Breast cancer is currently the most prevalent cancer among women and a leading cause of cancer-related deaths. In 2020, there were 2.3 million new cases of breast cancer and 685,000 deaths globally (1). China is experiencing a shift towards cancer patterns seen in developed nations, marked by elevated incidences of lung, colorectal, breast, and prostate cancers. According to GLOBOCAN 2020 data, breast cancer is the most commonly diagnosed cancer in females (2). Numerous evidence-based medical studies indicate that early detection and treatment through screening can lower mortality rates and reduce the intensity of required treatment (3). X-ray mammography (MG) is a primary imaging tool for early breast cancer detection in Western countries and is widely utilized for breast cancer screening. However, in dense breast tissue, MG’s diagnostic sensitivity decreases by about 50% (4). Asian women, particularly those under 40 years old, often have smaller and denser breasts compared to Western women. Since MG is less effective for dense breasts, US plays a crucial role in screening for breast disease among Asian women (5).
Ultrasound is non-radioactive and adept at distinguishing between fat and gland tissue echoes, as well as characterizing lesion morphology and boundaries (6). Previous studies have shown that in large-scale screenings, US and MG demonstrate comparable efficiency, with US exhibiting higher sensitivity and specificity in screening dense breasts (7–9). However, conventional ultrasound examinations using handheld probes may result in local omissions due to size limitations and inspector experience. The repeatability and standardization of traditional hand-held ultrasound (HHUS) need enhancement. Introduced in 2009, the automatic breast volume scanner ultrasound (ABUS) offers advantages such as reduced time consumption, objectivity, and consistent retention of inspection data and plane graphics, in contrast to HHUS (10). Hence, ABUS may serve as a valuable tool for breast cancer screening and diagnosis. This study represents the first large-scale population opportunistic screening employing ABUS and compares its diagnostic performance with HHUS.
MethodsStudy designThis study was a multi-center randomized trial conducted in 10 breast centers across 9 districts of Beijing (Dongcheng, Chaoyang, Fengtai, Haidian, Tongzhou, Shunyi, Pinggu, Daxing, and Yanqing District). The lead center was PUMCH. The study was approved by the ethics committee of PUMCH (No. JS-1029) and registered in ClinicalTrials.gov (ChiCTR1900023916). Informed consent was obtained from all participants. Between January 1, 2016, and December 31, 2019, a total of 10,537 women aged 35–75 years from 9 districts were evaluated for study eligibility (Figure 1). All enrolled patients were required to fill out a high-risk factor questionnaire according to the PUMCH risk-assessment model (11). The collected data included age, height, weight, age at menarche, age at first live birth, age at menopause, breastfeeding history, lifestyle, reproductive history, and family history. Participants were randomized into two groups for HHUS or ABUS examination. Each group was further divided into subgroups based on the presence or absence of MG screening. Random number tables were used for randomization. Results of physical and ultrasound examinations, as well as follow-up outcomes, were recorded. Women with negative screening results or benign biopsy findings were invited for rescreening one year later. Medical records, telephone calls, mails, emails, or face-to-face interviews were used for annual follow-ups.
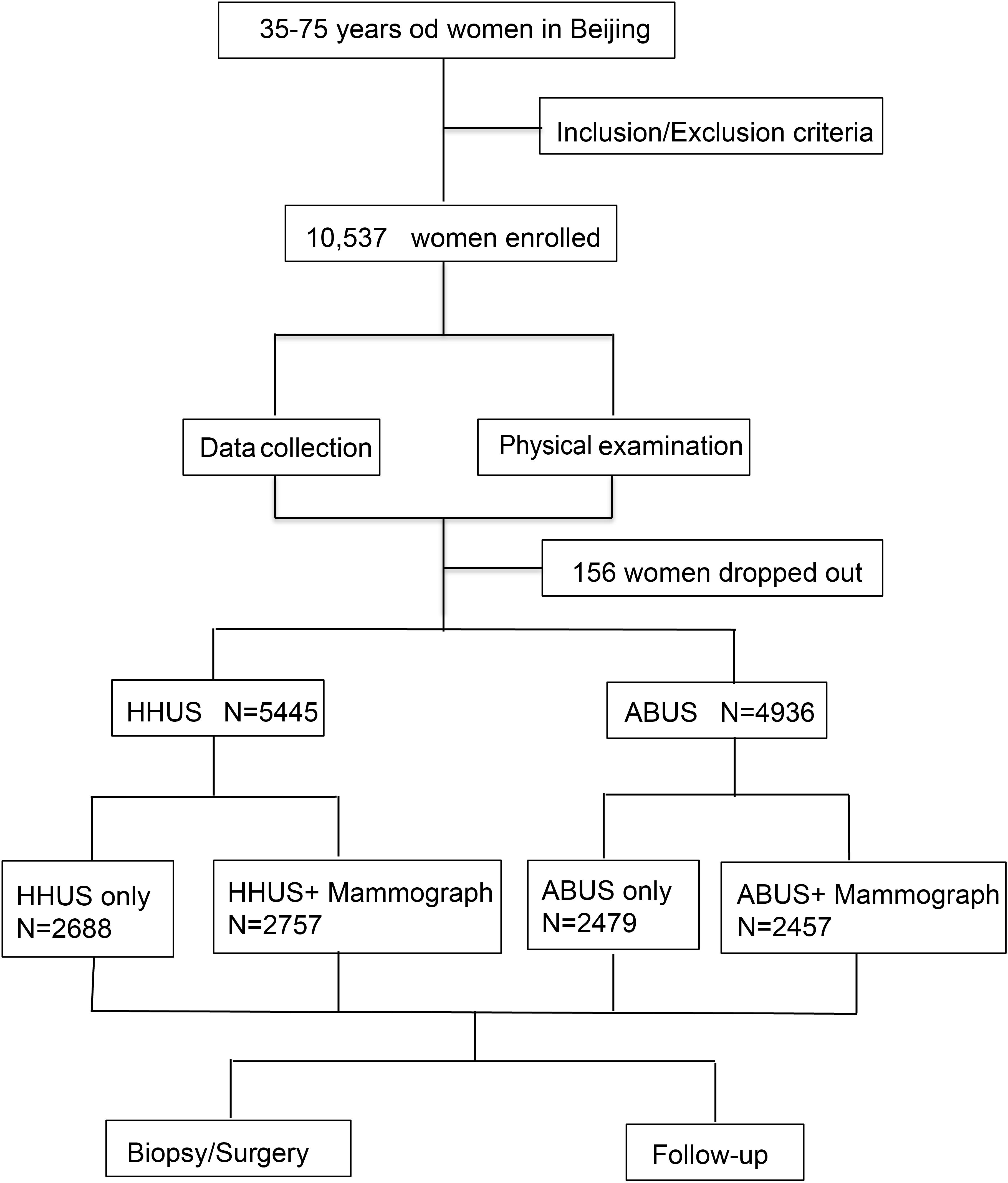
Figure 1. Flowchart of study design: A total of 10,537 women aged 35–75 years were enrolled in our study. After excluding 156 participants who were lost to follow-up, the number of participants enrolled in the two screening groups were 5,445 and 4,936, respectively.
Inclusion and exclusion criteriaInclusion criteria: (1) Female, aged 35–75 years. (2) No history of breast cancer. (3) Willingness to provide truthful information. (4) Sign informed consent. (5) Willingness to participate in follow-up by researchers. Exclusion criteria: (1) Pregnant or breastfeeding women. (2) History of breast prosthesis implantation. (3) History of breast cancer or other malignant tumors. (4) Breast surface damage, including obvious ulcers, purulent infections, or other infections. (5) History of hysterectomy and inability to determine menstrual status.
Screening methods and quality assuranceFor HHUS screening, Resona 7 or 8 devices (Mindray Medical, Shenzhen, China) equipped with 5-14 MHz linear-array transducers were utilized. Screening with the ABUS was conducted using Siemens equipment, comprising a scanning unit and a diagnostic workstation. The scanning unit featured a high-frequency (10–15 MHz) linear transducer. All professionals involved, including physicians and technicians, underwent standardized technical, theoretical, and practical training provided by Siemens Healthcare and the Department of Ultrasound affiliated with Peking Union Medical College Hospital. Digital mammography was performed using a standard two-view technique.
Lesions were evaluated based on size, shape, border, edge, orientation, internal and posterior echo, calcification, and presence of axillary lymph nodes, among other manifestations. The Breast Imaging Reporting and Data System (BI-RADS), proposed by the American College of Radiology (ACR) in its 2013 edition, was used to classify lesions into five categories (12). Breast density was assessed via ultrasound and categorized according to BI-RADS ACR classifications and quantifications: BI-RADS A: almost entirely fat (low density of mammary gland parenchyma), BI-RADS B: scattered fibroglandular densities (average density of gland parenchyma), BI-RADS C: heterogeneously dense (high density of gland parenchyma), and BI-RADS D: extremely dense (very high density of gland parenchyma) (13).
Experienced radiologists underwent further training in ABUS and the appropriate and clear utilization of BI-RADS for quality and consistency assurance. Mammography, HHUS, and ABUS were performed and interpreted separately by different physicians with over five years of experience. Women classified as BI-RADS 4b-c or 5 underwent core needle biopsy or surgical biopsy for pathological diagnosis. For those classified as BI-RADS 3 or 4a, biopsy or follow-up was conducted as necessary. Regular annual follow-up was conducted until January 2020, with interval cancers documented during follow-up.
Statistical analysisSensitivities and specificities were determined using the positive results of biopsies and follow-ups until January 2020 as the ‘gold standard’. The positive predictive value (PPV) was calculated as the malignancy rate among cases that tested positive by each screening modality. Sensitivity[True positive/(True positive+false negative)], specificity[True negative/(True negative+false positive)], accuracy[(True positive+true negative)/all], PPV(True positive/detected positive), and negative predictive value (NPV)(True negative/detected negative) for different diagnostic methods were quantified, and comparisons were made using the Chi-square test. The Receiver Operating Characteristic (ROC) curve illustrated the diagnostic performance of ABUS and HHUS, with or without mammography. A comparison of the Area Under the Curve (AUC) was conducted using the DeLong test. Statistical analyses were performed using R software (version 4.2.2). All statistical tests were two-sided, and a P-value < 0.05 was considered indicative of a significant difference.
ResultsParticipants enrollment and characteristicsA total of 10,537 women with a mean age of 47.00 ± 8.32 completed high-risk assessment and were eligible for inclusion in the study. After excluding 156 participants lost to follow-up, 10,381 participants were included in the analysis. All participants underwent a total of 35,395 breast screenings. The number of participants enrolled in the two screening groups (HHUS and ABUS) were 5,445 and 4,936, respectively. Each group was further divided into two subgroups based on the presence or absence of MG screening. The number of participants in the four subgroups (HHUS only, HHUS+MG, ABUS only, ABUS+MG) were 2,688, 2,757, 2,479, and 2,457, respectively. The demographic characteristics of the four subgroups are shown in Supplementary Table S1. The demographic characteristics of participants who received HHUS and ABUS are shown in Table 1. Generally, the demographics of the two ultrasound screening groups were comparable (P > 0.05).
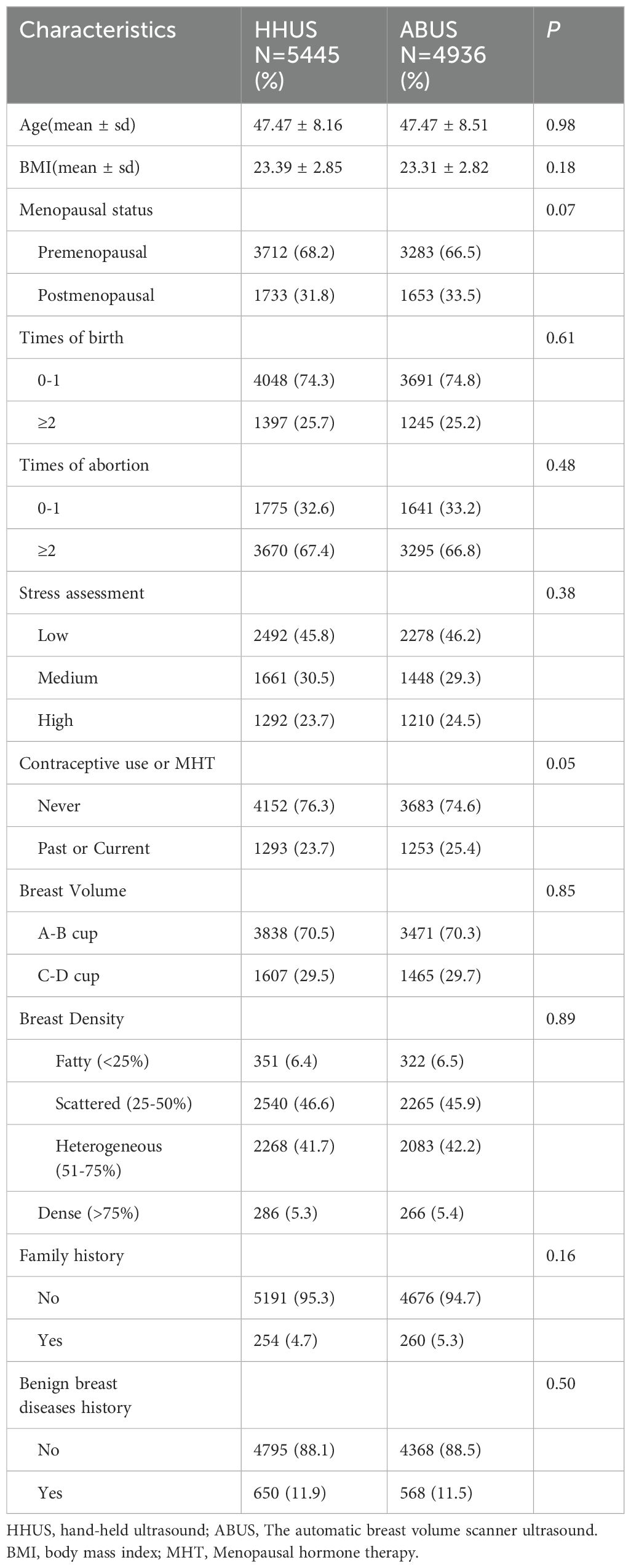
Table 1. Demographic and clinical characteristics of enrolled patients.
Screening finding and Cancer detectionUltrasound screening findings were classified according to BI-RADS score (1–3 as negative and 4–5 as positive). Among the 5,445 participants in the HHUS group, 376 subjects were diagnosed as ultrasound-positive, and 382 participants underwent biopsy or surgery. Among the 4,936 participants in the ABUS group, 350 subjects were diagnosed as ultrasound-positive, and 249 participants underwent biopsy or surgery. Overall, the HHUS group and the ABUS group identified 90 carcinomas and 292 benign lesions or 71 carcinomas and 178 benign lesions, respectively. There was no significant difference in the positive rate of ultrasound and the detection rate of carcinomas between the two groups, while the biopsy/surgery rate of the HHUS group was higher (Table 2). The addition of mammography to ultrasound improved the sensitivity of breast cancer screening but slightly decreased the specificity of screening (Supplementary Table S2).
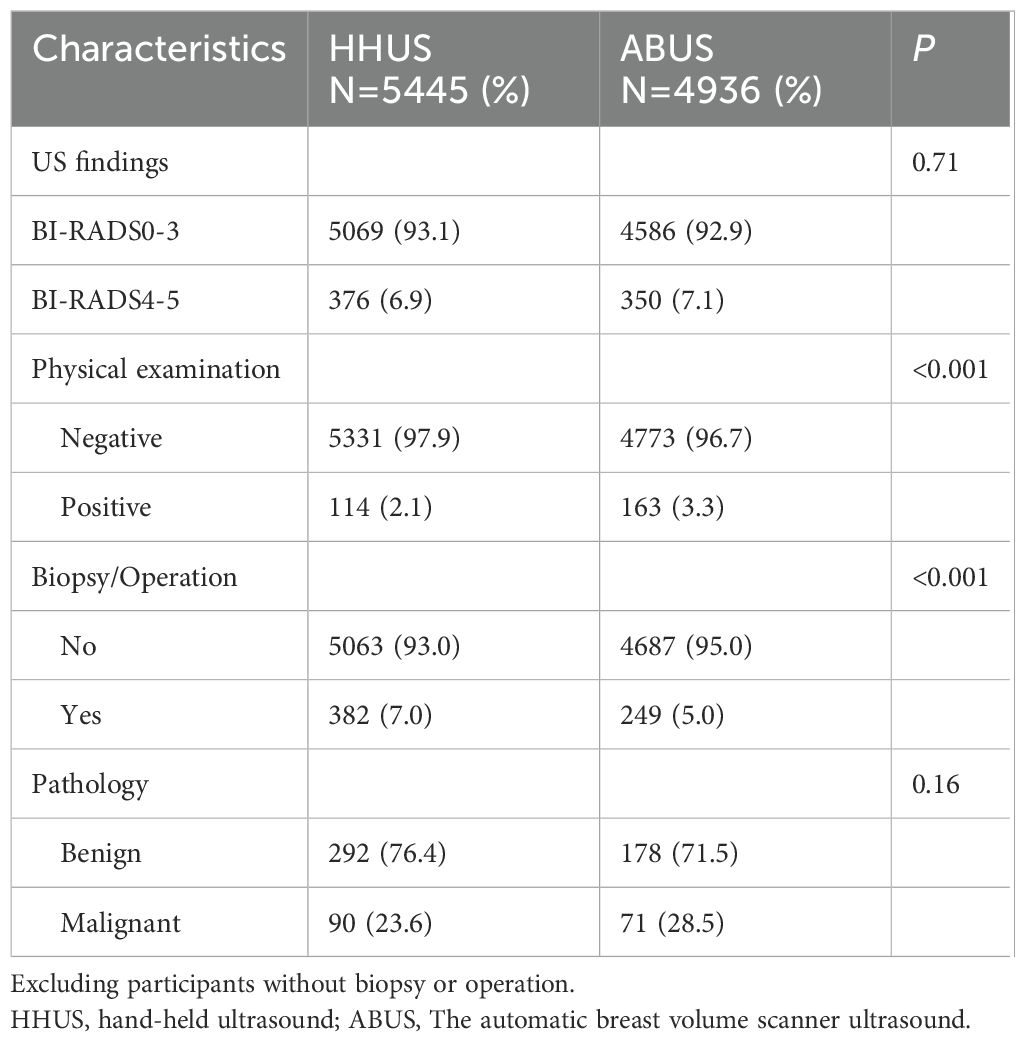
Table 2. Screening findings of HHUS and ABUS.
The demographic characteristics of participants who underwent biopsy/surgery are shown in Table 3. A total of 161 malignant lesions were confirmed by pathology. Compared with 470 participants with benign lesions, patients with malignant tumors were approximately 5 years older (46.13 vs. 51.34). The proportion of postmenopausal patients was higher in patients with malignant lesions (P < 0.001). Participants with a history of benign breast disease tended to have benign lesions in this biopsy/surgery (P < 0.001). There was no significant difference in BMI, parity, abortion history, stress assessment, contraceptive use or MHT, breast volume, breast density, or family history.
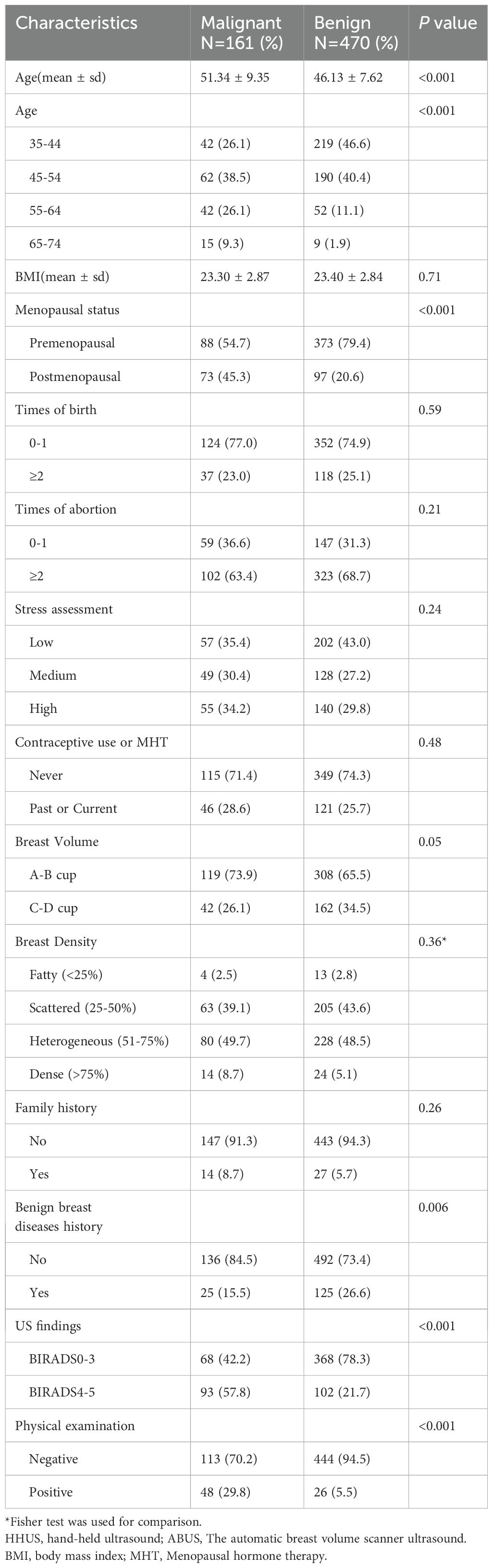
Table 3. Demographic and clinical characteristics of participants underwent biopsy/operation.
Diagnosis performance of HHUS and ABUSThe sensitivity (SE), specificity (SP), accuracy (AC), positive predictive value (PPV), and negative predictive value (NPV) of HHUS and ABUS are calculated and presented in Table 4. For HHUS, the SE, SP, AC, PPV, and NPV were 51.11%, 93.84%, 93.13%, 12.23%, and 99.13%, respectively. For ABUS, these values were 66.20%, 93.77%, 93.38%, 13.43%, and 98.98%, respectively. The diagnostic performance indices between the two groups were similar (P > 0.05). ROC curves were depicted to illustrate the diagnostic performance (Figure 2). The AUC values of HHUS and ABUS were 0.72 (95% CI: 0.67-0.78) and 0.86 (95% CI: 0.82-0.91), respectively, indicating better diagnostic performance of ABUS compared to HHUS (Delong test, P < 0.05). The diagnostic performance indices among the four subgroups (HHUS only, HHUS+MG, ABUS only, ABUS+MG) are shown in Supplementary Table S3. Among the 5,214 participants who underwent mammography, the SE, SP, AC, PPV, and NPV for HHUS were 21.50%, 99.43%, 97.58%, 16.14%, and 98.37%, respectively (Supplementary Table S4). Compared with mammography screening, HHUS and ABUS screening demonstrated higher sensitivity in Chinese women.
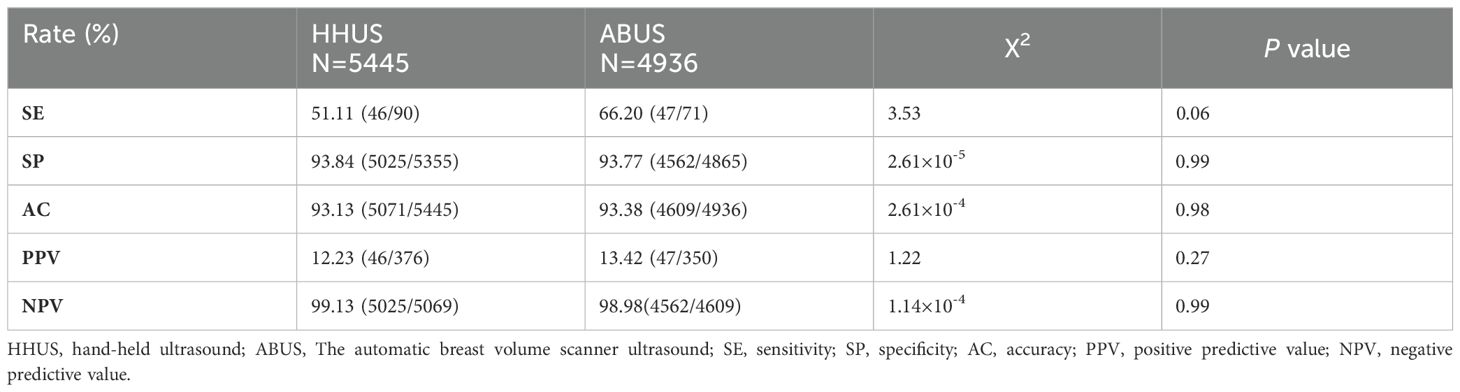
Table 4. Diagnostic performance of HHUS and ABUS.
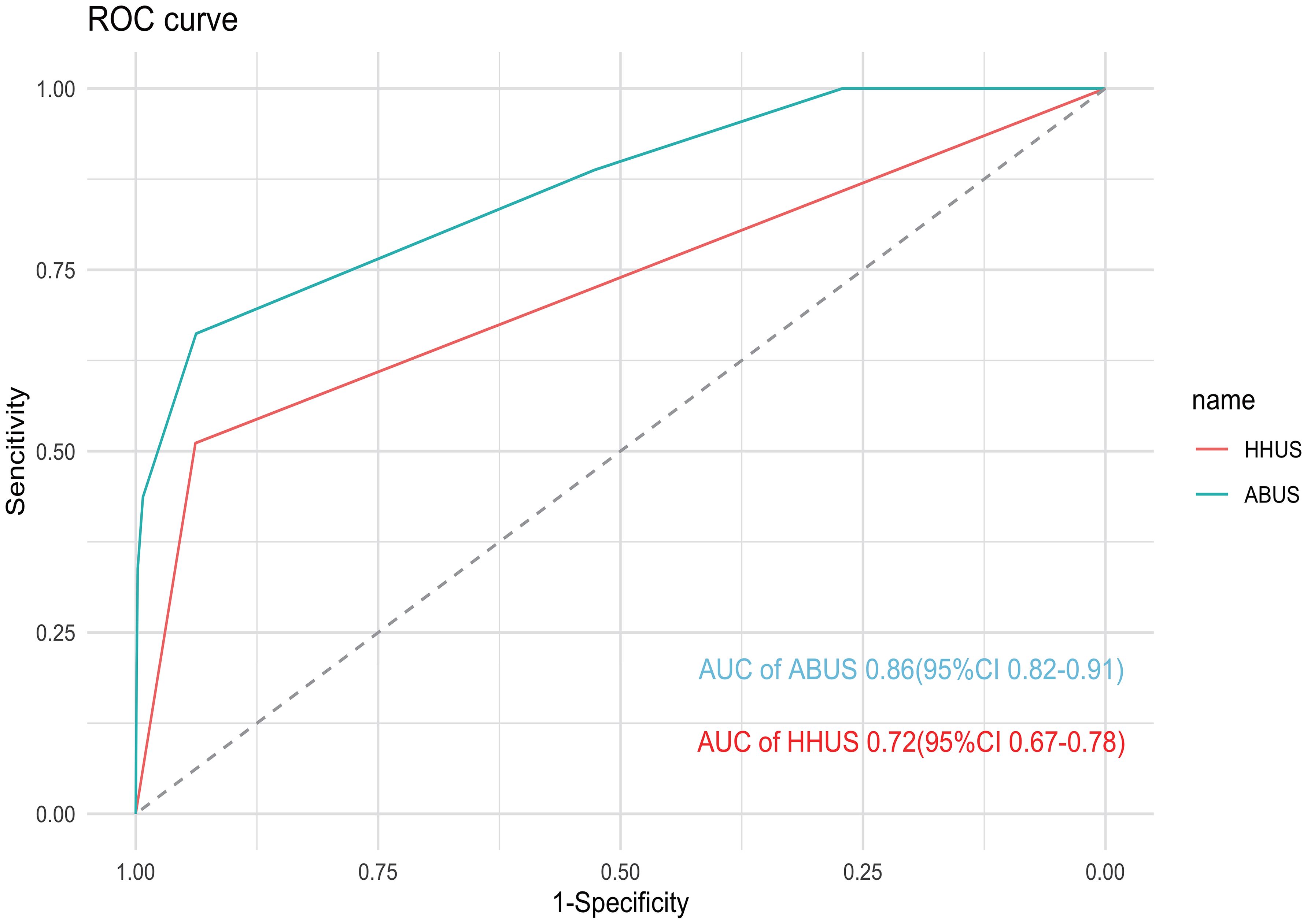
Figure 2. ROC curve of HHUS and ABUS for enrolled patients. The AUC of ABUS and HHUS were 0.86 (95% CI: 0.82–0.91) and 0.72 (95% CI: 0.67–0.78), respectively.
Subgroup analyses were conducted to assess the diagnostic performance of HHUS and ABUS across patient subpopulations (Table 5). Participants were divided into three age subgroups: 35–44 years old (4,224 participants), 45–54 years old (4,052 participants), and 55–74 years old (2,015 participants). There was no significant difference in the diagnostic performance index between the two groups in the three different age subgroups. Similarly, the diagnostic performance index between the two groups in breast density subgroups and menopausal status subgroups was also similar (P > 0.05). Additionally, participants were divided into A-B Cup and C-D Cup subgroups based on cup size. ABUS demonstrated better sensitivity than HHUS in the C-D Cup subgroup, while there was no significant difference in SP, AC, PPV, and NPV.
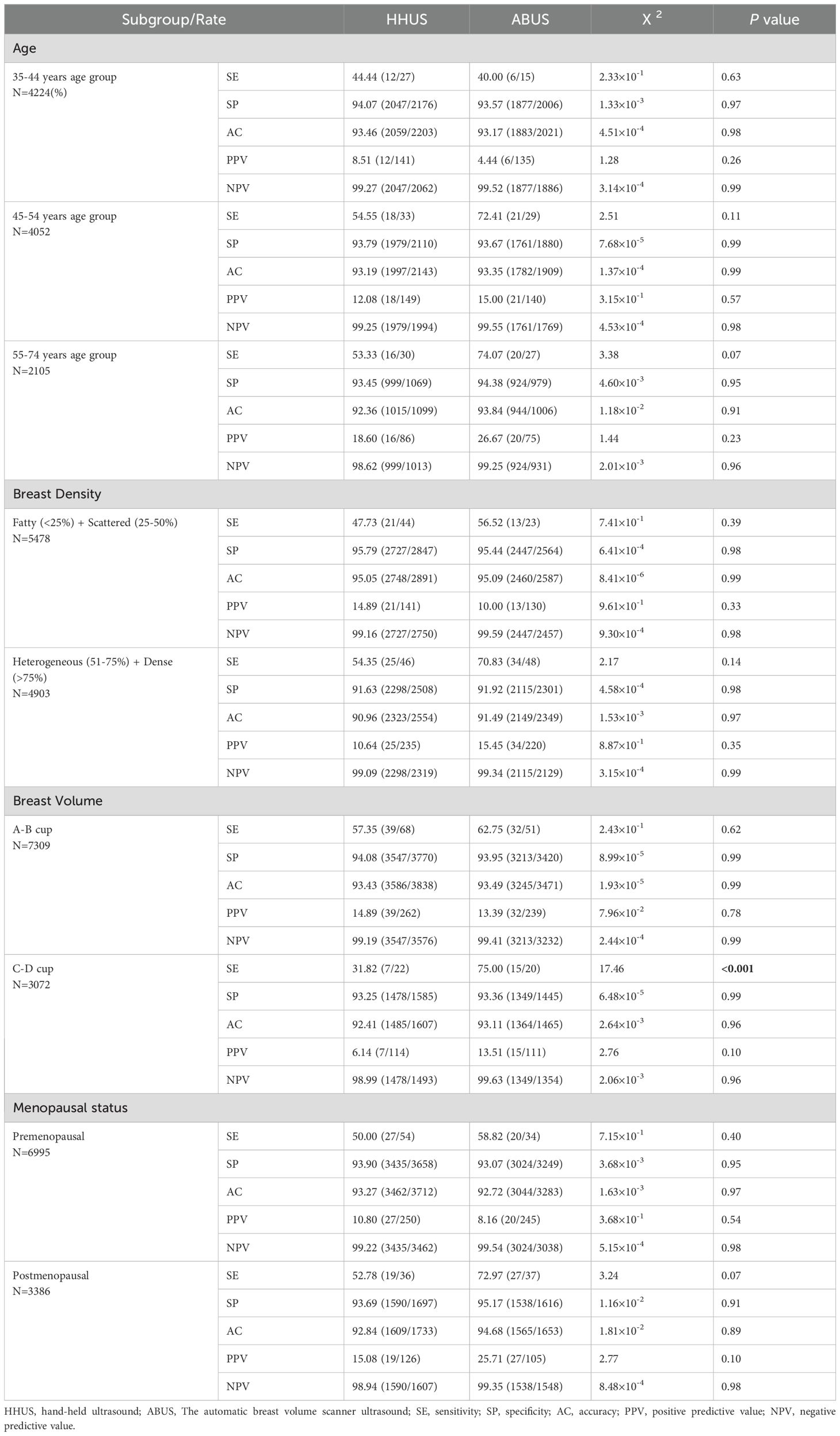
Table 5. Subgroup analyses of diagnostic performance of HHUS and ABUS.
DiscussionEarly detection of breast cancer has significantly reduced breast cancer mortality by 15% and led to down-staging of breast cancer at diagnosis (14). While MG is the preferred screening method in Western countries, previous research has shown that ultrasound examination is sensitive, specific, and cost-effective for detecting breast cancer in Chinese women (9). In our study, ultrasound screening demonstrated higher sensitivity compared to MG. HHUS is operator-dependent and has poor repeatability, whereas ABUS overcomes the limitations of manual scanning and offers repeatability. In 2012, ABUS was approved for use in the United States and subsequently introduced to China. It stands as the sole ultrasound system globally to have obtained FDA certification for the early diagnosis of breast cancer. ABUS offers standardized tomographic images of the breast that have already found applications in clinical practice. ABUS has emerged as a novel breast cancer diagnostic technology and shows no statistically significant difference from HHUS in terms of interobserver variability and diagnostic performance (15). In this multi-center randomized trial, ABUS was used as a diagnostic tool for the first time in large-scale population opportunistic screening, and its diagnostic performance was similar to HHUS (P > 0.05). Combining ultrasound with mammography in our study improved screening sensitivity but slightly reduced specificity, though the difference in specificity was not significant. Previous studies have demonstrated that ABUS has better or at least comparable diagnostic capabilities to HHUS. For instance, in a study with over 400 lesions, ABUS showed a sensitivity and specificity of 92.16% and 87.05%, respectively, while HHUS demonstrated a sensitivity and specificity of 90.20% and 84.17%, respectively, with no significant difference from ABUS (16). Another study conducted in Beijing reported a sensitivity of 91.8% and 94.7% and a specificity of 92.9% and 89.4% for ABUS and HHUS, respectively, with similar diagnostic accuracy. The authors attributed these findings to ABUS’s ability to display more coronal plane-related information, such as mass margins, shape, spiculations, and tissue retraction distortion (17). A meta-analysis of 14 studies showed that ABUS has a sensitivity range of 0.72–1.0 and a specificity range of 0.52–0.98, while HHUS has a sensitivity range of 0.62–1.0 and a specificity range of 0.49–0.99 (10). In summary, ABUS has demonstrated consistent sensitivity and specificity in our study and previous research, indicating its utility for breast disease diagnosis and breast cancer screening.
Another advantage of ABUS in breast cancer screening its potential for teleconsultation compared to HHUS. ABUS images can be stored for diagnosis and consultation by different radiologists, addressing issues of result deviation due to variations in radiologists’ experience in breast screening. In our study, ABUS demonstrated higher sensitivity than HHUS in breast cancer screening, with no compromise in specificity, indicating superior diagnostic performance. Subgroup analysis revealed no significant differences in diagnostic indicators between the two ultrasound examinations across age and breast density subgroups, consistent with findings from Xin’s research (17). Xin’s study showed that ABUS sensitivity and specificity are comparable to HHUS across different age and breast density subgroups. In different breast volume subgroups, ABUS exhibited higher sensitivity in the C-D cup group. This could be attributed to manual ultrasound potentially missing areas in larger breasts, while ABUS, with its machine scanning, does not encounter such issues. ABUS is more advantageous in screening larger breasts compared to smaller ones. Although the AUC of mammograms for non-dense breasts is significantly better than that for dense breasts (18), the similar imaging principles of both types of ultrasound result in no significant difference in sensitivity and specificity between HHUS and ABUS across different breast density subgroups. Patients undergoing screening ultrasound typically have higher breast density, younger age, and potentially higher breast cancer risk compared to those with non-dense breasts (3). Mammographic breast density exhibits a significant negative correlation with age (19), and there is no significant difference in the utilization of the two types of ultrasound between premenopausal and postmenopausal women.
From a health economics perspective, ABUS can significantly reduce labor costs, provided there is no significant difference in diagnostic ability between the two types of ultrasound. ABUS requires lower experience and technical expertise from operators, thus reducing training costs for screening physicians. Moreover, with machine learning, complete ABUS imaging records can be diagnosed and analyzed with the assistance of artificial intelligence, further lowering labor costs (20).
This study has several limitations. Firstly, despite randomizing screened patients into ABUS and HHUS groups, there may still be some selection bias, although no significant differences in baseline data were observed between the two groups. Additionally, due to the less clinical use of ABUS compared to HHUS, some participants assigned to the ABUS group declined examination and follow-up, resulting in a slightly lower number of participants in the ABUS group. Secondly, mortality was not assessed in this study; instead, the detection rate was used as an alternative endpoint. Thirdly, the follow-up time in this study is relatively short, which precludes reporting on mortality rate. Repeated screening or extended follow-up could provide more informative data for breast cancer screening.
In summary, data from this multi-center study suggest that ABUS could be more sensitive and potentially superior to HHUS in breast cancer screening. ABUS is user-friendly, requires less training, and reduces the reliance on examiner experience compared to traditional ultrasound. The integration of automatic ultrasound with artificial intelligence holds promising prospects for future large-scale breast cancer screening efforts.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by The ethics committee of Peking Union Medical College Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsYX: Data curation, Formal Analysis, Writing – original draft, Conceptualization, Investigation. YLX: Data curation, Investigation, Methodology, Writing – original draft. SS: Formal Analysis, Methodology, Supervision, Writing – original draft. FM: Investigation, Project administration, Supervision, Validation, Writing – review & editing. YL: Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing. XZ: Formal Analysis, Methodology, Writing – review & editing. YNZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. QS: Conceptualization, Funding acquisition, Investigation, Writing – review & editing. YDZ:Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This article was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-C-066).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1421425/full#supplementary-material
AbbreviationsABUS, The automatic breast volume scanner ultrasound; AC, accuracy; AUC, Area under the curve; HHUS, hand-held ultrasound; MG, mammography; NPV, the negative predictive value; PPV, the positive predictive value; SE, Sensitivities; ROC, Receiver operator characteristic; SP, specificities; PPV, the positive predictive value; US, Ultrasound; BMI, body mass index; MHT, menopausal hormone therapy.
References1. Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). (2021) 41:1183–94. doi: 10.1002/cac2.v41.11
PubMed Abstract | Crossref Full Text | Google Scholar
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
PubMed Abstract | Crossref Full Text | Google Scholar
3. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the american cancer society. JAMA. (2015) 314:1599–614. doi: 10.1001/jama.2015.12783
PubMed Abstract | Crossref Full Text | Google Scholar
5. Rajaram N, Mariapun S, Eriksson M, Tapia J, Kwan PY, Ho WK, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat. (2017) 161:353–62. doi: 10.1007/s10549-016-4054-y
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zhang X, Chen J, Zhou Y, Mao F, Lin Y, Shen S, et al. Diagnostic value of an automated breast volume scanner compared with a hand-held ultrasound: a meta-analysis. Gland Surg. (2019) 8:698–711. doi: 10.21037/gs.2019.11.18
PubMed Abstract | Crossref Full Text | Google Scholar
7. Berg WA, Bandos AI, Mendelson EB, Lehrer D, Jong RA, Pisano ED. Ultrasound as the primary screening test for breast cancer: analysis from ACRIN 6666. J Natl Cancer Inst. (2016) 108(4):djv367. doi: 10.1093/jnci/djv367
PubMed Abstract | Crossref Full Text | Google Scholar
8. Omidiji OA, Campbell PC, Irurhe NK, Atalabi OM, Toyobo OO. Breast cancer screening in a resource poor country: Ultrasound versus mammography. Ghana Med J. (2017) 51:6–12. doi: 10.4314/gmj.v51i1.2
PubMed Abstract | Crossref Full Text | Google Scholar
9. Shen S, Zhou Y, Xu Y, Zhang B, Duan X, Huang R, et al. A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br J Cancer. (2015) 112:998–1004. doi: 10.1038/bjc.2015.33
PubMed Abstract | Crossref Full Text | Google Scholar
10. Ibraheem SA, Mahmud R, Mohamad Saini S, Abu Hassan H, Keiteb AS, Dirie AM. Evaluation of diagnostic performance of automatic breast volume scanner compared to handheld ultrasound on different breast lesions: A systematic review. Diagnostics (Basel). (2022) 12(4):541. doi: 10.3390/diagnostics12020541
PubMed Abstract | Crossref Full Text | Google Scholar
11. Xu YL, Sun Q, Shan GL, Zhang J, Liao HB, Li SY, et al. A case-control study on risk factors of breast cancer in China. Arch Med Sci. (2012) 8:303–9. doi: 10.5114/aoms.2012.28558
PubMed Abstract | Crossref Full Text | Google Scholar
12. Radiology. ACo.ACR BI-RADS Atlas. 5th ed. Reston: ACR (2013).
14. Mittra I, Mishra GA, Dikshit RP, Gupta S, Kulkarni VY, Shaikh HKA, et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. BMJ. (2021) 372:n738. doi: 10.1136/bmj.n256
PubMed Abstract | Crossref Full Text | Google Scholar
15. Choi EJ, Choi H, Park EH, Song JS, Youk JH. Evaluation of an automated breast volume scanner according to the fifth edition of BI-RADS for breast ultrasound compared with hand-held ultrasound. Eur J Radiol. (2018) 99:138–45. doi: 10.1016/j.ejrad.2018.01.002
PubMed Abstract | Crossref Full Text | Google Scholar
16. Liu J, Zhou Y, Wu J, Li P, Liang X, Duan H, et al. Diagnostic performance of combined use of automated breast volume scanning & hand-held ultrasound for breast lesions. Indian J Med Res. (2021) 154:347–54. doi: 10.4103/ijmr.IJMR_836_19
PubMed Abstract | Crossref Full Text | Google Scholar
17. Xin Y, Zhang X, Yang Y, Chen Y, Wang Y, Zhou X, et al. A multicenter, hospital-based and non-inferiority study for diagnostic efficacy of automated whole breast ultrasound for breast cancer in China. Sci Rep. (2021) 11:13902. doi: 10.1038/s41598-021-93350-1
PubMed Abstract | Crossref Full Text | Google Scholar
18. Omoleye OJ, Woodard AE, Howard FM, Zhao F, Yoshimatsu TF, Zheng Y, et al. External evaluation of a mammography-based deep learning model for predicting breast cancer in an ethnically diverse population. Radiol Artif Intell. (2023) 5:e220299. doi: 10.1148/ryai.220299
PubMed Abstract | Crossref Full Text | Google Scholar
19. Chandrasiri L, Hewavithana B, Jayasinghe A, Bimali W, Gunathilake P, Abeysundara S. A quantitative approach to assess the correlation of mammographic breast density with selected affecting factors. Ceylon Med J. (2022) 67:102–7. doi: 10.4038/cmj.v67i3.9697
PubMed Abstract | Crossref Full Text | Google Scholar
20. Tan T, Rodriguez-Ruiz A, Zhang T, Xu L, Beets-Tan RGH, Shen Y, et al. Multi-modal artificial intelligence for the combination of automated 3D breast ultrasound and mammograms in a population of women with predominantly dense breasts. Insights Imaging. (2023) 14:10. doi: 10.1186/s13244-022-01352-y
留言 (0)