Neoplasms affecting the female reproductive organs, such as ovarian, cervical, and endometrial types, constitute a substantial global health challenge due to their high incidence and associated mortality (Bertone et al., 2004). These malignancies are distinguished by distinct biological characteristics, causes, and treatment responses (Carninci et al., 2005). Despite the progress in medical research, the outlook for individuals with late-stage reproductive neoplasms remains unfavorable, primarily due to emerging drug resistance. Deciphering the underlying mechanisms of this resistance is crucial for improving treatment efficacy and extending patient survival (Li et al., 2019a).
Exosomes, small extracellular vesicles derived from endosomes, have emerged as key players in intercellular communication (Zhang et al., 2018). They carry a diverse range of molecular constituents, including proteins, lipids, DNA, mRNA, and various non-coding RNAs. In the field of oncology, exosomes contribute to numerous processes such as tumor progression, metastasis, immune response modulation, and the development of drug resistance (Lakhotia et al., 2020). The role of exosomes in the tumor milieu and their potential as biomarkers and therapeutic agents have become focal points in cancer research (O’Brien et al., 2020).
Long non-coding RNAs (lncRNAs), a class of non-coding RNAs that exceed 200 nucleotides in length and are not predominantly involved in protein synthesis, were initially perceived as transcriptional noise (Bertone et al., 2004; Carninci et al., 2005). However, they are now recognized for their critical roles in gene expression regulation at several levels, including chromatin reconfiguration, transcription, and post-transcriptional processing. In cancer research, lncRNAs are known to play essential roles in regulating oncogenic and tumor suppressive pathways (Li et al., 2019a; Zhang et al., 2018). The discovery of lncRNAs in exosomes has opened new avenues for understanding drug resistance in cancer. These exosomal lncRNAs, which can be transferred among cancer cells and other cells within the tumor microenvironment, influence drug sensitivity and resistance (Bertone et al., 2004; Carninci et al., 2005). The mechanisms by which exosomal lncRNAs contribute to drug resistance are multifaceted, involving the modulation of cell death, drug efflux, DNA repair processes, and interactions in the tumor microenvironment (Lakhotia et al., 2020).
A primary obstacle in treating cancers of the female reproductive system is the onset of resistance to established chemotherapy protocols. This resistance, which can be inherent or acquired, frequently results in treatment failure and cancer progression. Investigating exosomal lncRNAs presents a promising approach to comprehend and potentially counteract this resistance. These entities may act as indicators for predicting therapeutic response and as novel targets to increase the susceptibility of cancer cells to treatments. Recent investigations are beginning to decode the intricate connections between exosomal lncRNAs and mechanisms of drug resistance in cancers of the female reproductive system. Though still in early stages, this research offers significant potential for devising new therapeutic approaches. The future trajectory in this domain involves a thorough analysis of exosomal lncRNAs, understanding their biological roles, and assessing their clinical application.
2 The basics of exosomesExosomes are a subpopulation of extracellular vesicles (EVs), nanoscale structures with a lipid bilayer membrane performing their biological functions mainly by transferring cargoes such as proteins, RNAs, DNAs, and lipids in an intercellular communication substrate (Figure 1). Based on their size, EVs are divided into four groups: exosomes, microvesicles, apoptotic bodies, and oncosomes (O’Brien et al., 2020). In cancer biology, among EVs, exosomes play the most important regulatory role (Chang et al., 2021; De Los Santos et al., 2019; Fontana et al., 2021). The term exosome (different from exosome complex, which plays a role in RNA degradation) was first applied to vesicles of unknown origin released from cultured cells with 5′-nucleotidase activity (van Niel et al., 2018), and they were thought to contain cellular waste (Harding and Stahl, 1983; Pan and Johnstone, 1983). Exosomes usually differ in size between 40 and 150 nm, and their surface contains marker proteins such as tetraspanins (CD63, CD81, CD82, and CD9), flotillin, and MHC, depending on their origin cell (Zhang et al., 2018; Hao et al., 2022). Generally, exosome biogenesis occurs within the endosomal system. Several steps of this process are regulated by intracellular and extracellular signals. At the start, the invagination of the plasma membrane forms a primary endosome, and the maturation of it goes on with continuous buddying of intraluminal vesicles (ILVs) into the primary endosome space. The primary endosome containing ILVs is known as the primary multivesicular body (MVB) at this stage (De Los Santos et al., 2019; Bebelman et al., 2018; Liu et al., 2022a). Two pathways have been identified in the process of ILV generation. One way requires ESCRT, a cluster of five subunits (ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and Vps4), and the other one is referred to as ESCRT-independent. It has been observed that the ESCRT-dependent pathway can actually be considered as the main pathway. There are also some other pathways dependent on ceramide or tetraspanins, which fall under the ESCRT-independent category. For example, nSMase2 produces ceramide via hydrolysis of sphingomyelin located in the MVB membrane, thus, playing a role in endosomal maturation (Fu et al., 2019; Trajkovic et al., 2008). In this stage, exosome cargoes are loaded into the exosome, and then transmembrane proteins such as tetraspanins are added to the exosome membrane, which means endosomal sorting is completed (van Niel et al., 2011). After that, the MVB may be destroyed via the lysosomal pathway or is converted into a mature MVB for exosomal release, which happens with the help of cytoskeleton proteins such as actin and microtubules (De Los Santos et al., 2019; Mashouri et al., 2019). Finally, Rab GTPase proteins such as Rab27a and Rab27b, which control vesicular transport, enable MVB binding and exosome release into the extracellular space (Mathieu et al., 2019; Ostrowski et al., 2010). Also, Rab27a and Rab27b have been shown to regulate the trafficking of the pro-invasive matrix metalloproteinase MMP14 (Macpherson et al., 2014). This process is aided by the SNARE complex that is responsible for MVB and cell membrane fusion (Hessvik and Llorente, 2018).
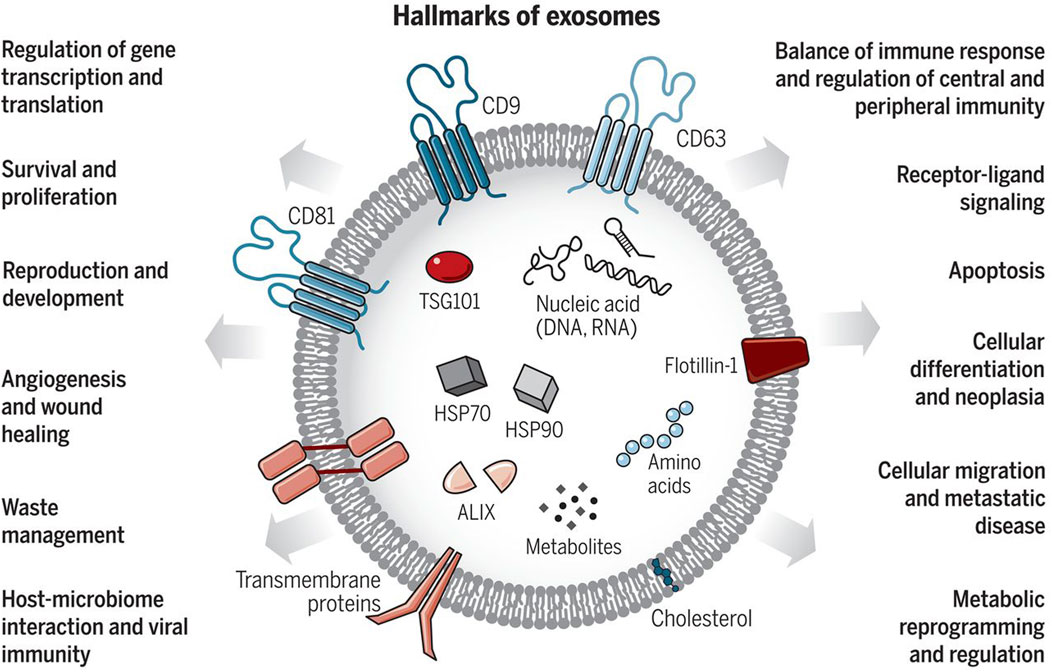
Figure 1. Exosomes: Sorting of Cellular Transport Network in Humans with Multifaceted Roles. Exosomes are tiny vesicles released by every cell type, encapsulating nucleic acids, proteins, lipids, and metabolic products. Serving as conduits for both paracrine and autocrine cell communication, they play critical roles in both healthy and diseased states, influencing numerous cellular processes. Re-printed from Science (Chang et al., 2021).
2.1 Role of exosomes in cell-cell communicationExosomes possess the ability to transport molecular cargos from a donor cell to a recipient cell; thus, they are considered a major piece of cell-cell communications (Hannafon and Ding, 2013). Dendritic cells, macrophages, cancer cells, and mesenchymal stem cells are only some of the cell types utilizing this mechanism as a means of local and systematic communication. Figure 2 describes the process of exosome biogenesis. As the cargos they carry to new cells are capable of exerting new biological changes, these exosomes can affect various mechanisms of cell biology in health and disease (Kalluri and LeBleu, 2020; Gurung et al., 2021). Table 1 outlines the crucial functions of exosomes in facilitating cell-cell communication, a fundamental process in both physiological and pathological contexts. After exosomes are released into the extracellular matrix (ECM), they proceed to their destination cell by taking one of the following pathways: autocrine, juxtacrine, paracrine, or endocrine (Hao et al., 2022). Uptake of exosomes is done depending on the cell they enter. Exosomes may bind directly to the surface receptors of a recipient cell through their surface ligands, such as glycans, lectins, integrins, or other cell adhesion molecules. This results in the activation of the downstream signaling pathway without internalization. In the second approach, exosome uptake is done through endocytosis, including clathrin-dependent endocytosis, clathrin-independent endocytosis, phagocytosis, or macropinocytosis. After exosomes enter recipient cells, endosomes containing them may be degraded within lysosomes, recycled through plasma membrane recombination, or released into target cells (Hessvik and Llorente, 2018; Gurung et al., 2021; Han et al., 2020; McKelvey et al., 2015).
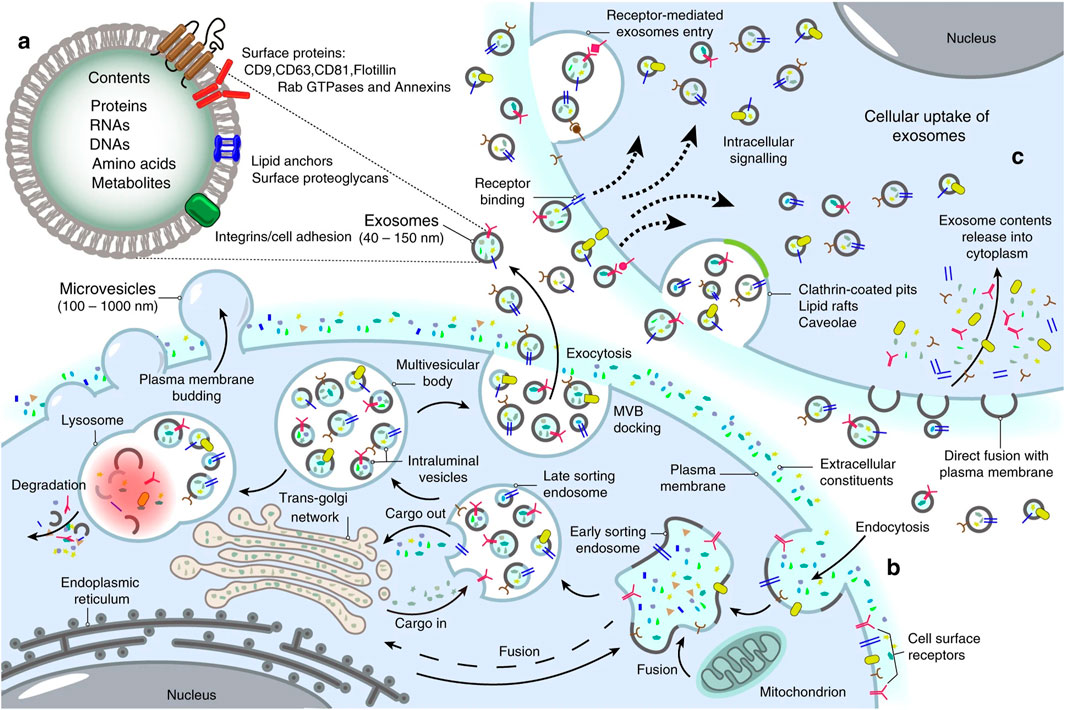
Figure 2. Landscape of exosome biogenesis. (A) Exosomes are composed of various proteins, nucleic acids, amino acids, and metabolites. Specific markers such as CD9, CD63, CD81, flotillin, and Annexins help identify them. (B) The process begins with the uptake of extracellular elements and cell surface proteins through endocytosis and invagination of the plasma membrane. This leads to the creation of early sorting endosomes (ESEs) through the merging of plasma membrane buds with components from the endoplasmic reticulum (ER), trans-Golgi network (TGN), and mitochondria. ESEs evolve into late sorting endosomes (LSEs), where further invagination and cargo modification result in the production of intraluminal vesicles (ILVs) and the development of multivesicular bodies (MVBs). Some MVBs merge with lysosomes for degradation of their contents, while others are transported to the cell surface, where they fuse with the plasma membrane. This fusion leads to the release of ILVs as exosomes outside the cell. (C) Exosomes can then enter other cells through various mechanisms, including direct fusion with the plasma membranes, receptor-mediated uptake, clathrin-coated pits, and lipid rafts. Re-printed from Springer Nature (De Los Santos et al., 2019).
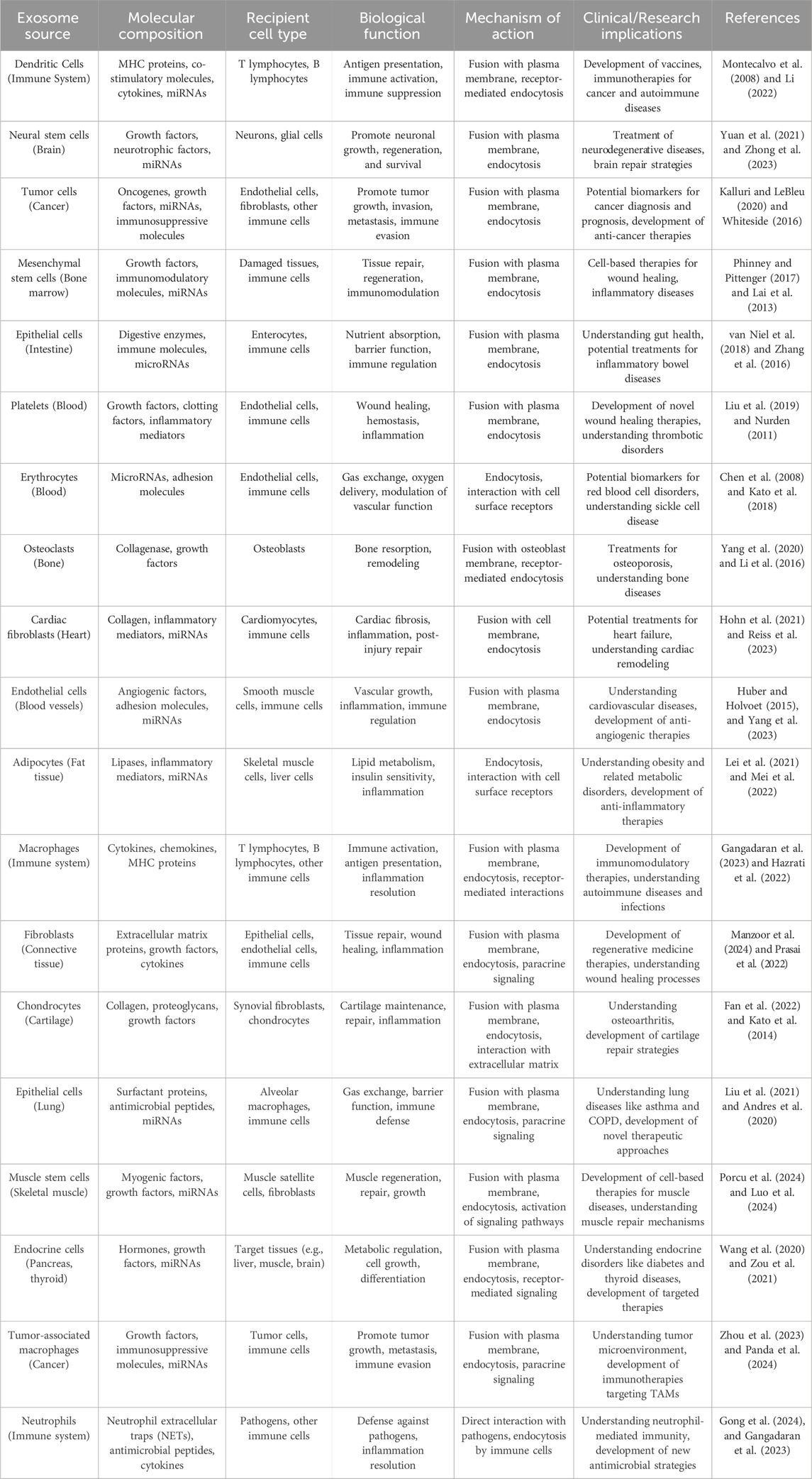
Table 1. Role of exosomes in cell-cell communication.
2.2 Molecular contents of exosomes: roteins, lipids, and RNAsFigure 3 describes the exosome and cargo recycling. Exosomes may partake in transferring various types of functional molecules such as lipids, proteins, and genetic material, and have been identified in different body fluids, including saliva, urine, breast milk, semen, blood, and bronchoalveolar lavage fluid (van Niel et al., 2011; Wang et al., 2016). These vesicles may play a different role based on their donor cell, their destination, or the cargos they carry. In addition, they can transfer bioactive molecules between cancer and other cells in the tumor microenvironment over near or long distances and are involved in cancer development by controlling processes such as immune system regulation, metastasis, angiogenesis, epithelial-mesenchymal transition (EMT), entry into quiescence (G0 phase), senescence, and drug resistance (Li, 2022; Soltész et al., 2021; Sundararajan et al., 2018; Tai et al., 2018). The fact that drug resistance can be induced in drug-sensitive cells through exosomes derived from exosome-resistant cells has been approved in several studies (Li and Nabet, 2019; Lobb et al., 2017; Mikamori et al., 2017; Takahashi et al., 2014a). Increasing the expression of multidrug resistance proteins, expelling chemotherapeutic drugs from cells, decreasing drug uptake, drug detoxification, and increasing DNA repair are some of the mechanisms through which exosomes increase drug resistance, all by releasing bioactive molecules in their target cells (Li, 2022; Sundararajan et al., 2018; Guo et al., 2020a; Li et al., 2020; Xie et al., 2019). Interestingly, some mediators of drug resistance, such as annexin 3 and RAB7, have been proven to participate in production of EVs in cancer cells. This means drug-resistant cells are eager to cause resistance in non-resistant cells (Guerra and Bucci, 2019; Xavier et al., 2020; Yin et al., 2012).
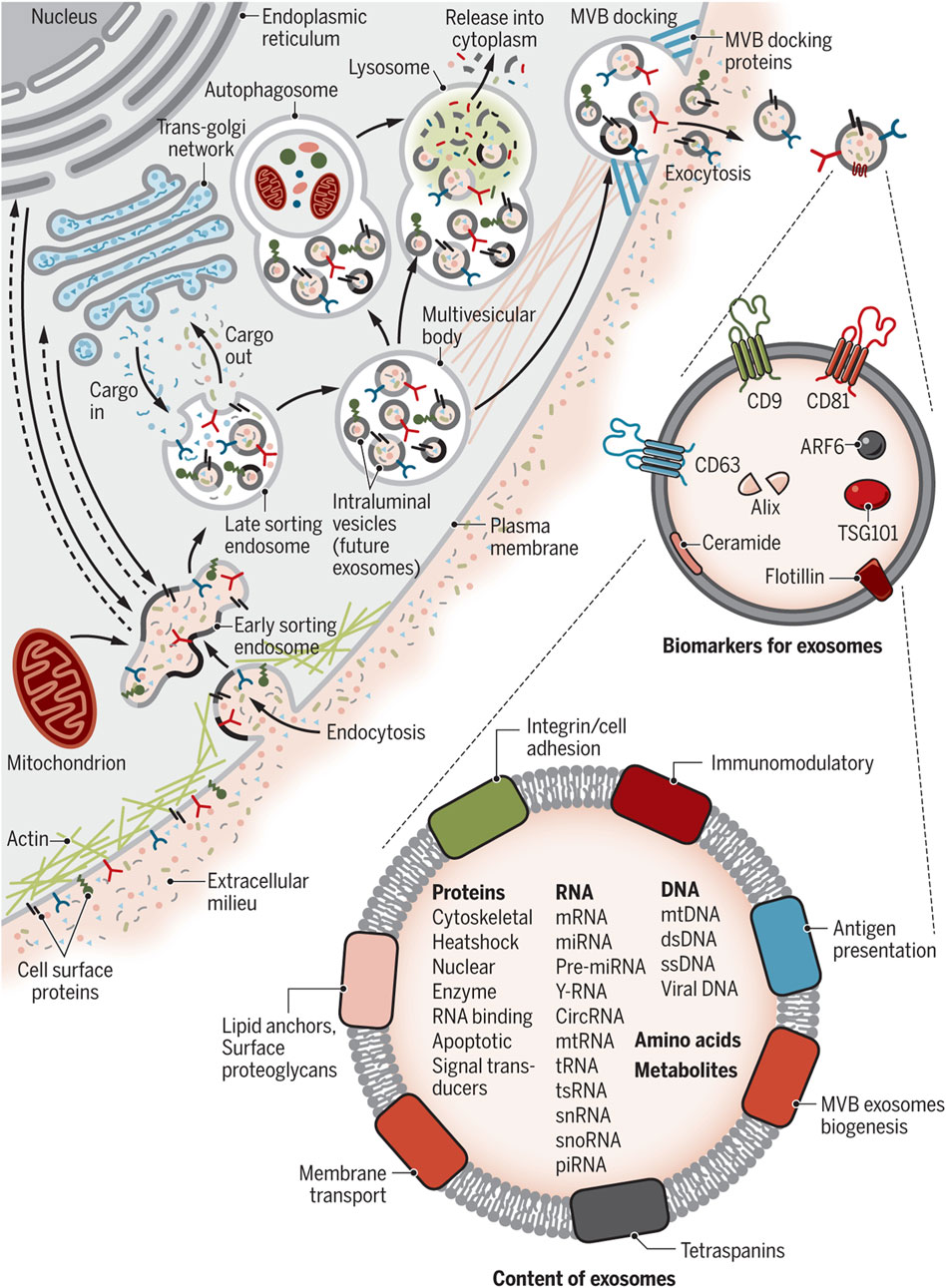
Figure 3. Exosome and cargo recycling. The process begins when various substances, including proteins, lipids, metabolites, and small molecules, enter cells through endocytosis or the inward folding of the cell membrane. This leads to the creation of early sorting endosomes (ESEs), either directly through budding or by merging with pre-existing ESEs formed from components of the endoplasmic reticulum (ER), trans-Golgi network (TGN), and mitochondria. These ESEs can merge with the ER and TGN, suggesting a method for the endocytic materials to reach these organelles. ESEs, containing a mix of membrane and internal materials from various origins, evolve into late sorting endosomes (LSEs). A second folding within the LSE forms intraluminal vesicles (ILVs), altering the composition of what will become exosomes, including the entry of cytoplasmic elements into these vesicles. This process can distribute cell surface proteins across ILVs in a unique manner and lead to ILVs of different sizes and compositions. LSEs then develop into multivesicular bodies (MVBs) that contain these ILVs. MVBs may merge with autophagosomes for content degradation in lysosomes, or directly with lysosomes. Alternatively, MVBs can move to the cell surface, where they release exosomes through exocytosis, maintaining a similar membrane orientation. The creation of exosomes involves several proteins, such as Rab GTPases and ESCRT proteins, along with markers like CD9, CD81, CD63, and others. Exosomes are characterized by their content, which includes various proteins, RNAs, DNAs, amino acids, and metabolites, as well as surface proteins like tetraspanins and integrins. Re-printed from Science (Chang et al., 2021).
3 Understanding long non-coding RNAsComplete sequencing of the human genome and developing technologies such as high-throughput next-generation sequencing have aided our understanding of coding and non-coding RNAs (Bertone et al., 2004; Carninci et al., 2005). About 75% of the human genome is transcribed into RNA, and of this amount of RNA, only 3% contains protein-coding mRNA (Yan and Bu, 2021). Non-coding RNAs (ncRNAs) can be classified into different types based on their transcript length, function, structure and position in the genome (Lakhotia et al., 2020). The ncRNAs that play an important role in regulating gene expression and have been studied in recent years include small interfering RNAs (siRNAs), microRNAs (miRNAs), Piwi-interacting RNAs (piRNAs), circular RNAs (circRNA), and long ncRNAs (lncRNA) (Yan and Bu, 2021). According to a classification based on transcript size, ncRNAs are divided into two groups: small ncRNAs (less than 200 nt) and long ncRNAs (more than 200 nt) (Kashi et al., 2016; St Laurent et al., 2015). ciRNAs and lncRNAs are both composed of more than 200 nucleotides, and the difference lies in their structure. LncRNAs are generally linear molecules that can form complex seconary structures, such as stem-loops and hairpins, while circRNAs are characterized by a covalently closed loop, lacking 5′ and 3′ ends (Yan and Bu, 2021; Zampetaki et al., 2018). LncRNAs are mRNA-like transcripts longer than 200 nt with limited protein-coding potential (Gao et al., 2020; Sahu et al., 2015). Most of them are transcribed by the RNA polymerase II and have a polyadenylated 3′ end and a m7G-cap at their 5′ end (Statello et al., 2021). Unlike mRNA, lncRNAs usually lack an open reading frame, have fewer exons, and are also expressed at lower levels with more tissue-specific expression patterns. These types of ncRNAs have been under weaker pressure of natural selection during evolution, and many of them are specific to mammals (Li et al., 2019a; Zhang et al., 2018). LncRNAs are classified into different classes according to their location in the genome, sequence, length, morphology, structure, and functional properties. Based on their genomic position, there are six general groups: intergenic (between two protein-coding genes), intronic (in the intronic regions of the protein-coding gene), enhancer (eRNA; transcribed from the DNA sequence of the enhancer regions), sense (transcribed from the same strand and in the same direction as the neighboring protein-coding gene), antisense (transcribed from the opposite strand and in the opposite direction of the neighboring protein-coding gene), and bidirectional (1 Kb away from the promoter region of a protein-coding gene, and it is transcribed from the opposite strand) (Hermans-Beijnsberger et al., 2018; Blokhin et al., 2018; Sun and Kraus, 2015; Borkiewicz et al., 2021). Table 2 outlines the diverse roles and mechanisms through which long non-coding RNAs (lncRNAs) are involved in biological functions.
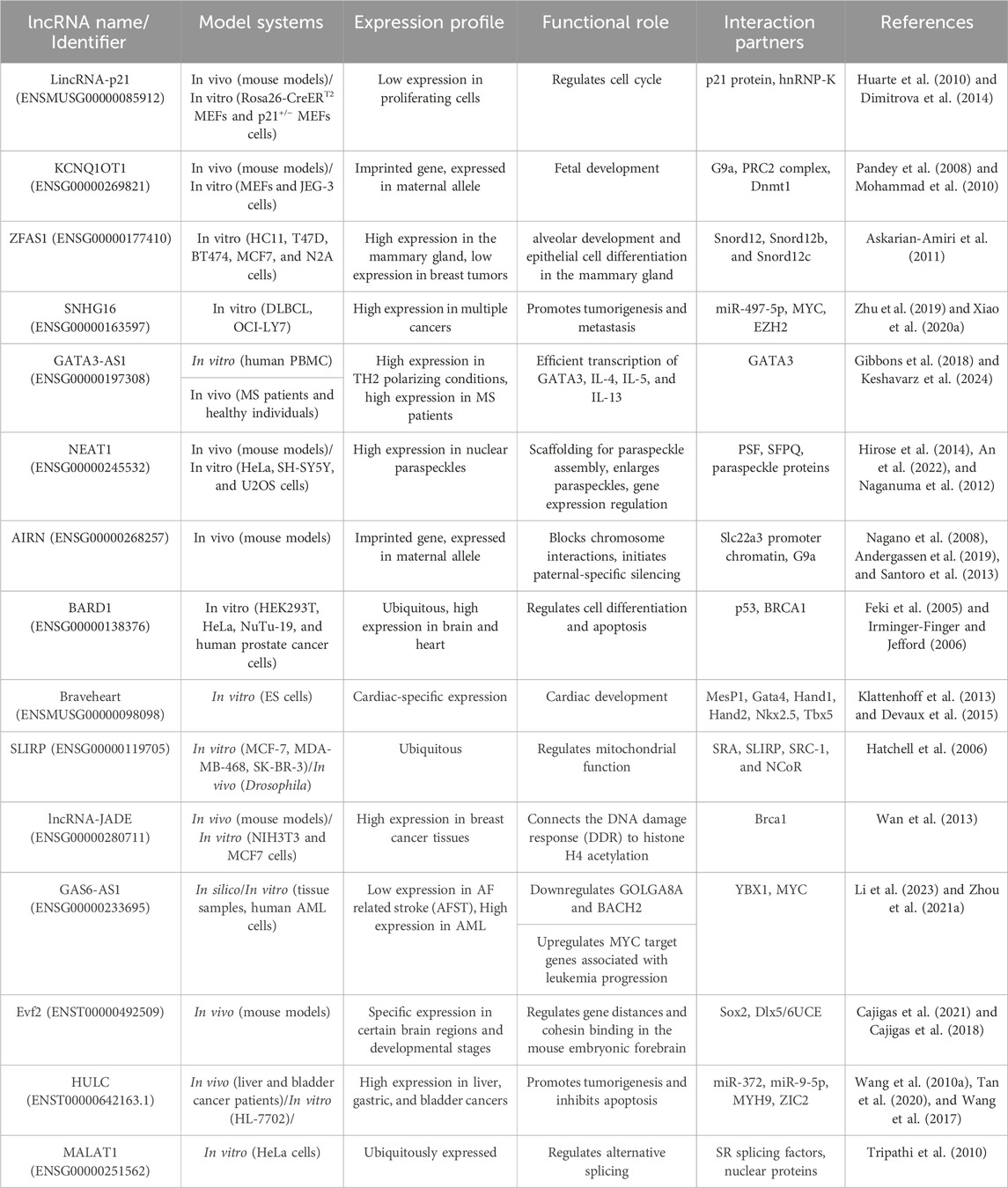
Table 2. Biological Functions and Mechanisms of lncRNAs.
3.1 The biological functions and mechanisms of lncRNAsLncRNAs can be observed in a variety of intracellular components such as the nucleus or cytoplasm (Gudenas and Wang, 2018). LncRNAs are capable of modifying a landscape of cellular process, such as replication, pre/post transcription, and translation. They are able to regulate chromatin structure at various functional stages prior to transcription, which include histone methylation and acetylation, DNA methylation, and chromatin remodeling (Li et al., 2019a; Liu et al., 2022b). For example, the lncRNA HOTTIP binds to the WDR5-MLL complex and then, by targeting the ΄5HOXA locus, mediates activation of HOXA transcription through H3K4 methylation (Wang et al., 2011). In addition, lncPRESS1 plays an important role in regulating the cell differentiation process through its interaction with the deacetylase SIRT6 (Jain et al., 2016). Furthermore, lncRNAs can play the role of a cis-regulator or a trans-regulator, or they can interfere with imprinting in pre-transcriptional regulation (Sun and Kraus, 2015; Jarroux et al., 2017; Ma et al., 2013; Wang et al., 2021a; Yao et al., 2019; Kornienko et al., 2013). LncRNAs can also regulate gene expression by binding directly to transcription factors or the polymerase machinery or by interfering with polymerase-promoter bonds (He et al., 2019). For example, the lncRNA 7SK represses elongation by binding to the transcription factor PTEFβ and preventing its phosphorylation for elongation (Peterlin et al., 2012). The mechanisms of lncRNAs interfering with post-transcriptional regulation include regulation of mRNA alternative splicing, mRNA stability, protein stability, DNA regulation, regulation of protein localization, and acting as a sponge for miRNA (Li et al., 2019a; Statello et al., 2021; Pisignano and Ladomery, 2021; Karakas and Ozpolat, 2021; Zhou et al., 2019). For instance, the lncRNA TINCR interacts with the staufen1 (STAU1) protein to promote the stability and expression of differentiation-related mRNAs such as KRT80 (Kretz et al., 2013). Figure 4 provides examples of long non-coding RNAs (lncRNAs) and their mechanisms that contribute to cancer progression.
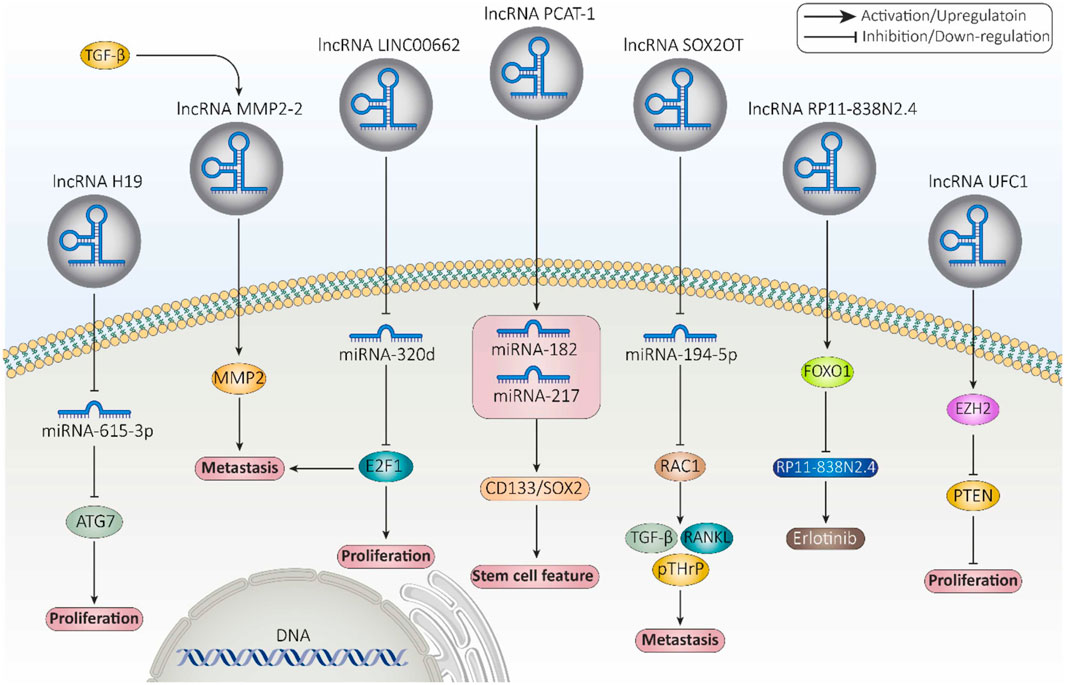
Figure 4. Examples of long non-coding RNAs (lncRNAs) and their mechanisms involved in cancer progression (Fontana et al., 2021).
3.2 lncRNAs in disease and cancer progressionRegarding the biological functions of lncRNAs and their significant contribution to both activation and inhibition of gene expression, lncRNAs have the potential to participate in various diseases such as cancer (Ahadi, 2021). According to multiple studies, dysregulation of lncRNAs may contribute to cancer initiation and development by affecting different biological processes and pathways such as proliferation, differentiation, metastasis, invasion, cell death, angiogenesis, cell cycle, and miRNA silencing (Ahadi, 2021; Smolarz et al., 2021). LncRNA SChLAP1 acts as an antagonist of SWI/SNF chromatin remodeling complex in prostate cancer cells and contributes to metastasis and aggressiveness of cancer cells (Prensner et al., 2013). TFAP2A-AS1, which is associated with decreased expression in breast cancer, acts as a miRNA sponge for miR-933 and this way regulates Smad2 expression (Zhou et al., 2019). Among the lncRNAs related to various cancers are MALAT1, HOTAIR, LUCAT1, MRPS30-DT, and IFNG-AS1 (Taniue and Akimitsu, 2021; Xing et al., 2021; Hajjari and Salavaty, 2015; Shirani et al., 2024; Yaghoobi et al., 2018). Therefore, lncRNAs can be used in diagnosis and targeted therapy in cancer.
4 Exosomal lncRNAs: the interfaceThere is still no detailed understanding of how lncRNAs are packaged into exosomes. However, studies conducted have been suggesting that several proteins, including the heterogeneous nuclear ribonucleoproteins (hnRNP) family, such as hnRNPA2B1 (Chen et al., 2020a; Lei et al., 2018; Zheng et al., 2019) and hnRNPK (Gao et al., 2018) as well as human antigen R (HuR) (Deng et al., 2020) are some of the proteins that aid the sorting of lncRNAs into exosomes (Fabbiano et al., 2020). What is clear is that in almost every situation, RNAs are present as a ribonucleoprotein (RNP) complex in cells or exosomes. Thus, proteins with the ability of shaping a complex with RNAs are undoubtedly essential for the encapsulation of ncRNAs into exosomes (Zang et al., 2020).
One protein whose role in regulating lncRNAs has been proven is hnRNPA2B1 (Qiu et al., 2021). In bladder cancer (BC) cells, hnRNPA2B1 specifically binds to lncRNA LNMAT2 and is packed into exosomes. This was approved because hnRNPA2B1 knockdown had no effect on the expression levels of LNMAT2 in BC cells, but exosome levels of this lncRNA was evidently decreased (Chen et al., 2020a). LncRNA H19 in human non-small cell lung cancer (NSCLC) also forms a complex with hnRNPA2B1. The investigation by Lei et al. further proved that evaluated expression levels of hnRNPA2B1 promoted exosomal levels of H19 secreted by gefitinib-resistance cells of NSCLC (Lei et al., 2018). In another study conducted by Chen et al., lncARSR could bind to hnRNPA2B1 and be packaged into exosomes. hnRNPA2B1-lncARSR complex was seen in cytoplasm and exosomes rather than nucleus, which hnRNPA2B1 was initially identified. This observation proved that hnRNPA2B1 is specifically involved in the wrapping of lncARSR into the exosomes (Qu et al., 2016). Zheng et al. also indicated that in human breast cancer cells, hnRNPA2B1 is upregulated and has a correlation with the expression of exosomal lncRNA AGAP2AS1, and silencing hnRNPA2B1 resulted in downregulation of AGAP2AS1. Ultimately, it was proved that encapsulation of AGAP2AS1 into exosomes and its secretion outside breast cancer cells is done in an hnRNPA2B1-dependent manner (Zheng et al., 2019). Also, Gao et al. showcased the contribution of hnRNPK in the packaging of lncRNA 91H in colorectal cancer (CRC) which caused aggressive tumor relapse and metastasis (Gao et al., 2018). The study conducted by Liu et al. highlights the crucial role of exosomal long noncoding RNAs (lncRNAs), with a particular focus on LINC01133, in the progression of pancreatic ductal adenocarcinoma (PDAC), an extremely aggressive and lethal form of cancer (Figure 5). It was found that LINC01133 is not just highly present in PDAC cases but also associated with more advanced cancer stages and lower survival rates in patients. The study explores how LINC01133 affects PDAC progression, showing that Periostin increases both exosome release and LINC01133 levels, which in turn activates several cancer-promoting processes such as cell growth, movement, invasion, and the transition from epithelial to mesenchymal states via the EGFR pathway and interaction with c-myc. Crucially, LINC01133 boosts the Wnt/β-catenin signaling pathway through its interaction with EZH2, resulting in AXIN2 suppression and β-catenin activation via H3K27 trimethylation. These insights highlight the central role of exosomal LINC01133 in PDAC and propose that targeting LINC01133 could be an effective approach for treating this cancer (van Niel et al., 2018).
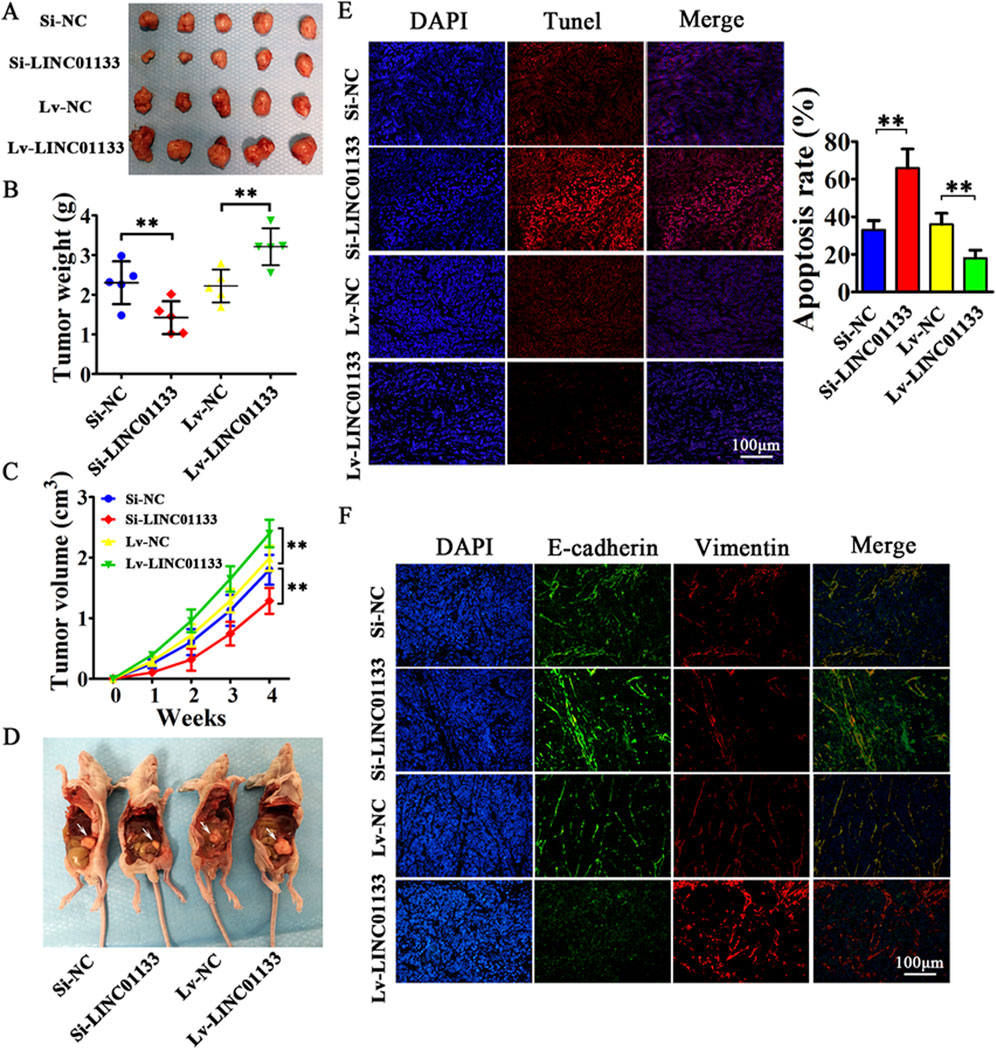
Figure 5. the results of an experiment where 4 million CFPAC-1 cells, treated with either Si-LINC01133, Si-NC, Lv-LINC01133, or Lv-NC, were injected into the right side of nude mice. (A) One month later, the mice were euthanized, and the tumor grafts were removed for analysis. (B) The findings showed that CFPAC-1 cells treated with Lv-LINC01133 resulted in heavier xenografts, while those treated with Si-LINC01133 led to lighter xenografts. (C) Additionally, CFPAC-1 cells exposed to Lv-LINC01133 demonstrated faster growth and larger tumor volumes compared to those treated with Si-LINC01133, which exhibited slower growth and smaller tumor volumes. (D) Furthermore, when 1 million CFPAC-1 cells treated with each of the substances were injected into the lower left abdomen quadrant, the spread within the abdominal cavity was assessed, with metastatic nodules identified by white arrowheads. (E) The study also found that LINC01133 significantly reduced apoptosis in the xenograft tumors of CFPAC-1 cells, as verified by TUNEL assay, with the apoptosis rate measured across five random fields in three repeated experiments. (F) Lastly, the expression levels of E-cadherin and Vimentin in the xenograft tumors were examined through immunofluorescence. Re-printed from Springer Nature (van Niel et al., 2018).
4.1 The selective sorting mechanism of lncRNAs into exosomesThe type of a cell and its homeostatic state define the ncRNAs that load into an exosome with other cargos; however, not much is known about the exact mechanisms and factors that contribute to the selective sorting of lncRNAs into exosomes (Qiu et al., 2021). Nevertheless, some nucleotide sequences have been identified on RNAs that can determine how RNAs are chosen and then packed into exosomes (Groot and Lee, 2020; Hoek et al., 1998; Hwang et al., 2007; Wang et al., 2010b). Ahadi et al., investigated some exosomal lncRNAs secreted from four different prostate cancer cell lines (VCaP, LNCaP, DU145, PC3) and one normal prostate cell line. The aim was to determine if any specific RBP binding sites on lncRNAs could be found. They were able to identify 126 different six-base motifs that were particularly expressed in the four prostate cancer cell lines but not the healthy prostate cell line (Ahadi et al., 2016). Chen et al. found that there is a sequence of GGAG on the 1930-1960 nt region of LNMAT2 that binds with hnRNPA2B1 specifically. This sequence is located on a stem-loop structure and is recognized by hnRNPA2B1 (Chen et al., 2020a). In the case of exosomal lncRNA H19 originated from NSCLC, Lei et al. found the same special sequence of GGAG at the 5′ -end of the lncRNA which was the specific binding site of hnRNPA2B1 (Lei et al., 2018). Moreover, in RCC cells, lncARSR could bind to hnRNPA2B1 through its special motifs (GGAG/CCCU) at the 5′ terminal region (Qu et al., 2016).
4.2 Role of exosomal lncRNAs in tumor microenvironment modulationA tumor microenvironment (TME) is a mixture of non-cancerous cells, blood vessels, secreted factors, and extracellular matrix (ECM), which collectively promote the growth of a tumor. From the beginning of the tumor growth, the cancer cells start to have interactions with their surrounding normal cells through secreting exosomes and will induce the transformation of their local environment to shape the desired TME that supports immunosuppression, cancer cell survival, local invasion, and metastatic dissemination (Chen et al., 2019a; Jin and Jin, 2020; Pathania and Challagundla, 2021). LncRNAs constitute a significant portion of this cell-cell communication between cancer cells and TME (Pathania and Challagundla, 2021). LncRNAs secreted from tumor cells can affect ECM, stromal cells, immune cells, endothelial cells, macrophages, and myeloid-derived suppressor cells (MDSCs) (Chen et al., 2019a). One major event during tumor progression is the lack of oxygen supply or hypoxia. This situation leads to the activation of hypoxia-inducible factor (HIF)-1α pathway and eventually a great deal of lncRNAs are produced and secreted into TME that elevate cell survival (Masoud and Li, 2015; Takahashi et al., 2014b). For instance, in BC cell line 5637, lncRNA urothelial cancer-associated 1 (UCA1) is secreted during hypoxia condition and aids tumor promotion via epithelial-mesenchymal transition (EMT) (Xue et al., 2017). In another study it was shown that CCAL (colorectal cancer-associated lncRNA) is transferred from cancer-associated fibroblasts (CAFs) to cancer cells via exosomes. Once inside the CRC cells, CCAL suppresses apoptosis, enhances chemoresistance, and activates the β-catenin pathway, both in vitro and in vivo (Deng et al., 2020). Furthermore, Liang et al., found that exosomes derived from CRC cells transport lncRNA RPPH1 into macrophages. This transfer promotes the polarization of macrophages into the M2 phenotype, which in turn facilitates metastasis and proliferation of CRC cells. Additionally, levels of exosomal RPPH1 in blood plasma were higher in treatment-naive CRC patients but decreased after tumor resection (Liang et al., 2019). Another study on CRC showed that the expression of lncRNA 91H in serum was closely associated with exosomes, both in vitro and in vivo. The association was likely to enhance tumor-cell migration and invasion during tumor development by altering HNRNPK expression. Also, CRC patients with high levels of lncRNA 91H expression had a higher risk of tumor recurrence and metastasis compared to patients with low lncRNA 91H expression (Gao et al., 2018). Moreover, Pan et al., found that the expression of ZFAS1 was elevated in tumor tissues, serum, and serum exosomes of GC patients, which was significantly correlated with lymphatic metastasis and the TNM stage of the disease (Pan et al., 2017).
Some other exosomal lncRNAs have been identified that can contribute to cell growth (Zhao et al., 2019a), proliferation (Zhao et al., 2019a; Xu et al., 2020a; Hu and Hu, 2019; Conigliaro et al., 2015), angiogenesis (Chen et al., 2020a; Conigliaro et al., 2015), migration (Chen et al., 2020a; Zhao et al., 2019a; Xu et al., 2020a; Hu and Hu, 2019; Chen et al., 2020b; Feng et al., 2019), invasion (Xu et al., 2020a; Chen et al., 2020b; Lu et al., 2020), metastasis (Chen et al., 2020a; Zhao et al., 2019a), drug resistance through activation of Wnt/β-catenin pathway in recipient cells (Deng et al., 2020), inhibition of apoptosis (Chen et al., 2020b; Yin et al., 2020; Song et al., 2020), and inhibition of inflammation (Song et al., 2020; Li et al., 2018a), all of which ultimately promotes tumor growth. Lastly, recent studies have pointed out the involvement of exosomal lncRNAs in autophagy. Tumor cells tend to upregulate autophagy in order to minimize environmental stress, protect themselves from chemotherapy, and maintain their fuel supply. Exosomal lncRNAs have been proven to play a significant role in inducing autophagy which leads to cell survival and proliferation (Pathania and Challagundla, 2021; Sun et al., 2013). For example, high lncRNA-CAF levels in normal stromal fibroblasts reinforce tumor proliferation, and are associated with poor prognosis in oral squamous cell carcinoma (OSCC) patients (Ding et al., 2018). Table 3 details the specific roles of long non-coding RNAs (lncRNAs) contained within exosomes in the context of tumor microenvironment modulation.
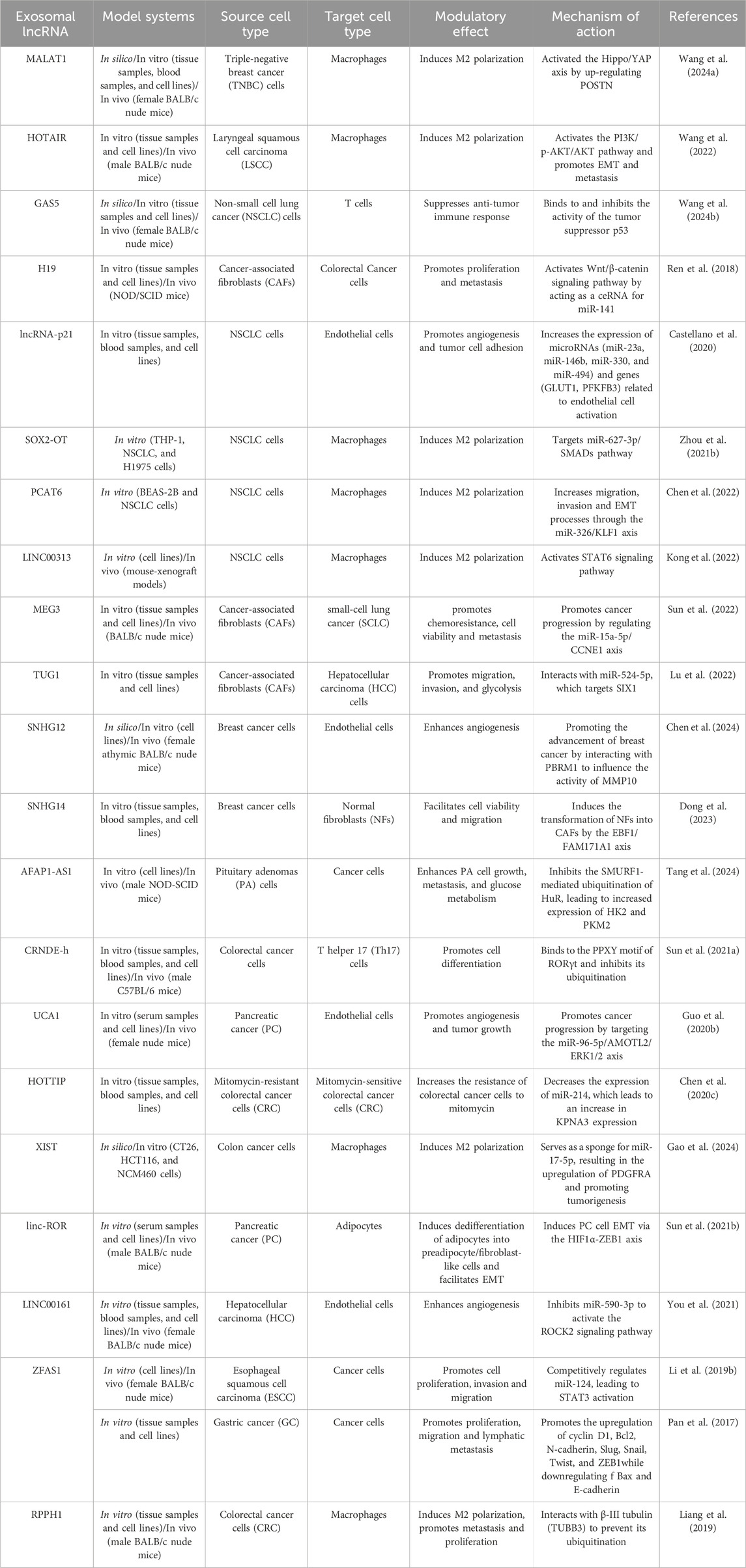
Table 3. Role of Exosomal lncRNAs in Tumor Microenvironment Modulation.
5 Drug resistance in gynecological cancers5.1 Mechanisms underlying drug resistance in cancer cellsDrug resistance has long been an issue in many cancer studies. Despite the efforts and successes in cancer treatment, anticancer drug resistance remains a major problem in the treatment of this disease and has a significant impact on the clinical outcome (Mondal and Meeran, 2021; Vasan et al., 2019). In general, there are two types of drug resistance: intrinsic, which exists before treatment starts, and acquired resistance. In acquired resistance, cancer cells that have already received the drugs can acquire resistance to the treatment at later stages, which is the most important cause of relapse and death in cancer patients (Wang et al., 2019a). Chemical resistance can be divided into two categories: single-drug resistance and multidrug resistance. In the case of single-drug resistance, the cancer cells are resistant to a specific drug. In contrast, multidrug resistance occurs when the cancer cells are resistant not only to the drugs currently being used, but also to other chemotherapeutic agents to which they have not previously been exposed (Gacche and Assaraf, 2018). MDR is one of the most important mechanisms in the development of drug resistance and is responsible for more than 90% of deaths in cancer patients receiving anticancer drugs. The major mechanisms in the development of MDR include overexpression of ABC transporters, defects in the apoptotic system, alterations in drug metabolism or drug targets, epigenetic changes, oncogene amplification, and increased DNA repair capacity (Bukowski et al., 2020; Yang et al., 2014; Vaidya et al., 2022).
5.2 Specific drugs and their resistance profiles in gynecological cancers5.2.1 Breast cancerCurrently, most breast cancer therapies are based on targeted treatments, as this disease has considerable heterogeneity. ER+ (estrogen receptor) and PR+ (progesterone receptor) breast cancers belong to the subtype of hormone-dependent cancers (Kinnel et al., 2023). Approximately 70%–80% of all breast cancers are HR+, which can be effectively treated by endocrine therapy or anti-estrogen therapy by modulating ER or lowering estrogen levels. One of the main drugs in the treatment of HR + breast cancer is tamoxifen. This drug is a selective estrogen receptor modulator that blocks the effect of estrogen on ER-positive breast cancer cells. Hormone therapy for premenopausal women includes administration of tamoxifen alone or a combination of a luteinizing hormone-releasing hormone analog with tamoxifen or an aromatase inhibitor (AI). Postmenopausal women are treated with aromatase inhibitors such as letrozole and exemestane or tamoxifen combined with SERDs (selective estrogen receptor degraders) like fulvestrant (Kay et al., 2021). Also, abemaciclib is an inhibitor of cyclin-dependent kinase 4 and 6 (CDK4/6) that was recently approved for HR + advanced breast cancer in combination with endocrine therapy (Johnston et al., 2020). The role of tyrosine kinase receptors (activation of the PI3K/AKT/mTOR pathway), transcription factors (activation of the C-MYC/HDAC5/SOX9 axis), cell cycle regulators (interaction of LEM4 with CDK 4/6 and Rb to accelerate the G1–S transition), and autophagy (activation of LAMP3) are among the mechanisms involved in tamoxifen resistance (Yao et al., 2020). 15%–20% of breast cancers have overexpression of the HER-2 gene and are more aggressive than HER-2 negative breast cancers (Le Du et al., 2021). Trastuzumab, a recombinant humanized monoclonal antibody, has been one of the first drugs used in the treatment of HER-2+ breast cancer. First-line therapy for most patients consists of a mix of trastuzumab, pertuzumab (another monoclonal antibody), and taxanes (a class of chemotherapeutic agents), while trastuzumab emtansine (T-DM1) is the second-line therapy (Kay et al., 2021; Le Du et al., 2021; Martínez-Sáez and Prat, 2021). Several novel treatments such as tucatinib, lapatinib, neratinib, fam-trastuzumab deruxtecan-nxki (DS-8201a), and margetuximab-cmkb that have recently been approved are among the third-line treatment options (Martínez-Sáez and Prat, 2021). Despite medical advances in the treatment of this type of breast cancer, approximately 22%–25% of HER-2-positive metastatic breast cancer patients have congenital or acquired drug resistance, which is associated with significant morbidity and mortality (Choong et al., 2020). Mechanisms of drug resistance in HER-2+ breast cancer include inadequate blockade of the HER-2 receptor, activation of downstream signaling pathways like PI3K and MAPK, inhibition of tumor suppressor genes, acquired HER-2 mutations, dysregulation of cell cycle regulators (high copy number of the CCND1 gene encoding cyclin D1), and non-cell autonomous mechanisms within the tumor microenvironment (Kay et al., 2021; Choong et al., 2020; Zhang, 2021). Triple negative breast cancer (TNBC) lacks ER, PR, and HER-2 receptors. It accounts for 15%–20% of all breast cancers, and due to its heterogeneity and complexity, treatment strategies are usually unsuccessful (Merikhian et al., 2021; Marra et al., 2020). Currently, chemotherapy is the first line of treatment, and most patients become resistant to chemotherapeutic agents (Cosentino et al., 2021). Conventional chemotherapy used in TNBC treatment can be divided into two categories: neoadjuvant therapy and adjuvant therapy. Anthracycline–cyclophosphamide drugs (AC regimen) such as doxorubicin, cyclophosphamide and paclitaxel are used in neoadjuvant therapy, which can improve the efficacy of treatment when combined with cisplatin. Other neoadjuvant drugs include carboplatin, Abraxane, and bevacizumab (a monoclonal antibody that inhibits VEGF activation). Neoadjuvant therapy also utilizes anthracycline-taxane like doxorubicin, docetaxel, and cyclophosphamide. In cases of advanced or metastatic breast cancers where these drugs are ineffective, capecitabine may be given alone or in combination with docetaxel (Medina et al., 2020). Some mechanisms involved in TNBC chemoresistance are upregulation of ABC transporters (an important mechanism), presence of cancer stem cells (CSCs), hypoxia, and TP53 mutations (Marra et al., 2020).
5.2.2 Ovarian cancerOvarian cancer treatment generally begins with surgical removal and is followed by chemotherapy, radiotherapy, or neoadjuvant chemotherapy (Alatise et al., 2022). High-dose chemotherapy usually leads to drug resistance development in patients, and about 80% of patients experience a relapse after treatment and gradually become resistant to chemotherapeutic agents (Alatise et al., 2022; Brasseur et al., 2017). Platinum-based and taxane-based drugs, including cisplatin (DDP), are mainly used to treat ovarian cancer, and resistance to these drugs has caused many problems so far (Alatise et al., 2022; Cui, 2022; Zhang et al., 2023). In advanced cases, a combination of cisplatin with gemcitabine is recommended to increase the response to cisplatin treatment (Garrido et al., 2021).
Ovarian cancer (OC) is categorized into two main types: epithelial OC and non-epithelial OC. Epithelial ovarian cancer (EOC) is the most common type, accounting for about 90% of all malignant ovarian tumors. EOC consists of several subtypes, including endometrioid, clear cell, serous carcinoma [low-grade serous carcinoma (LGSOC) and high-grade serous ovarian carcinoma (HGSOC)], undifferentiated, and mucinous. Among these, HGSOC is the most common subtype and is responsible for 75% of EOC cases (Lukanović et al., 2022; Gaona-Luviano et al., 2020; Shih et al., 2021). Recently, polyadenosine diphosphate ribose polymerases (PARP) inhibitors (niraparib, cediranib, olaparib, and pembrolizumab) and anti-angiogenic options (trebananib, sorafenib, entrectinib) have been shown to be effective in the treatment of epithelial origin (EOC) (Garrido et al., 2021). The endometrioid and clear cell subtypes make up 20%–25% and 5%–10% of EOCs, respectively (Zhou et al., 2021c). In one study, a signaling pathway was discovered that can be targeted to increase the sensitivity of platinum-resistant ovarian endometrioid cancer cells to chemotherapy. The study revealed that using non-receptor tyrosine kinase Lymphocyte Cell-Specific Protein-Tyrosine Kinase inhibitors (LCKi) followed by co-treatment with cisplatin resulted in lower cell viability and increased cell death in laboratory tests. This effect was associated with increased DNA adduct formation and reduced tumor growth in vivo (Crean-Tate et al., 2021). Clear cell carcinoma (CCC) is one of the most common chemoresistant cancers. However, the precise mechanisms underlying drug resistance in this type of cancer are not fully understood. Studies have demonstrated that genes associated with the epithelial-mesenchymal transition (EMT) pathway are markedly elevated in cases of ovarian CCC that do not respond to chemotherapy. This suggests that the EMT pathway plays a crucial role in the development of chemotherapy resistance (Takahashi et al., 2023). Although 60%–80% of patients with HGSOC initially exhibit a positive response to treatment, most will eventually develop resistance to platinum-based therapies. Epigenetic modifications like DNA methylation, histone deacetylation, and microRNA expression have been linked to the development of chemoresistance in HGSOC. For instance, hypomethylation of MSX1 contributes to EMT by promoting cancer cell transition to a mesenchymal phenotype, resulting in chemoresistant HGSOC patients. These modifications could be targeted by epigenetic modulation therapies to overcome chemoresistance (Matthews et al., 2021).
Non-epithelial ovarian cancers (NEOC) are rare malignancies, accounting for approximately 10% of all ovarian cancer cases. This group mainly comprises germ cell tumors (GCT), sex cord-stromal tumors (SCST), and a few exceptionally rare tumor subtypes (Martínez-Sáez and Prat, 2021). GCTs and SCSTs represent 2%–5% and 2% of ovarian cancers, respectively. Cisplatin-based chemotherapy with surgery can effectively treat most GCTs. However, resistance to cisplatin (CDDP) can develop due to changes in the levels of critical factors such as p53, mouse double minute 2 homolog (MDM2), octamer-binding transcription factor 4 (Oct4), and cytoplasmic p21. Due to the lack of high-quality studies in this area, current practice guidelines recommend using chemotherapy regimens established for testicular germ cell tumors due to their similar origin (De Giorgi et al., 2019; Uccello et al., 2020). The current targeted therapy approaches being studied in clinical trials for GCTs and SCSTs include tyrosine kinase inhibitors, angiogenesis inhibition, immunotherapy, and endocrine therapy. However, there is still limited research on many aspects of non-epithelial ovarian tumors, especially their resistance to chemotherapy (Maoz et al., 2020).
Examples of chemotherapy resistance mechanisms in ovarian cancer include expression of anti-apoptotic proteins, overexpression of MAPK-activating death domain protein (MADD), disruption of major DNA repair pathways, increased efflux due to high expression of APC transporters, and the presence of a small subset of cancer stem cells (CSCs) (Alatise et al., 2022; Ortiz et al., 2022).
5.2.3 Cervical cancerCervical cancer has two main types: squamous cell carcinoma (SCC), accounting for about 75% of cases, and adenocarcinomas, responsible for approximately 25% of cervical cancers.
High-risk human papillomavirus (HPV) infection, predominantly types 16 and 18, is considered a major factor in the development of most SCCs (Volkova et al., 2021; Liu et al., 2023). Although, HPV-independent tumors are associated with both adenocarcinomas and squamous histologic subtypes (Fernandes et al., 2022). HPV-negative cervical cancers have a more aggressive course, which is clinically significant (Volkova et al., 2021). HPV oncoproteins like E6 and E7, which inactivate tumor suppressor genes p53 and Rb, further promote resistance by allowing cells to evade apoptosis (Xiong et al., 2022).
Surgery and radiotherapy are the main treatments of cervical cancer, and chemotherapy is often used if metastasis or tumor recurrence happens (Li et al., 2017). One of the main chemotherapeutic agents used to treat cervical cancer is cisplatin, which exerts its effects by attacking DNA and forming irreparable bonds with it (Mahapatra et al., 2022). Research has shown that the combination of platinum and paclitaxel has better efficacy in treatment response compared with cisplatin alone (Della Corte et al., 2020). In advanced cervical cancer, patients become resistant to these drugs, and cancer cells evade its anti-cancer effects; therefore, finding a solution to overcome the acquired resistance is of great importance (Mahapatra et al., 2022; Liu et al., 2016a). Acquired cisplatin resistance in cervical cancer therapy is due to decreased uptake of cisplatin (downregulation of the transmembrane protein CTR1), increased efflux (overexpression of MRP1), inactivation of the apoptotic pathway, inactivation of proteins containing thiol groups, and activation of the epithelial-mesenchymal transition (EMT) (Bhattacharjee et al., 2022).
5.2.4 Endometrial cancerEndometrial cancer is a common gynecological cancer that affects the uterus. According to Bokhman’s classification, it is divided into two types: estrogen-independent, which has a poorer outlook and is often diagnosed late, and estrogen-dependent endometrial cancers. Moreover, WHO classifies endometrial cancer into four types: endometrioid endometrial cancer (EEC), serous adenocarcinoma, clear cell adenocarcinoma and mixed endometrial cancer and uterine carcinosarcoma. EECs are generally estrogen-dependent tumors and are the most common type of endometrial cancer, accounting for more than 80% of cases (Yang et al., 2024). Treatment typically involves surgery, vaginal brachytherapy, external beam radiotherapy (EBRT) and in advanced cases, carboplatin, paclitaxel, adriamycin, and cisplatin may be administered (Saito et al., 2021). For hormone receptor-positive cancers, hormone therapies such as progestins, gonadotropin-releasing hormone agonists, or aromatase inhibitors are used (Kailasam and Langstraat, 2022). Emerging treatments include immune checkpoint inhibitors like pembrolizumab, particularly for cancers with microsatellite instability (MSI) or mismatch repair deficiency (dMMR), and targeted therapies such as PI3K/AKT/mTOR inhibitors (Yang et al., 2024; Jeong et al., 2023; Venkata et al., 2022; Colombo et al., 2024). Resistance to treatment in endometrial cancer often occurs due to activation of the PI3K/AKT/mTOR pathway, especially in tumors with mutations in the PIK3CA gene or loss of PTEN function. These mutations promote cell survival and reduce the effectiveness of hormonal and chemotherapeutic treatments (Yang et al., 2024; Lee, 2021). Additionally, some endometrial cancers that initially respond to immunotherapy can develop resistance by upregulating immune evasion mechanisms, such as increased PD-L1 expression, while hormone therapy resistance is often linked to mutations in estrogen and progesterone receptors (Yang et al., 2024; Hao et al., 2024). Table 4 provides a list of various drugs and how they cause drug resistance in different types of cancers.
留言 (0)