the prevention, diagnosis, and management of mental health disorders within this demographic (87).
A supervised exercise program in primary care has been shown to significantly improve the quality of life and emotional and social well-being of patients in advanced stages of their disease. One study identified both potential barriers and facilitators related to participation in exercise and its maintenance. However, it is crucial to promote intersectoral coordination in the socio-healthcare field to ensure integrated and continuous care for chronic patients (94).
Shuo Chan and colleagues, in 2022, investigated the effects of physical exercise on children with attention deficit hyperactivity disorder (ADHD). The researchers examined how exercise affected various aspects of ADHD, such as attention, impulsivity, and hyperactivity. The results showed that physical exercise had beneficial effects on children with ADHD. Improvements were observed in attention, concentration, and control of impulsivity after participating in regular physical exercise programs. Additionally, exercise also helped reduce symptoms of hyperactivity in these children (95).
6.2 DepressionMore than three decades ago, physical inactivity was recognized as a significant risk factor for depression among adults. Since that time, numerous studies have reinforced the notion that exercise serves as an effective intervention for alleviating depressive symptoms. Randomized controlled trials have demonstrated that physical activity, whether performed alone or in conjunction with antidepressant medications, leads to a notable reduction in depressive symptoms compared to those observed in sedentary individuals (96). Furthermore, physical activity has been found to have an inverse relationship with anxiety sensitivity in young adults, contributing to improvements in symptoms across various anxiety disorders, including schizophrenia, autism, depression, and bipolar disorder (97). A critical element in this context is the role of psychological stress, which has been identified as a significant factor in the observed reductions in both depression and anxiety symptoms (98) Moreover, research indicates that individuals with depression often exhibit lower serum levels of brain-derived neurotrophic factor (BDNF), a relationship supported by genetic studies linking BDNF dysfunction to depressive disorders (99). Additionally, analyses have shown a marked decrease in BDNF levels within the hippocampus of patients diagnosed with major depression, further underscoring the importance of BDNF in the pathophysiology of depressive conditions (100, 101).
Conversely, antidepressant therapy has been shown to enhance BDNF expression in specific brain regions, resulting in increased levels of BDNF mRNA within just a few days of treatment. This elevation in BDNF is mediated by TrkB signaling pathways in areas such as the hippocampus and midbrain (102). Research conducted on rodent models indicates that direct administration of BDNF protein into the hippocampus may produce antidepressant-like effects (103). Furthermore, variations in BDNF function appear to affect hippocampal activity in individuals with depression, suggesting that BDNF levels may serve as potential biomarkers for the early diagnosis and timely intervention for depression and associated suicidal behaviors (104, 105).
Evidence indicates that anxiolytic medications can substantially enhance BDNF expression in the hippocampus and prefrontal cortex, resulting in increased levels of BDNF mRNA or BDNF protein (103, 106). In support of this, Jemni et al. investigated the biological and molecular mechanisms connecting various types, intensities, and durations of exercise to BDNF expression and the alleviation of depressive symptoms. Their findings highlight the potential of exercise as an effective intervention for depression (97). Additionally, Murawska et al. explored the interaction between BDNF and biological markers of depression, emphasizing the influence of physical exercise and training. Their review synthesized existing literature to elucidate how BDNF affects depression-related biomarkers, including gene expression, neuroplasticity, and overall brain function (97, 107).
6.3 Parkinson’s diseaseParkinson’s disease (PD) is a progressive neurological disorder characterized by the degeneration of brain cells, which negatively impacts motor control and cognitive abilities. Key pathological features of PD include the loss of dopaminergic neurons in the substantia nigra—affecting up to 70% of these cells—and the formation of Lewy bodies, which are aggregates of α-synuclein protein found in the remaining neurons (108). These neurobiological alterations contribute to the motor and cognitive impairments observed in PD patients (109).
Evidence indicates that exercise therapy may alleviate symptoms associated with Parkinson’s disease by enhancing neuroplasticity, improving dopaminergic function, and decreasing the accumulation of α-synuclein (110–113). Additionally, exercise therapy shows direct benefits for individuals with acquired brain injuries (114). Brain-derived neurotrophic factor (BDNF) is thought to serve as a protective agent against neurodegeneration in PD, with its levels increasing in response to exercise (115–117). This elevation in BDNF may be a key mechanism through which exercise improves clinical outcomes in PD patients. Notably, high-intensity interval training appears to have a more pronounced effect on BDNF levels than moderate-intensity continuous exercise (108, 118–120).
In animal models of Parkinson’s disease, exercise has been shown to enhance motor function by preserving nigrostriatal dopaminergic neurons, protecting mitochondrial integrity, and inhibiting the formation of Lewy bodies within the nigrostriatal pathway. These benefits contribute to the alleviation of motor symptoms, providing neuroprotection and potentially slowing disease progression through mechanisms such as improved mitochondrial function and reduced pathological accumulation of α-synuclein (83, 121, 122).
Studies conducted in 2016 revealed significant cognitive deficits in patients with Parkinson’s disease, evidenced by lower scores on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) compared to healthy controls. Additionally, patients with Parkinson’s disease exhibited significantly lower serum BDNF levels (109). A positive correlation was found between BDNF levels and scores on the five RBANS indices, suggesting that diminished BDNF is associated with cognitive impairments in PD. These findings underscore the importance of BDNF in hippocampal neuroplasticity, which is critical for learning and memory, implying that reduced BDNF may contribute to the cognitive decline observed in Parkinson’s disease (9).
Despite its largely sporadic and unknown etiology, post-mortem studies have documented decreased BDNF levels in the substantia nigra compacta (SNc) of PD patients. Furthermore, evidence suggests that BDNF supports the survival of dopaminergic neurons in animal models of Parkinson’s disease. Recent studies emphasize the significance of BDNF signaling via its receptor, TrkB, in the survival of nigrostriatal dopaminergic neurons during brain aging (109). involving TrkB hypomorphic mice, which exhibit reduced receptor expression, revealed substantial loss of dopaminergic neurons in the SNc, along with diminished dopaminergic axonal terminals in the striatum and gliosis in both the SNc and striatum. Additionally, these dopaminergic neurons displayed heightened sensitivity to the neurotoxin MPTP, indicating that impaired BDNF–TrkB signaling may play a role in the progression of Parkinson’s disease (109).
Palasz et al. investigated the therapeutic potential of BDNF in the context of Parkinson’s disease, demonstrating that BDNF may offer promising therapeutic benefits by protecting dopaminergic neurons, enhancing motor function, and promoting neuronal survival in animal models of the disease. The authors also discussed potential strategies to elevate BDNF levels in the brain as a therapeutic approach for managing Parkinson’s disease (83).
6.4 Alzheimer’s diseaseAlzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disorder characterized by the degeneration and loss of neurons and synapses in the cerebral cortex and specific subcortical regions, primarily leading to symptoms such as memory loss, anxiety, and confusion (123). Research indicates that a sedentary lifestyle may contribute to an earlier onset of AD. Recent studies have shown that exercise interventions can alleviate neurodegenerative symptoms in patients with AD, and similar effects have been observed in rodent models (124). Moreover, multimodal exercise has been associated with improvements in functional capacity, suggesting that physical activity is beneficial for older adults diagnosed with Alzheimer’s disease (AD) (125).
In terms of biomarkers, aerobic training has been found to elevate plasma levels of the myokine Cathepsin B (CTSB), with changes in CTSB levels correlating with cognitive performance, while levels of BDNF have been observed to decrease. Metabolic analyses have indicated changes in polyunsaturated free fatty acids, ceramides, sphingolipids, phospholipids, and gut microbiome metabolites, alongside alterations in redox homeostasis. Notably, around 30% of the identified metabolites showed correlations with changes in BDNF levels, suggesting a possible exercise-induced metabolic regulation. The positive relationship between CTSB and cognitive performance, along with the modulation of lipid metabolites, underscores the beneficial effects of exercise on brain function, positioning CTSB as a potential marker for cognitive changes in middle-aged adults at risk for dementia (126).
In a study by Baranowski et al., both physical exercise and BDNF injections were found to positively influence APP processing enzymes and enhance cognitive function in animal models, indicating their potential as therapeutic interventions for neurodegenerative disorders like Alzheimer’s disease (127, 128).
However, discrepancies exist in the literature. For instance, Gaitán et al. explored the impact of aerobic exercise training on systemic biomarkers and cognitive function in middle-aged adults at risk for Alzheimer’s disease through a randomized controlled trial. After 26 weeks of treadmill training, an increase in the exercise-related biomarker CTSB was noted, alongside a decrease in BDNF levels. The correlation between CTSB and cognitive performance indicates a connection to brain function, with additional metabolic changes, such as increased fatty acid levels and decreased certain lipids, also observed (126).
Furthermore, Baranowski et al. investigated BDNF’s effect on the activity of the BACE1 enzyme, which is responsible for producing beta-amyloid peptides associated with Alzheimer’s disease, alongside the effects of exercise in animal models. Their findings revealed that direct BDNF treatment significantly reduced BACE1 activity in the prefrontal cortex, independent of other changes induced by exercise. Additionally, a high-fat diet was shown to lower BDNF levels in the hippocampus, while acute exercise was found to elevate BDNF levels in the prefrontal cortex (129).
6.5 SchizophreniaSchizophrenia is marked by significant changes in brain morphology, including enlarged ventricles and reductions in various brain regions (130, 131). These structural alterations have been linked to positive symptoms, negative symptoms, and cognitive deficits associated with the disorder (132, 133). The activity of brain-derived neurotrophic factor (BDNF) is believed to be influenced by genetic factors, particularly the Val66Met polymorphism (134, 135). BDNF plays a vital role in neurogenesis and synaptic connectivity in the brains of individuals with schizophrenia (136). Particular attention has been directed toward the Val66Met polymorphism within the BDNF gene, which is associated with reduced brain volume, especially in areas like the hippocampus (137–139). Studies indicates that individuals carrying the Met allele tend to exhibit a smaller overall brain volume and greater reductions in specific regions compared to Val/Val homozygotes (140, 141).
Investigations into the relationship between serum BDNF levels and brain volume changes in schizophrenia have been conducted. Evidence suggests that peripheral BDNF levels are generally lower in individuals with schizophrenia compared to healthy controls (139). Nonetheless, the precise correlation between serum BDNF levels and brain volume reductions remains unclear (142–144). Further research examining the connection between BDNF and brain volume changes in schizophrenia could enhance our understanding of the disorder’s pathophysiology and potentially inform the development of new therapeutic strategies (145).
6.6 Multiple sclerosis (MS)Amato and colleagues investigated (146) the effects of physical exercise, specifically lactate threshold training, in patients with multiple sclerosis (MS). In a study with eight subjects, the effects of a biweekly training program over 12 weeks were evaluated at three time points: baseline (T0), after the program (T1), and 9 months later (T2), considering physical, psychological, hematochemical parameters and dietary habits. The results showed a significant increase in brain-derived neurotrophic factor (BDNF) levels, suggesting that exercise could help improve neuronal function and reduce the progression of MS. Although dehydroepiandrosterone sulfate (DHEAS) showed a trend towards increase, it did not reach statistical significance but was associated with reduced fatigue, a common symptom in MS. Furthermore, a decrease in lactate levels was observed, indicating an improvement in lactate reutilization as an energy substrate, contributing to greater metabolic efficiency. Exercise also favored patients’ self-efficacy, improving their perception of their ability to cope with the disease’s challenges and enhancing their self-esteem. These findings support the hypothesis that physical exercise has synergistic effects on inflammation, neuroprotection, and neuronal plasticity, although further studies with larger samples and longer intervention periods are needed to confirm these results.
In addition, Brain-derived neurotrophic factor (BDNF) is a key regulator of neuroprotection, neuroplasticity, and remyelination, processes that are critically important in the context of multiple sclerosis (MS) (147). BDNF facilitates repair mechanisms within the central nervous system, which are often impaired in MS, resulting in progressive neuronal damage and increasing disability over time (147). Despite the still unresolved mechanisms underlying remyelination (148) and its variable extent among MS patients, BDNF is believed to play an essential role in these regenerative processes. Recent findings indicate that BDNF might exert beneficial effects on MS, as research showing favorable clinical improvements and MRI results predominates over studies suggesting negative effects. The role of the Val66Met polymorphism in MS remains uncertain, with studies divided on whether it represents a protective or detrimental factor (149, 150). However, the prevailing hypothesis suggests that the polymorphism may modulate BDNF secretion and exert anti-inflammatory effects, contributing to the disease’s progression in an inflammatory environment. To clarify BDNF’s impact on MS and refine therapeutic strategies, additional research involving larger patient cohorts and extended longitudinal studies is required.
7 Neuroprotection and exercise: therapeutic potentialIn recent decades, a paradox has emerged: although life expectancy continues to rise, there has also been a concomitant increase in chronic conditions, leading to significant functional costs (151). These costs have serious implications for both health and socioeconomic factors. Cognitive decline is one condition that illustrates this issue, affecting nearly fifty million individuals globally, as reported by the World Health Organization (WHO) (152). Currently, pharmacological treatments for cognitive decline lack sufficient efficacy and often produce limited cognitive benefits (153–155). Consequently, research is increasingly focusing on non-pharmacological approaches for managing dementia and mild cognitive impairment (156). Accumulating evidence indicates that physical exercise represents the most effective non-pharmacological intervention for enhancing brain health. Studies demonstrate that exercise can diminish the risk of cognitive decline and delay the onset of dementia (157). Regular physical activity not only mitigates age-related cognitive decline but also reduces the likelihood of developing neurodegenerative diseases and psychiatric disorders (158). These protective effects are attributed to enhanced neurogenesis and neuroplasticity, which improve learning and memory. At a molecular level, brain-derived neurotrophic factor (BDNF) is integral to the neuroprotective effects of physical activity (158). Individuals who do not engage in regular exercise exhibit a significantly elevated risk of cognitive decline (159). Thus, physical exercise is proposed as an accessible, low-cost intervention with minimal side effects, allowing for benefits without requiring extensive physical activity (160, 161).
Numerous studies have shown that consistent physical activity not only enhances cardiovascular and metabolic health but also fosters neuroplasticity and neurogenesis, both of which are essential for maintaining cognitive function and preventing neuronal degeneration. The roles of physical exercise and BDNF have become central to understanding neurodegenerative disease modulation (162, 163). Furthermore, research indicates that exercise interventions positively influence BDNF levels in healthy individuals (164, 165). BDNF is crucial for neuronal survival and growth, underscoring its significance in neurological health (29). However, some researchers argue that while physical exercise promotes beneficial effects via BDNF expression, the precise cellular sources and molecular mechanisms underlying the elevated BDNF production in the brain resulting from exercise are still being elucidated (159, 161, 166, 167).
An increase in BDNF levels due to physical activity is thought to enhance adult neurogenesis and synaptogenesis, preventing neuronal loss and potentially leading to cognitive improvements and reductions in psychiatric symptoms. It is noteworthy that peripheral BDNF levels may reflect central nervous system health to some degree, with lower concentrations typically observed in individuals with psychiatric and metabolic disorders (29).
Neurodegenerative diseases are often associated with diminished BDNF levels. A recent meta-analysis examined the effects of exercise on plasma BDNF levels in individuals with various neurodegenerative disorders, including multiple sclerosis, Parkinson’s disease, mild cognitive impairment (MCI), and Alzheimer’s disease. This meta-analysis, encompassing 18 randomized controlled trials (RCTs), found that exercise interventions significantly elevated plasma BDNF levels. Notably, this increase was particularly evident in individuals with multiple sclerosis and Parkinson’s disease, while MCI showed a non-significant trend. The elevation in BDNF levels was consistent across different types of exercise, including combined, aerobic, or strength training, as well as varying weekly exercise volumes and intervention durations. The authors concluded that physical exercise interventions can enhance plasma BDNF levels in individuals with neurodegenerative disorders, indicating potential therapeutic benefits for these conditions (86).
Supporting this notion, BDNF is posited to act as a crucial mediator linking physical activity with the alleviation of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. A recent meta-analysis by Hirsch et al. (206) reaffirmed that exercise positively impacts BDNF levels in individuals with Parkinson’s disease, although it included uncontrolled studies that may introduce bias (168). Similarly, increases in BDNF levels have been documented in patients with neurological disorders, including stroke, following aerobic exercise interventions (169).
Among the physiological mechanisms investigated, exercise is recognized for its neuroprotective role, enhancing neuronal survival, promoting angiogenesis and neurogenesis, reducing inflammation, and significantly lowering cerebrovascular risk factors (157, 168, 170–172).
In animal models, research by Nagahara et al. demonstrated that BDNF provides neuroprotective effects on neural circuits affected by Alzheimer’s disease. In transgenic mice exhibiting amyloidosis, BDNF gene administration post-disease onset reversed synaptic loss, partially normalized abnormal gene expression, enhanced cellular signaling, and restored learning and memory, independent of amyloid plaque load. Moreover, BDNF infusion in aged rats reversed cognitive decline and improved age-related gene expression disruptions. Additionally, BDNF was shown to prevent lesion-induced death of entorhinal cortical neurons in rats and adult primates, while reversing neuronal atrophy and improving cognitive decline in aged primates (173).
8 DiscussionBrain-derived neurotrophic factor (BDNF) plays a crucial role in neuroplasticity, neurogenesis, and neuronal protection, all of which are fundamental for maintaining optimal brain function and preventing neurodegenerative diseases. The available data suggest that physical exercise is a highly effective intervention for reducing the risk of neurological and psychological disorders, as well as preserving mood stability and neuronal integrity. Integrating exercise into treatment plans alongside conventional therapies has the potential to decrease mortality rates without introducing the side effects commonly associated with pharmacological treatments.
Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, are increasing public health challenges globally. Over 55 million individuals currently suffer from dementia, and Parkinson’s disease prevalence has doubled in recent decades, significantly impacting quality of life. Numerous scientific studies have established BDNF as a pivotal factor in promoting brain health.
One of the most promising aspects of ongoing research focuses on the regulatory effects of physical exercise on BDNF. Recent investigations have explored how various exercise types—differing in intensity and duration—modulate BDNF levels and the associated outcomes on cognition, emotional well-being, and neuroplasticity. Studies have consistently shown that high-intensity exercise induces a significant, immediate increase in BDNF levels in healthy young adults, highlighting that physical activity not only yields long-term neuroprotective effects but also induces acute changes in neurotrophic markers. Research by Dadkhah et al. (174) and Antunes et al. (175) has provided experimental and clinical evidence on the role of exercise in BDNF regulation and its positive effects on cognitive function. These findings underscore the potential of incorporating physical exercise as a complementary strategy in the prevention and treatment of neurodegenerative diseases.
Additionally, the interaction between BDNF and genetic factors, such as the BDNF Val66Met polymorphism, adds further complexity to the relationship between exercise and cognitive health. The BDNF Val66Met polymorphism has been shown to influence cognitive function, particularly when combined with regular physical exercise, suggesting that individual genetic profiles may modulate the brain’s response to exercise-induced BDNF regulation. This growing body of evidence supports the notion that physical activity can significantly impact BDNF regulation, providing protection against age-related cognitive decline and neurodegenerative disorders. However, more research is necessary to elucidate the specific mechanisms involved and optimize exercise-based interventions for diverse populations, including those with neurological conditions.
This expanding field of research holds the potential to revolutionize our understanding and management of neurodegenerative diseases, offering new strategies to enhance brain health and improve the quality of life for millions worldwide.
Author contributionsAR: Writing – original draft. VD: Writing – original draft. EJ: Writing – original draft. JN: Writing – original draft, Writing – review & editing. LM: Writing – original draft, Supervision, Validation, Writing – review & editing, Conceptualization, Investigation.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this
留言 (0)