Type 2 diabetes mellitus (T2DM) is associated with an elevated risk of fractures and increased post-fracture mortality. Despite having higher bone mineral density (BMD), individuals with T2DM face a paradoxically higher risk of fractures, complicating the prediction and management of fracture risk in this population (1). Hyperglycemia contributes to increased bone matrix rigidity and reduced bone toughness by promoting the accumulation of advanced glycation end products (AGEs) (2). Additionally, insulin resistance or insufficient insulin secretion further suppresses osteoblast function, leading to decreased bone formation (3). Furthermore, patients with T2DM often exhibit a state of chronic low-grade inflammation, characterized by elevated levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). These cytokines disrupt bone metabolic balance by enhancing osteoclast activity and inhibiting osteoblast differentiation (4). Simultaneously, oxidative stress and microvascular complications indirectly impair bone health by damaging osteocytes and reducing bone blood supply (5).
Commonly utilized clinical bone turnover markers, such as type 1 collagen amino-terminal propeptide (P1NP) and carboxy-terminal collagen crosslinks (CTX), effectively reflect bone turnover changes and predict fracture risk in non-diabetic individuals (6). However, these markers do not correlate with fracture risk in T2DM patients, thus limiting their utility in this context. Furthermore, there is a significant gap in the understanding of the molecular mechanisms that underlie changes in bone density during the progression of T2DM.
Sirtuin 1 (SIRT1), a class III histone deacetylase, is known for its extensive biological activities, primarily through the modulation of various transcription factors such as p53, FoxOs, NF-κB, and PPARγ, all of which play critical roles in multiple disease processes (7). In the context of diabetes mellitus, SIRT1 serves as a protective factor for pancreatic β-cells, enhancing insulin secretion in response to glucose, promoting gluconeogenesis, reducing oxidative stress, and ameliorating insulin resistance in key metabolic tissues such as the liver, adipose tissue, and skeletal muscle (8, 9). Studies focusing on bone metabolism have revealed that SIRT1 plays a role in promoting osteoblast differentiation while inhibiting osteoclast maturation, In the molecular regulation of bone metabolism, SIRT1 plays a critical protective role by promoting osteoblast differentiation through activation of the Wnt/β-catenin signaling pathway. SIRT1 also suppresses osteoclast maturation and reduces the production of inflammatory cytokines, contributing significantly to the regulation of bone metabolism. However, its precise role in bone density and metabolism within the context of type 2 diabetes mellitus (T2DM) pathogenesis remains poorly understood (10). This study offers an initial investigation into the potential involvement of Sirtuin 1 in alterations of bone density among patients with T2DM, and its potential utility as a biomarker for evaluating bone metabolism. Through this exploration, the study aims to contribute clinical evidence towards identifying novel biomarkers for assessing bone metabolism in individuals with T2DM.
2 Materials and methods2.1 Research objectsThe study was approved by the Ethics Committee of Chinese 903rd Hospital of PLA, conducted in accordance with the Declaration of Helsinki. Prior to this study, written informed consent was obtained from all the subjects. Data were collected from both outpatients and inpatients at the Department of Endocrinology, 903rd Hospital of PLA, China, between March 2015 and August 2023.
According to the inclusion and exclusion criteria of the 1999 World Health Organization (WHO) criteria for diabetes diagnosis and classification, the specific method for the oral glucose tolerance test involves administering a solution containing 75 grams of anhydrous glucose to adults, a total of 69 newly diagnosed T2DM patients were included as the T2DM group (mean age 48 years)and 82 patients were carried as the NGT group(mean age 49 years). Then divide into four subgroups on the basis of BMD levels [normal BMD subjects (−1.0 < T/Z < 1.0) and decreased BMD subjects (T/Z ≤ −1.0, including osteopenia and osteoporosis)] for further analysis. Exclude type 1 diabetes patients and type 2 diabetes patients who have used hypoglycemic drugs, chronic renal insufficiency, history of parathyroid or thyroid diseases, with a fracture history, long-term use of glucocorticoids, heparin, antiepileptic drugs, antipsychotic medications, thiazides, calcium supplements, or other medications affecting bone metabolism and anti-osteoporotic drugs.
2.2 Anthropometric and biochemical parametersAnthropometric measurements were conducted with high precision following standardized methodologies. Height was recorded in a standing position using a wall-mounted stadiometer (VM Electronics, Chicago, IL, USA), while body weight was obtained to the nearest 0.1 kg with a digital scale. Hip circumference was accurately measured at the maximal protrusion of the gluteal region, and waist circumference (WC) was precisely determined at the widest point between the xiphoid process and the iliac crest. Key indices of adiposity, including waist-to-hip ratio (WHR) and body mass index (BMI), were calculated with rigorous attention to detail. BMI was derived by dividing weight (kg) by height squared (m²), and WHR was calculated as the ratio of waist circumference (cm) to hip circumference (cm), both important metrics for assessing body composition. After anthropometric assessments, blood pressure measurements, including systolic (SBP) and diastolic blood pressure (DBP), were obtained under standardized conditions.
Blood samples were collected after a minimum 10-hour overnight fast and stored at -80°C for subsequent analysis. Advanced biochemical parameters were measured using an automatic biochemical analyzer (Abbott, Aeroset C16000, USA) for accurate quantification of total triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), and 2-hour plasma glucose (2hPG). Fasting insulin (FINS) was measured via a chemiluminescence immunoassay analyzer (Abbott, I4000, USA), and glycosylated hemoglobin (HbA1c) was assessed using the Afinion AS100 analyzer (Alere, Afinion AS100, USA). Additionally, insulin resistance (HOMA-IR) and β-cell function (HOMA-β) were rigorously calculated using the homeostasis model assessment (HOMA) equations: HOMA-IR = FBG (mmol/L) × FINS (μU/mL)/22.5, and HOMA-β = 20 × FINS (μU/mL)/[FBG (mmol/L) − 3.5].
2.3 Measurements of Sirtuin 1, bone turnover markers, and BMDEnzyme-linked immunosorbent assays (ELISAs) were utilized for the quantification of sirtuin 1 levels (Dingguo, Guangzhou, China), expressed in umol/L. Serum levels of osteocalcin (OC), procollagen type 1 intact N-terminal propeptide (P1NP), β-C-terminal telopeptide of type I collagen (β-CTX), and 25-hydroxyvitamin D (25(OH)D) were determined via chemiluminescence immunoassay (CLIA) kits following the manufacturers’ instructions (Beckman, USA). Bone mineral density (BMD) of the L1–L4 vertebrae (Lumbar BMD) was assessed using dual-energy X-ray absorptiometry (DXA) (GE Medical systems lunar, USA) and expressed as absolute values (g/cm2), T-scores, or Z-scores.
2.4 Statistical analysisThe continuous variables with a normal distribution were expressed as means ± standard deviation (SD) and analyzed using independent samples t-test. For variables deviating from a normal distribution, data were presented as median [interquartile range (IQR)] and analyzed using the Mann–Whitney test. Subgroup comparisons were conducted using one-way ANOVA followed by post hoc analysis. Pearson correlation analysis was applied to assess the association between sirtuin1 levels and parametric variables, while Spearman correlation analysis was used for nonparametric variables. Binary logistic regression analysis was utilized to determine risk factors for osteoporosis. A significance level of p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 29.0 software.
3 Results3.1 The clinical parameters and serum SIRT1 levels of the two groupsAll collected data are summarized in Tables 1 and 2. Compared to the control subjects, patients with T2DM exhibited significantly higher diastolic blood pressure (DBP), weight, BMI, waist circumference, triglycerides (TG), and total cholesterol (TC), alongside lower levels of procollagen type 1 N-terminal propeptide (PINP) and high-density lipoprotein cholesterol (HDL-C) (P < 0.01 or P < 0.05). Notably, serum SIRT1 levels in the T2DM group were markedly reduced than those in the control group (P < 0.05).No significant differences were observed between the two groups regarding lumbar BMD, osteocalcin (OC), 25-hydroxyvitamin D [25(OH)D], and β-C-terminal telopeptide of type I collagen (β-CTX) (P > 0.05). The incidence of osteoporosis was higher in the T2DM group compared to the control group (34.8% vs. 18.3%, P = 0.026). Furthermore, SIRT1 levels in T2DM patients with osteoporosis (DMo) were substantially lower than in NGT patients with osteopenia or osteoporosis (NGTo) (27.50 ± 7.95 vs. 32.34 ± 8.18 μmol/L, P < 0.05).However, no significant difference in serum SIRT1 levels was found between NGT patients with osteopenia or osteoporosis (NGTo) and those with normal BMD (NGTn) (32.34 ± 8.18 vs. 32.90 ± 7.32 μmol/L, P > 0.05), as illustrated in Figure 1.
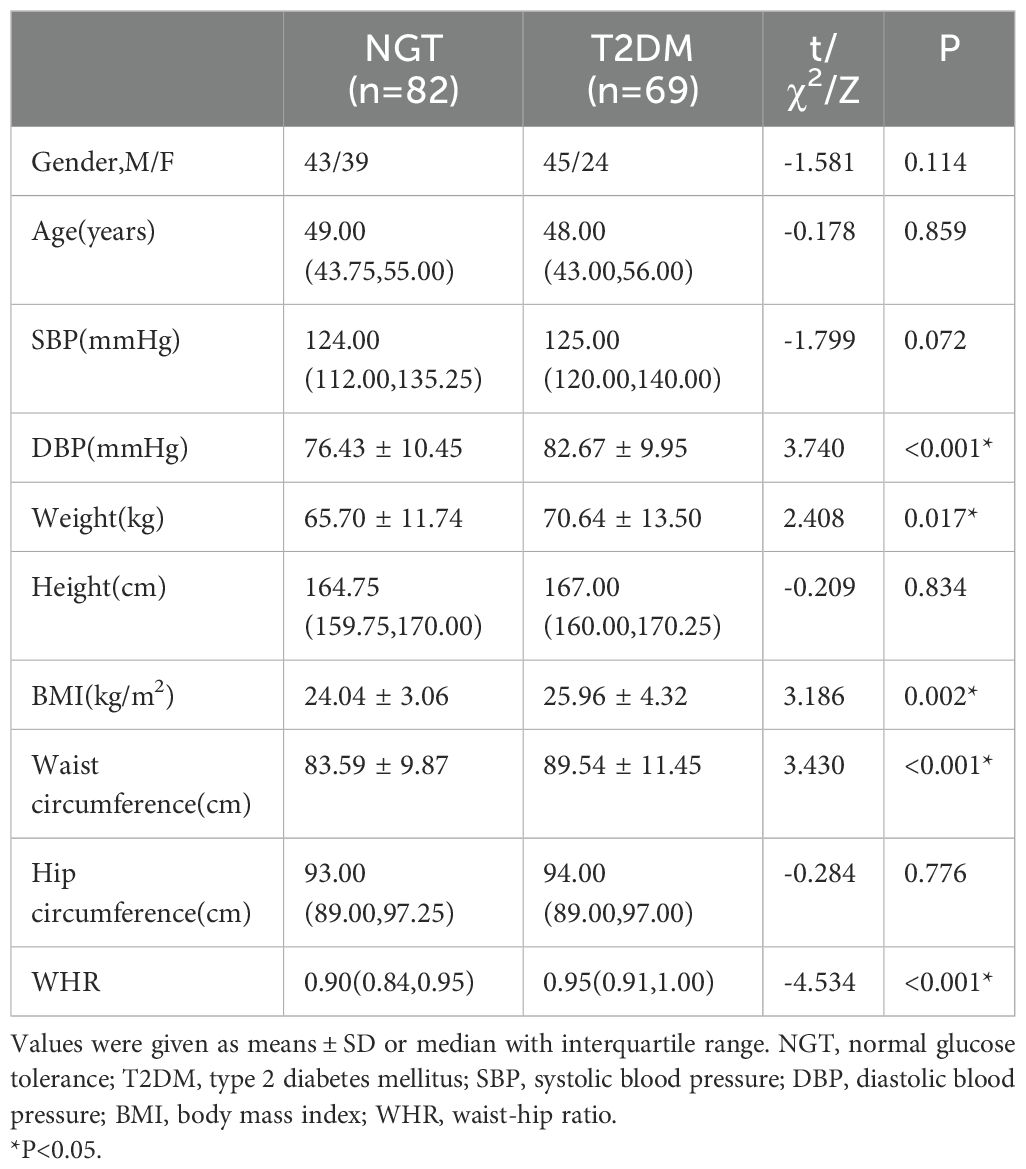
Table 1. Clinical physical parameters of controls and T2DM subjects.
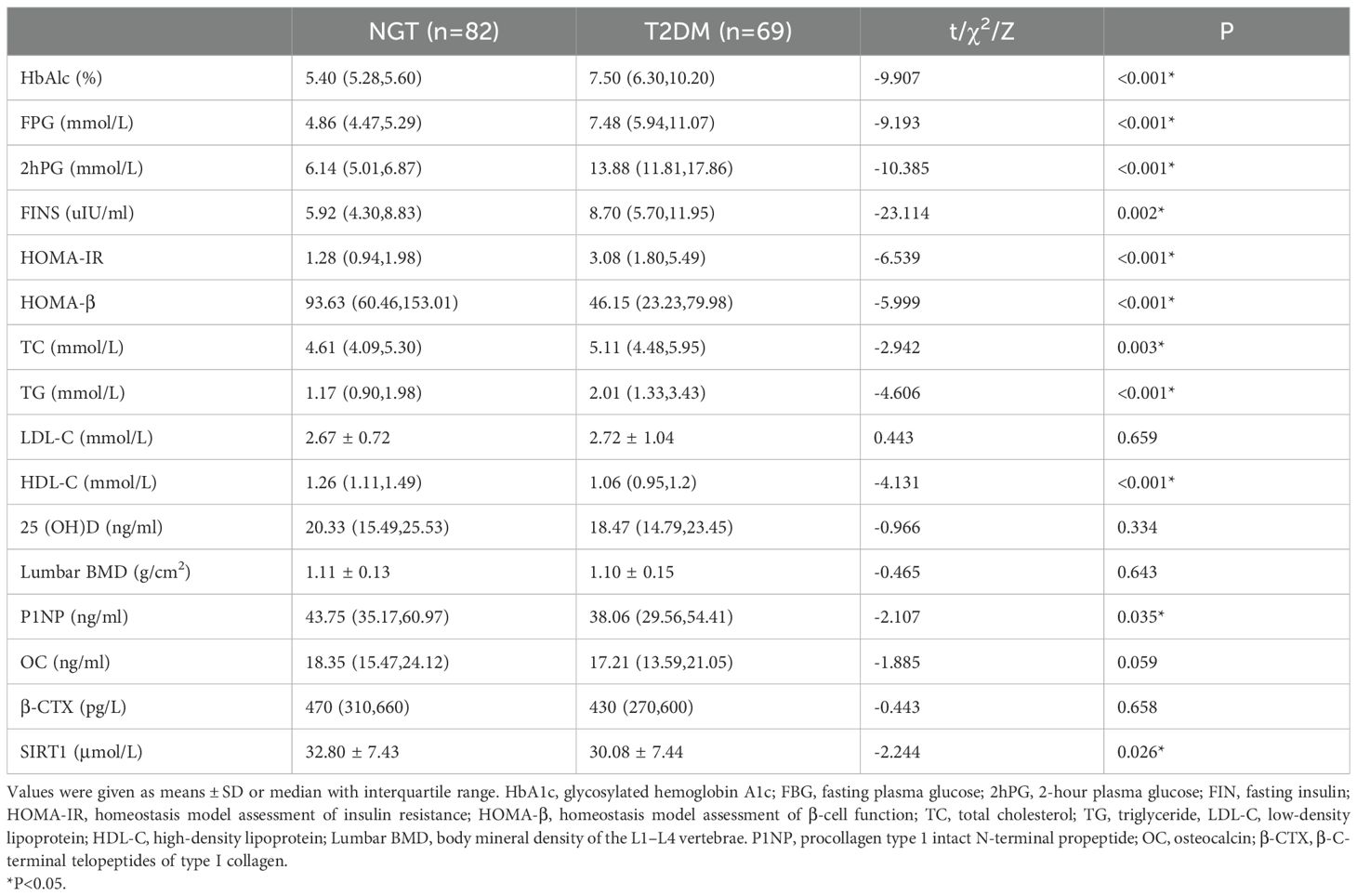
Table 2. serum test parameters of controls and T2DM subjects.
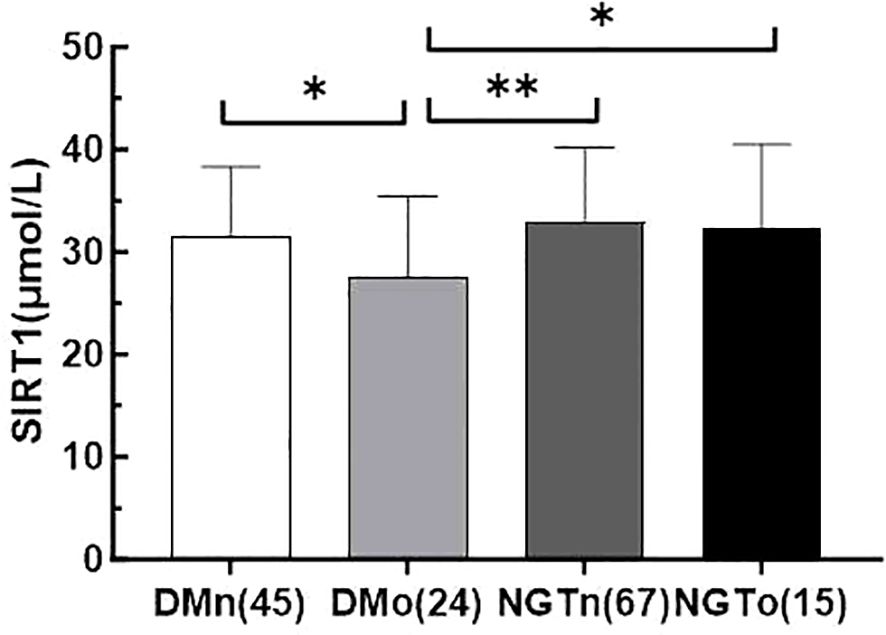
Figure 1. Serum irisin levels in four subgroups of DMn (T2DM with normal BMD), DMo (T2DM with osteopenia or osteoporosis), NGTn (NGT with normal BMD), and NGTo (NGT with osteopenia or osteoporosis). The number of subjects was shown in parentheses. values are evaluated by a Tukey (HSD) test. * indicate P< 0.05, ** indicate P < 0.01.
3.2 Relevant factors in pathogenesis of osteoporosisIn the NGT group, lumbar BMD was found to have a significant negative correlation with PINP (r = -0.236, P < 0.05). In contrast, in the T2DM group, lumbar BMD was inversely correlated with age (r = -0.313, P < 0.01). Additionally, lumbar BMD in the T2DM group showed positive correlations with waist circumference (r = 0.279, P < 0.05), weight (r = 0.333, P < 0.01), height (r = 0.272, P < 0.01), and hip circumference (r = 0.245, P < 0.05) (Table 3), correlations that were not observed in the NGT group. Stepwise binary logistic regression analysis further suggested that SIRT1, age, HDL-C, PINP, and β-CTX were independently associated with the pathogenesis of osteoporosis (P < 0.01 or P < 0.05) (Table 4).
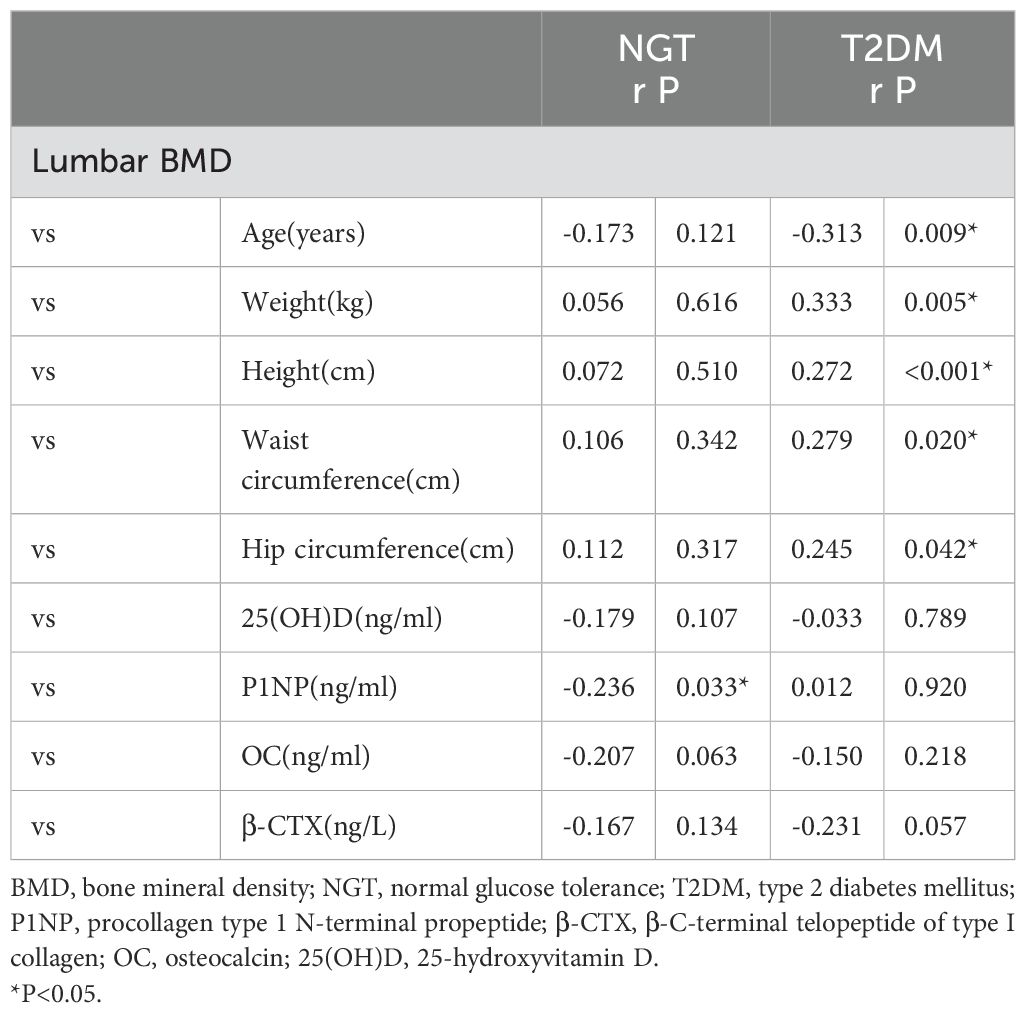
Table 3. Correlations of BMD with clinical characteristics in the two groups.
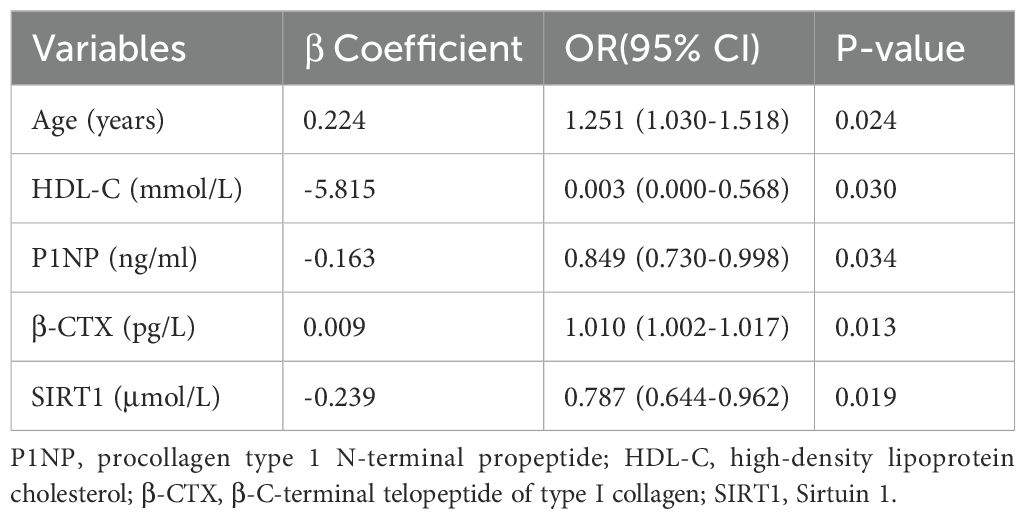
Table 4. Binary logistic regression analysis of risk factors for developing osteoporosis in T2DM group.
4 DiscussionSirtuin 1 is a deacetylase enzyme with extensive biological functions, capable of targeting and regulating numerous post-translational regulatory factors, including p53, FoxOs, nuclear transcriptional regulator NF-κB, and peroxisome proliferator-activated receptor γ (PPARγ), and is associated with various diseases (11, 12). The role of Sirtuin 1 in diabetes is well-established; it acts as a protective factor for pancreatic β-cells and promotes glucose-dependent insulin release (13, 14). Additionally, it enhances gluconeogenesis, reduces oxidative stress, and improves insulin resistance in the liver, adipose tissue, and skeletal muscle. Studies on bone metabolism regulation have shown that Sirtuin 1 can promote osteogenic differentiation and inhibit osteoclast maturation through various mechanisms (15, 16). However, changes in Sirtuin 1 in patients with T2DM and abnormal bone mass remain unclear (17).
The findings of this study demonstrate that newly diagnosed T2DM patients exhibit lower serum levels of Sirtuin 1 and P1NP compared to the normal control group. Subgroup analysis further reveals that T2DM patients with osteoporosis (DMo) have significantly lower serum Sirtuin 1 levels compared to those with normal bone mineral density (DMn). Logistic regression analysis highlighted that reduced SIRT1 levels, alongside age, HDL-C, P1NP, and β-CTX, were independent risk factors for osteoporosis in T2DM patients. However, conflicting results exist regarding the expression of SIRT1 in diabetic patients (18). These discrepancies may be attributed to varying stages of diabetes and differences in disease severity. Research has shown that one month after the onset of diabetes in rats, SIRT1 expression in cardiac tissue increases, while after three months, SIRT1 levels decrease (18). Studies reporting decreased SIRT1 levels often involve cases with greater diabetes severity (19). Moreover, SIRT1 deficiency has been associated with increased oxidative stress, as well as progression of diabetic retinopathy and cognitive dysfunction (20, 21). In our study, all participants were newly diagnosed, untreated T2DM patients, with an HbA1c level of approximately 7.5%, indicating a moderate degree of disease severity (22).
The logistic regression results of this study indicate that hypercholesterolemia also serves as an independent protective factor against osteoporosis in diabetic patients (23). However, the impact of HDL-C on osteoporosis remains inconclusive (24). In the presence of HDL, osteoblast lysosomes are more likely to maintain their integrity. Increased expression of scavenger receptor class B type I (SR-BI) reduces oxLDL uptake and enhances selective cholesterol absorption, thereby regulating oxLDL concentration. Studies at the cellular level suggest that high HDL levels are beneficial for osteoblast survival, contributing to improved bone density. HDL-C can inhibit LDL-C oxidation and prevent the associated multi-organ functional damage, thus exhibiting a protective effect. However, whether HDL-C has a definitive protective role against osteoporosis warrants further investigation (25).
The results of this study indicate no significant difference in bone mineral density (BMD) between the diabetic and non-diabetic groups. However, the diabetic group exhibited a notable decline in SIRT1 levels compared to the non-diabetic group, suggesting that alterations in SIRT1 may precede BMD reductions as an indicator of bone metabolism abnormalities. Reports on BMD changes in T2DM patients are inconsistent, with some studies finding decreased BMD, while others report no change or even an increase. Given that bone mass changes in T2DM patients are influenced by various factors, the mechanisms of diabetes-induced osteoporosis are currently thought to be related to sex, age, measurement site, and BMI, as well as insulin deficiency or resistance, diabetes complications, and hyperlipidemia. However, reports on the effects of these factors on osteoporosis remain inconclusive. Using chi-square analysis, this study found a higher prevalence of abnormal bone mass in the diabetic group compared to the non-diabetic group. As BMD was used in the initial comparison between groups, and chi-square analysis was based on T-scores to determine bone mass abnormalities, the positive correlation between BMD and T-scores may contribute to the discrepancy between these results. The inconsistency may primarily stem from two factors: First, the sample size is relatively small. Second, due to the limited number of osteoporosis patients, we combined those with reduced bone mass and osteoporosis into a single abnormal bone mass group, resulting in a minimal BMD difference. This limitation should be noted as a shortcoming of the study.
5 ConclusionThis study demonstrates that serum Sirtuin 1 levels are significantly lower in T2DM patients compared to non-diabetic controls, with an even greater reduction observed in those with osteoporosis. Sirtuin 1 has been identified as an independent protective factor against osteoporosis in T2DM patients and may serve as an earlier indicator of bone metabolism abnormalities than declines in bone mineral density (BMD). Additionally, hypercholesterolemia appears as an independent protective factor against osteoporosis in diabetic patients. Although no difference in BMD was observed between diabetic and non-diabetic groups, the prevalence of abnormal bone mass was higher in diabetic patients. Further investigation into the mechanistic role of Sirtuin 1 and the protective effects of other related factors in diabetic osteoporosis may provide valuable insights into the pathogenesis of T2DM-associated bone metabolic abnormalities and inform early intervention strategies.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the ethics committee of No.903 Hospital of PLA Joint Logistic Support Force (Ethics number :20211119/10/01/012). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsYX: Data curation, Funding acquisition, Writing – original draft. TH: Funding acquisition, Visualization, Writing – original draft. PJ: Writing – review & editing. XW: Data curation, Writing – original draft. JY: Writing – review & editing. HS: Writing – original draft. ZZ: Writing – original draft. BZ: Writing – original draft. TW: Writing – original draft. YR: Writing – original draft. JW: Writing – original draft. QT: Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Health Science and Technology Project of Hangzhou Health Commission (No. B20220384) and the Medical and Health Science and Technology Project of Zhejiang Province (Nos. 2019RC253 and 2019KY537) and The Natural Science Foundation of China (grant no. 81701481).
AcknowledgmentsThank you to every participant in this study, as well as the clinical laboratory staff of the 903rd Hospital of the People’s Liberation Army who assisted in blood sample testing and data recording. We would like to thank Huiling Shen, Zhenying Zhang, Bojing Zheng, Ting Wang, Yanxia Ren, and Jing Wang for their contributions.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sheu A, Greenfield JR, White CP, Center JR. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol Metab. (2022) 33:333–44. doi: 10.1016/j.tem.2022.02.006
PubMed Abstract | Crossref Full Text | Google Scholar
2. Gao Q, Jiang Y, Zhou D, Li G, Han Y, Yang J, et al. Advanced glycation end products mediate biomineralization disorder in diabetic bone disease. Cell Rep Med. (2024) 5:101694. doi: 10.1016/j.xcrm.2024.101694
PubMed Abstract | Crossref Full Text | Google Scholar
3. Zhang J, Li Y, Lai D, Lu D, Lan Z, Kang J, et al. Vitamin D status is negatively related to insulin resistance and bone turnover in Chinese non-osteoporosis patients with type 2 diabetes: A retrospective cross-section research. Front Public Health. (2021) 9:727132. doi: 10.3389/fpubh.2021.727132
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kitaura H, Marahleh A, Ohori F, Noguchi T, Shen WR, Qi J, et al. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci. (2020) 21:5169. doi: 10.3390/ijms21145169
PubMed Abstract | Crossref Full Text | Google Scholar
5. Mangialardi G, Spinetti G, Reni C, Madeddu P. Reactive oxygen species adversely impacts bone marrow microenvironment in diabetes. Antioxid Redox Signal. (2014) 21:1620–33. doi: 10.1089/ars.2014.5944
PubMed Abstract | Crossref Full Text | Google Scholar
6. Napoli N, Conte C, Eastell R, Ewing SK, Bauer DC, Strotmeyer ES, et al. Bone turnover markers do not predict fracture risk in type 2 diabetes. J Bone Miner Res. (2020) 35:2363–71. doi: 10.1002/jbmr.4140
PubMed Abstract | Crossref Full Text | Google Scholar
7. Rasha F, Mims BM, Castro-Piedras I, Barnes BJ, Grisham MB, Rahman RL, et al. The versatility of sirtuin-1 in endocrinology and immunology. Front Cell Dev Biol. (2020) 8:589016. doi: 10.3389/fcell.2020.589016
PubMed Abstract | Crossref Full Text | Google Scholar
8. Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). (2019) 10:187. doi: 10.3389/fendo.2019.00187
PubMed Abstract | Crossref Full Text | Google Scholar
9. Velagapudi S, Karsai G, Karsai M, Mohammed SA, Montecucco F, Liberale L, et al. Inhibition of de novo ceramide synthesis by Sirtuin-1 improves beta-cell function and glucose metabolism in type 2 diabetes. Cardiovasc Res. (2024) 120:1265–78. doi: 10.1093/cvr/cvae100
PubMed Abstract | Crossref Full Text | Google Scholar
10. Zhang W, Zhou X, Hou W, Chen E, Ye C, Chen M, et al. Reversing the imbalance in bone homeostasis via sustained release of SIRT-1 agonist to promote bone healing under osteoporotic condition. Bioact Mater. (2023) 19:429–43. doi: 10.1016/j.bioactmat.2022.04.017
PubMed Abstract | Crossref Full Text | Google Scholar
13. Pacifici F, Di Cola D, Pastore D, Abete P, Guadagni F, Donadel G, et al. Proposed tandem effect of physical activity and sirtuin 1 and 3 activation in regulating glucose homeostasis. Int J Mol Sci. (2019) 20:4748. doi: 10.3390/ijms20194748
PubMed Abstract | Crossref Full Text | Google Scholar
14. Shen P, Deng X, Chen Z, Ba X, Qin K, Huang Y, et al. SIRT1: A potential therapeutic target in autoimmune diseases. Front Immunol. (2021) 12:779177. doi: 10.3389/fimmu.2021.779177
PubMed Abstract | Crossref Full Text | Google Scholar
15. Li Y, Nie J, Wu Q, Yang X, Jiang P. Circ-Sirt1 promotes osteoblast differentiation by activating Sirt1 and Wnt/β-catenin pathway. Acta Biochim Pol. (2023) 70:51–7. doi: 10.18388/abp.2020_6035
PubMed Abstract | Crossref Full Text | Google Scholar
16. Liu Z, Liu H, Liu S, Li B, Liu Y, Luo E. SIRT1 activation promotes bone repair by enhancing the coupling of type H vessel formation and osteogenesis. Cell Prolif. (2024) 57:e13596. doi: 10.1111/cpr.13596
PubMed Abstract | Crossref Full Text | Google Scholar
17. Chen Y, Xiao H, Liu Z, Teng F, Yang A, Geng B, et al. Sirt1: an increasingly interesting molecule with a potential role in bone metabolism and osteoporosis. Biomolecules. (2024) 14:970. doi: 10.3390/biom14080970
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kaviarasan K, Jithu M, Arif Mulla M, Sharma T, Sivasankar S, Das UN, et al. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism. (2015) 64:958–66. doi: 10.1016/j.metabol.2015.04.005
PubMed Abstract | Crossref Full Text | Google Scholar
19. Othman R, Berbari S, Vaucher E, Couture R. Differential expression of kinin receptors in human wet and dry age-related macular degeneration retinae. Pharm (Basel). (2020) 13:130. doi: 10.3390/ph13060130
PubMed Abstract | Crossref Full Text | Google Scholar
20. He M, Long P, Chen T, Li K, Wei D, Zhang Y, et al. ALDH2/SIRT1 contributes to type 1 and type 2 diabetes-induced retinopathy through depressing oxidative stress. Oxid Med Cell Longev. (2021) 2021:1641717. doi: 10.1155/2021/1641717
PubMed Abstract | Crossref Full Text | Google Scholar
21. Vezza T, Víctor VM. SIRT1 and miR-34a-5p: valuable biomarkers for the early detection of cognitive impairment in type 2 diabetes mellitus. J Clin Endocrinol Metab. (2024) 109:e1546–7. doi: 10.1210/clinem/dgad740
PubMed Abstract | Crossref Full Text | Google Scholar
22. Yeganeh-Hajahmadi M, Najafipour H, Rostamzadeh F, Naghibzadeh-Tahami A. Klotho and SIRT1 changes from pre-diabetes to diabetes and pre-hypertension to hypertension. Diabetol Metab Syndr. (2021) 13:115. doi: 10.1186/s13098-021-00736-2
PubMed Abstract | Crossref Full Text | Google Scholar
23. Brodeur MR, Brissette L, Falstrault L, Moreau R. HDL3 reduces the association and modulates the metabolism of oxidized LDL by osteoblastic cells: a protection against cell death. J Cell Biochem. (2008) 105:1374–85. doi: 10.1002/jcb.v105:6
PubMed Abstract | Crossref Full Text | Google Scholar
24. Yamauchi M, Yamaguchi T, Nawata K, Tanaka K, Takaoka S, Sugimoto T. Increased low-density lipoprotein cholesterol level is associated with non-vertebral fractures in postmenopausal women. Endocrine. (2015) 48:279–86. doi: 10.1007/s12020-014-0292-0
PubMed Abstract | Crossref Full Text | Google Scholar
25. Hui N, Barter PJ, Ong KL, Rye KA. Altered HDL metabolism in metabolic disorders: insights into the therapeutic potential of HDL. Clin Sci (Lond). (2019) 133:2221–35. doi: 10.1042/CS20190873
留言 (0)