Brain metastases (BM) are common among patients with advanced solid tumors. Estimates of the incidence of BM in the United States have varied, but approximately 200,000 new cases of BM are diagnosed in the United States every year and that 8% -10% of patients with cancer will develop BM (1). BM appear to be 10-fold more common than primary malignant brain tumors. In adults, lung, breast, skin (melanoma) and kidney are the most frequent sources of BM (2). Lung cancer, especially non-small cell lung cancer (NSCLC), is the most common primary cancer to develop BM. Approximately 50% of all patients with NSCLC metastasize to the brain (3, 4). The prognosis of patients with NSCLC BM is extremely poor. Even with the latest integrative treatment, the 5-year survival rate remains less than 5% (5).
Modern treatment for BM has dramatically changed their expected prognosis, and current available treatment options for NSCLC BM include surgery, whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), immunotherapy and targeted therapy. However, these treatments for NSCLC BM have certain limitations. For example, although WBRT can alleviate neurological symptoms and prevent new BM (6), its dose is limited due to potential serious toxicities (such as cognitive deterioration). Patients with NSCLC BM treated with WBRT alone generally have a poor prognosis with a median survival of less than 6 months (7). SRS delivers a high dose of conformal radiation with image guidance to minimize dose to surrounding normal brain tissue, and appears to promote anti-tumor immunity that could control tumor growth and symptoms without the neurocognitive side effects of WBRT (8). However, SRS is preferred for patients with one to three BM, and was shown to not improve the overall survival of BM (9). Chemotherapy is an active treatment for BM, however, the blood-brain barrier has limited the effective penetration of many systemic therapies into the brain. Novel systemic targeted therapies in patients with driver mutations have shown impressive intracranial efficacy, however, this treatment is largely for patients who have been pre-treated with local therapies and are asymptomatic (10). Craniotomy and tumor resection is considered for patients with a single metastasis measuring 3 cm or more, those with smaller tumors such as cerebellar neoplasms associated with severe neurologic symptoms due to cerebral edema, or those with multiple tumors with advanced neurologic symptoms in whom prompt improvement of neurologic symptoms is expected from surgery (11, 12). Lung adenocarcinoma (LUAD) is the most prevalent NSCLC cancer type that accounts for 85% of lung cancer, and the brain is the main organ prone to LUAD metastasis (13, 14). However, no retrospective data suggest that surgical resection may provide better intracranial progression-free survival in LUAD BM.
To clarify the impacts of surgery of LUAD BM, we retrospectively analyzed the clinical data, survival time and prognostic factors of the patients with BM originated from LUAD who had undergone surgical resection for the brain lesions and discussed future treatment strategies for this disease.
2 Patients and methods2.1 PatientsWe retrospectively reviewed a consecutive series of patients operated for BM from the lung, treated at Zhengzhou University People’s Hospital, Henan province, China, between March 2005 and October 2022. Our inclusion criteria for craniotomy were as follows: a surgically accessible tumor location and distinct negativity for cancer in other distant regions (except primary lung cancer). The inoperability criteria included the following: the overall condition of the patient was poor and the likelihood of craniotomy resulting in severe neural complications.
In this study, the inclusion criteria included: (1) aged 18 years and older; (2) underwent craniotomy for LUAD BM; (3) histological confirmation of cancer cell origin from lung; (4) lung cancer diagnosis with functional imaging including computed tomography (CT) and magnetic resonance imaging (MRI). The exclusion criteria included: (1) Only intracranial tumor biopsy was performed, with no intracranial tumor resection; (2) Pathological or imaging data were incomplete to prove that the intracranial tumor originated from the lung; (3) With other primary intracranial tumors.
Two hundred eight patients were eventually included in this study (Figure 1).
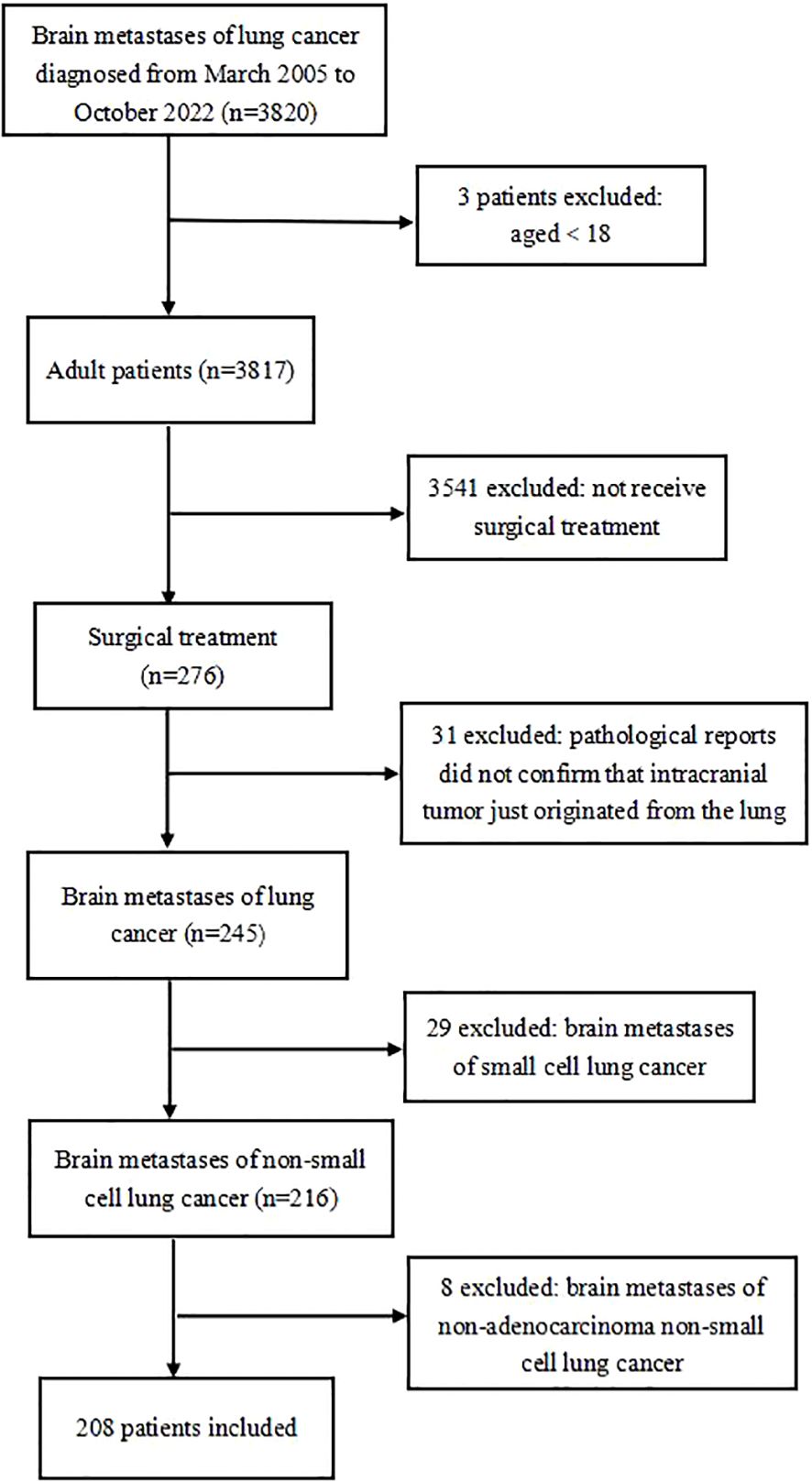
Figure 1. Flow chart of exclusion criteria and study design.
The clinical data on these patients were collected retrospectively from their medical records, including operative and pathological reports, clinical data and information from the office files of their neurosurgeons and thoracic surgeons. Detailed follow-up study of these individuals was conducted by letter and telephone contact with patients, family members, friends, or personal physicians.
This study was conducted by following the Declaration of Helsinki and was approved by the Ethics Committee of Zhengzhou University People’s Hospital, Henan province, China. All patients provided written informed consent before the surgery, and medical records of patients were anonymized.
2.2 Driver mutation testingGenetic mutations were tested through polymerase chain reaction (PCR) or Next-generation sequencing (NGS) using clinical tumor samples. Detections were applied in either plasma DNA or in paraffin-embedded tumor tissue. In the vast majority of patients (75%) in this study, Amplification Refractory Mutation System-qPCR method from AmoyDx, China, was used to detect hotspot mutations in accordance with the manufacturer’s instructions. The panel of PCR included: EGFR, ALK, ROS1, RET, KRAS, NRAS, BARF, HER2, PIK3CA, and MET. In other patients, the Illumia-Nextseq 550 system (Burning Rock, Guangzhou, China) was used to detect driver mutations. It covers 168 cancer genes or 139 cancer genes and can detect structural and copy number changes in addition to hotpot and other coding/splice mutations.
2.3 Statistical analysisAll analyses were performed by SPSS 26.0 (IBM Corp., Armonk, NY, USA) software. Descriptive statistics were expressed as numbers and percentages. The end date for the survival analysis for patients lost to follow-up was the time they were last seen in the department or last contacted by telephone. Overall survival was defined as the percentage of patients who were alive at the end of the study period. Survival curves were analyzed using the Kaplan–Meier method with the log-rank test. The univariable and multivariable Cox regression models were conducted to determine the prognostic factors for patients with LUAD BM. Factors with a P-value less than 0.05 in the univariable regression analysis were incorporated into the multivariable regression model. All hypothesis tests were conducted against a 2-sided alternative. P values were considered statistically significant when less than 0.05.
3 Results3.1 Patients characteristicsA total of 208 patients who underwent craniotomy for LUAD BM were identified from 2005 to 2022. The clinical information of this cohort is shown in Table 1. Among the patients, 110 patients (52.9%) were males and 98 females (47.1%), and their median age was 61.4 years (23-81years). The majority of patients were farmers (153, 73.6%). In addition, smoking history, alcohol drinking history and the family history of tumor accounted for 40.4% (84 cases), 42.8% (89 cases) and 14.9% (31 cases), respectively. Before craniotomy, only 5 patients (2.4%) had received lung cancer-related treatment at an early stage, of which 2 patients (1.0%) had undergone radical resection of lung cancer, 2 patients (1.0%) had undergone radical resection of lung cancer combined with targeted drug therapy, and 1 patient (0.4%) had only received targeted drug therapy. With respect to symptoms, 203 cases (97.6%) had neurological symptoms, including headache, dizziness, nausea, vomiting, vision loss, slurred speech, limb weakness and seizure. 5 patients (2.4%) had no neurological symptoms, in which PET-CT and physical examination found BM in 3 and 2 patients, respectively.
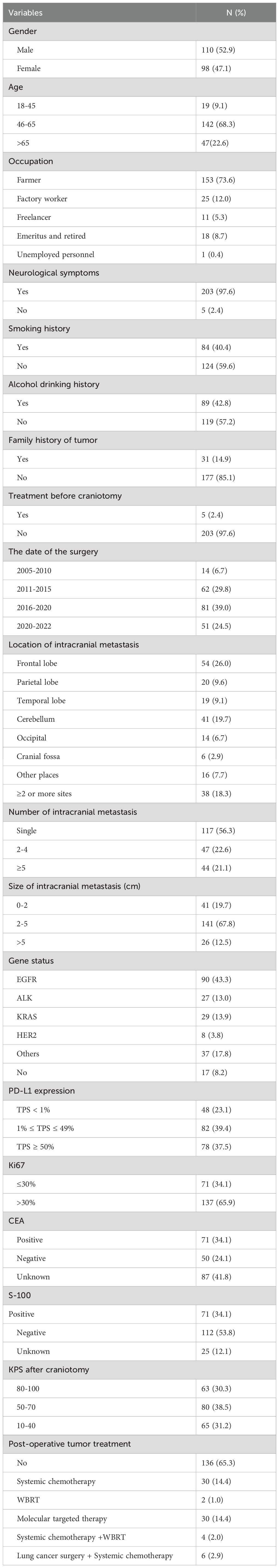
Table 1. Characteristics of our patients who underwent surgical resection for BM of LUAD.
Surgery was performed in 14 patients (6.7%) from 2005 to 2010, in 62 patients (29.8%) from 2011 to 2015, in 81 patients (39.0%) from 2016 to 2020, and in 51 patients (24.5%) between 2020 and 2022.
3.2 Characteristics of intracranial metastasisIntracranial metastases were located in the frontal lobe (54, 26.0%), followed by the cerebellum (41, 19.7%), parietal lobe (20, 9.6%), temporal lobe (19, 9.1%), occipital part (14, 6.7%), cranial fossa (6, 2.9%), thalamus (3, 1.4%) and corpus callosum (3, 1.4%), ramus (3, 1.4%), quadrilateral ventricles (2, 1.0%), pineal gland (2, 1.0%), sagittal sinus (2, 1.0%), cingulate gyrus (1, 0.4%). In addition, 38 patients (18.3%) had intracranial metastases in 2 or more sites.
With respect to the number of intracranial metastases, 117 patients (56.3%) had a solitary BM and 91 (43.7%) had multiple metastatic lesions. The tumors were divided into 3 grades according to their maximum diameters. 41 cases (19.7%) had a maximum diameter of 0 to 2 cm, 141 cases (67.8%) had a maximum diameter of 2 to 5 cm, and 26 cases (12.5%) had a maximum diameter of more than 5 cm.
Nearly half of the patients (90, 43.3%) had EGFR mutations; 29 cases (13.9%) had KRAS mutations, 27 cases (13.0%) ALK rearrangement, 8 cases (3.8%) Her2 mutations, and 37 cases (17.8%) other mutation. In addition, 17 cases (8.2%) had non-actionable mutations.
One of the commonly used methods for assessing PD-L1 expression is the Tumor Proportion Score (TPS), which considers the percentage of tumor cells expressing PD-L1. PD-L1 status was categorized into positive (TPS ≥1%) and negative (TPS <1%) expression. Immunohistochemical results of BM showed that PD-L1 was positive in 160 patients (76.9%) and negative in 48 patients (23.1%). There were 137 patients (65.9%) with Ki67 > 30%, and 71 patients (34.1%) with Ki67 ≤ 30%. S-100 was positive in 71 patients (34.1%) and negative in 112 patients (53.8%), and the result was missing in 25 patients (12.1%).
3.3 Karnofsky performance score after craniotomyPatients enrolled had Karnofsky Performance Score (KPS) assessed one week post-discharge. KPS was categorized into low (10-40), intermediate (50-70) and high (80-100). The mean ± standard deviation of KPS in the entire cohort was 58 ± 24. Low, intermediate and high performance status were seen in 65 cases (31.2%), 80 cases (38.5%), and 63 cases (30.3%) of the cohort, respectively.
3.4 Treatment characteristicsAll 208 (100%) patients underwent surgery for LUAD BM but only 72 (34.7%) continued additional tumor treatment after craniotomy. In these cases, 30 patients (14.4%) underwent postoperative chemotherapy (pemetrexed and cisplatin based), 2 patients (1.0%) underwent WBRT, 30 patients (14.4%) underwent molecular targeted therapy (28 patients receiving EGFR tyrosine kinase inhibitors (TKIs) and 2 patients ALK TKIs), 4 patients (2.0%) received chemotherapy combined with WBRT, 6 patients (2.9%) underwent lung cancer resection combined with chemotherapy. No patients received post-operative immunotherapy.
3.5 Survival outcomesA total of 190 patients (90.7%) were followed up successfully. Among these patients, survival time ranged from 0 to 55 months, with the median survival time being 11.5 months. The 3-month, 6-month, 1-year, 3-year and 4-year survival rates were 88.77%, 72.09%, 48.78%, 15.17% and 12.64%, respectively (Figure 2A). In the univariate Cox analysis, smoking history, Ki67 percentage, KPS after craniotomy, and molecular targeted therapy after craniotomy were significantly associated with overall survival (OS) (P<0.05) (Table 2). Figures 2B-E shows the Kaplan–Meier survival plots generated from curves stratified according to the smoking history, Ki67 percentage, KPS after craniotomy, and molecular targeted therapy after craniotomy. Gender, age, neurological symptoms, alcohol drinking history, history of lung cancer treatment, the number of intracranial metastases, size of metastases, PD-L1 expression, EGFR mutation, and systemic chemotherapy after craniotomy did not significantly affect the survival. Multivariate analysis revealed that smoking history (HR=0.677, P=0.02), Ki67 percentage (HR=0.594, P=0.004), KPS after craniotomy (HR=0.279, P=0.000) and molecular targeted therapy after craniotomy (HR=3.589, P=0.000) were independent factors affecting the survival time of patients (Table 2).
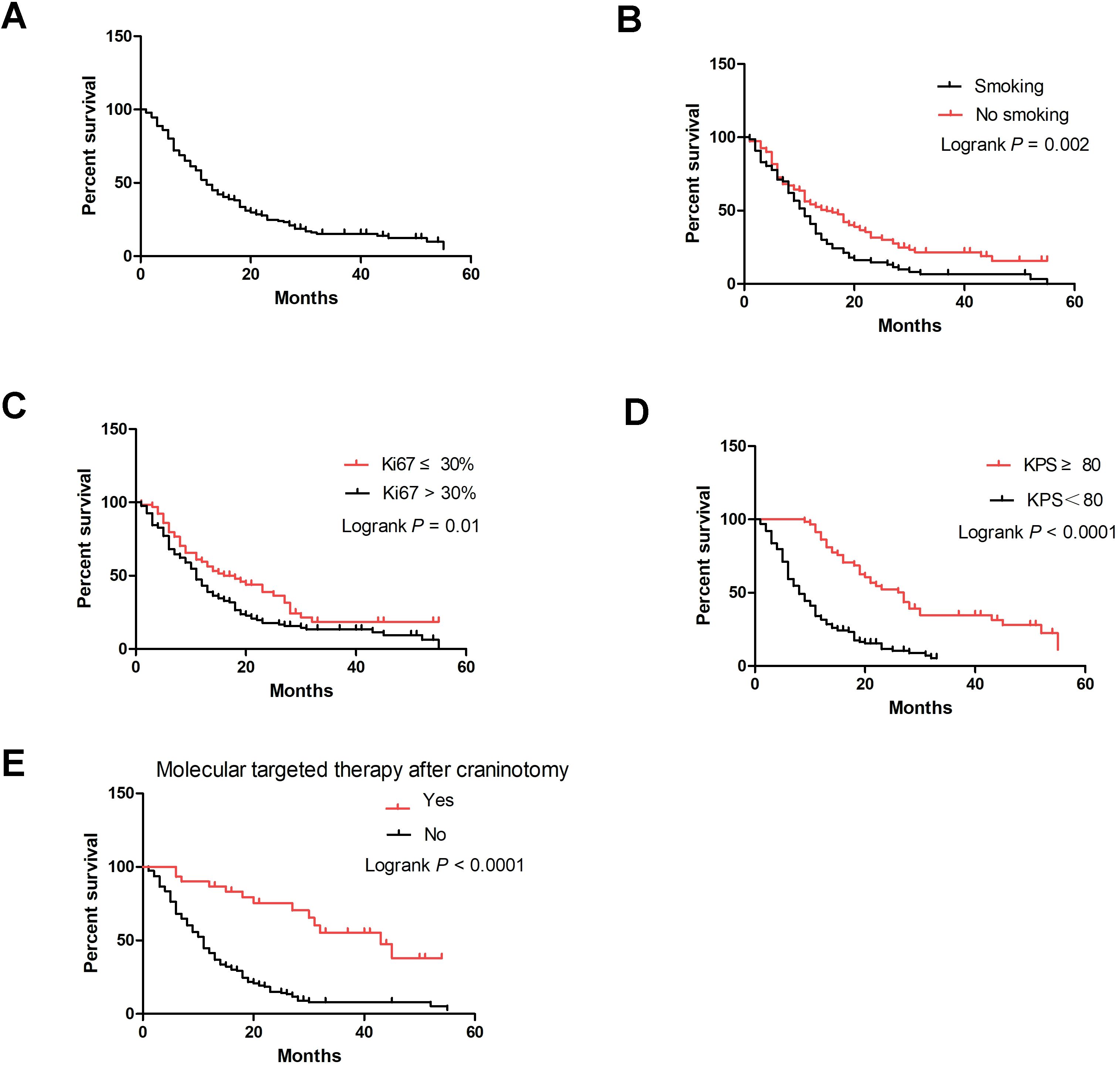
Figure 2. Kaplan–Meier curve of LUAD brain metastases patients undergoing surgical resection. (A) Overall survival curve of 190 patients; (B) Smoking-specific survival curves of patients; (C) Ki67 percentage–specific survival curves; (D) KPS-specific survival curves; (E) Molecular targeted therapy after craniotomy-specific survival curves.
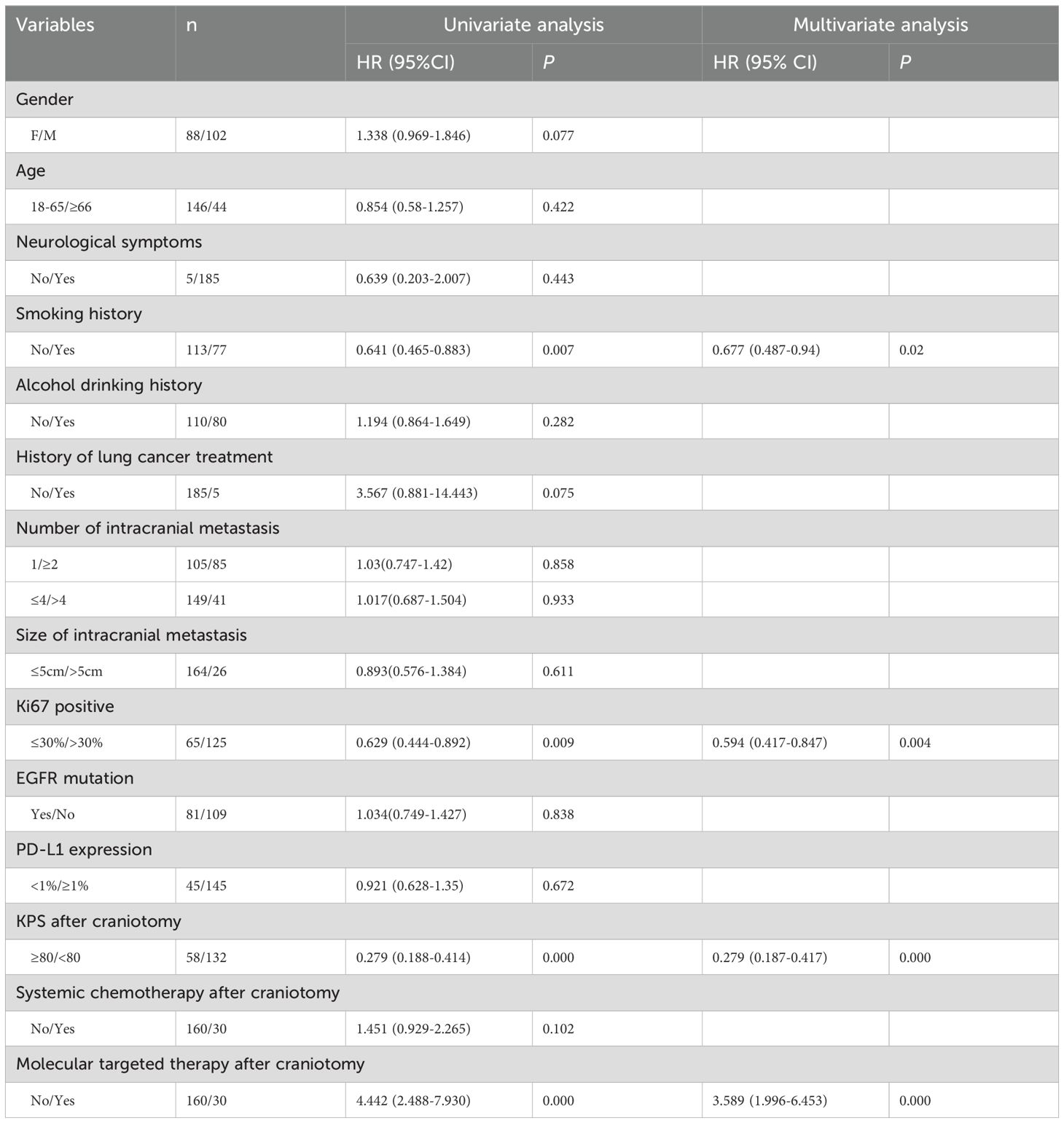
Table 2. Univariate and multivariate Cox proportional hazard models for overall survival in 190 cases who had surgical resection for BM of LUAD.
4 DiscussionLung cancer ranks first in terms of morbidity and mortality among all tumors worldwide, and the brain is a common metastasis site of lung cancer (15, 16). The incidence of BM in patients with lung cancer is approximately 20%-65%, which seriously affects the prognosis and quality of life (QOL) of patients (3, 4). Despite treatment, the majority of patients still die due to progressive BM. Although prognosis in patients with BM from lung cancer is usually poor, prolonged survival can be observed in a subgroup of patients with favorable prognostic factors (8, 9). In these favorable subgroups, more aggressive treatment modalities can be used to increase QOL and influence survival. BM are a complex condition with multiple factors contributing to diagnosis and prognosis. The blood-brain barrier has limited the effective penetration of many systemic therapies into the brain. Surgical option is feasible as an emergency strategy and also suggested as an elective procedure to improve neurological status, QOL, and survival.
In this study, the clinical data of 208 patients with LUAD BM undergoing surgical resection were retrospectively collected and analyzed. Patients age ranged from 23 to 81 years old, among which 46-65 years old (142 cases, 68.3%) accounted for a large proportion of patients. COX regression model found that age was not a factor affecting the prognosis of patients. Similarly, in Shen’s study, they analyzed the Surveillance, Epidemiology, and End Result (SEER) database and identified that age was not an independent prognostic factor of lung cancer BM (17). Other studies have reported that the incidence of LUAD BM increases with age, and this phenomenon may be related to living standards and physical conditions (18, 19). However, since the population included in this study was LUAD BM who underwent craniotomy, some patients who were old and frail and whose overall physical condition was not suitable for surgery were excluded. Evaluation of age on the prognosis of LUAD BM who underwent craniotomy requires a larger sample size. In addition, people aged 46-65 years old are the major society contributors, and attention should be paid to the characteristics of people in this stage to achieve early detection and early treatment.
Cigarette use is the major cause of morbidity and mortality in developed countries, increasing all cause mortality (20, 21). Smoking intervention could reduce the frequency of postoperative complications and improve the prognosis of lung cancer patients (22–24). However, there is limited data on the effects of smoking on LUAD BM. In our study, we found that smoking is an important factor affecting the prognosis of patients with LUAD BM; the survival time of smokers was significantly shorter than that of non-smokers. We speculate that this reason may be that tobacco smoke contains hundreds of known and probable human carcinogens (25), which accelerate the metastasis of cancer cells and aggravate the nervous system damage in LUAD BM patients. Shenker has reported that in patients with BM from lung cancer who received SRS, current smoking status and pack-year history of smoking had no effect on overall survival, however, current smokers with non-adenocarcinoma lung cancers had a trend toward greater neurologic death than nonsmokers and cumulative pack years smoking is associated with a greater BM velocity (26). These results indicated the dangers of smoking and the need for smoking cessation in the general population as well as in patients with BM from LUAD.
Ki67 is a well-known marker for the evaluation of cell proliferation. Numerous studies have indicated that Ki67 index independently predicts cancer progression. Immunostaining for Ki67 expression is the gold standard, and the higher positivity of Ki67 expression is, the stronger invasion ability of tumor cells (27). Berghoff et al. (28) found a correlation between Ki67 and survival time; high Ki67 expression showed a shorter survival time. In line with these findings, in our study, the prognosis of LUAD patients with BM with high expression of Ki67 was poor, and Ki67 was an independent factor affecting the prognosis of patients.
KPS has been used broadly in clinical practice to assess the overall performance status of cancer patients’ activity, work, and self-care abilities (29). A multicenter, retrospective cohort study showed that neurosurgical resection improved OS and was associated with a significantly better prognosis in patients with lung cancer BM and poor KPS (30). We assessed LUAD BM KPS one week post-discharge and found that patients with higher KPS had a better prognosis, which indicated that patients with preserved functional capacity benefit from craniotomy over those with lower KPS.
Our study cohort is unique since the overwhelming majority (97.6%) of the patients (203 patients) were admitted to the neurosurgery department due to neurological symptoms, without prior diagnosis of lung cancer. Three (1.4%) of the other 5 patients (2.4%) were diagnosed with lung cancer after relevant examination, which suggests the occult occurrence of lung cancer and the importance of early screening. Among the 208 patients, 5 patients (2.4%) had received lung cancer-related treatment at an early stage, including 2 patients (0.9%) with radical resection of lung cancer, 2 (0.9%) with radical resection of lung cancer combined with targeted drug therapy, and 1 (0.45%) with molecular targeted therapy. The Univariate COX model found that whether the patients underwent lung cancer-related treatment before BM surgery was not a factor affecting the postoperative survival time of patients with LUAD BM. The reason may be related to the small number of patients who underwent lung cancer-related treatment before BM surgery (just 5 cases), and the sample size will be expanded in future analysis.
The incidence of brain metastasis has increased in parallel with LUAD incidence, accuracy of imaging examination and people’s attention to physical examination. The role of surgery in the management of LUAD BM is indisputable. Surgery can quickly alleviate life-threatening symptoms caused by tumor effects, cerebrospinal fluid obstruction, and edema around the tumor. Surgical resection of BM is typically indicated for solitary intracranial metastasis (31, 32). Surgical treatment was not considered for patients with multiple metastases (more than 3 locations) that were considered to have uncontrolled primary tumors and poor prognosis (33). However, rapid advances in systemic therapy are improving outcomes for this patient population across multiple tumor types, generating new interest in local control strategies (34). Sauvageot et al. published a retrospective cohort study of 184 patients with 72 patients (39.1%) having more than 2 BM. The median OS was 19.2 months and the median cerebral progression-free survival was 8.4 months. The number of BM and tumor cerebral burden remained significant prognostic factors for OS (35). Pollock et al. published a retrospective cohort study of 52 patients with a median of three BM. The result showed that 5 patients who received resection alone survived a median of 19 months; 31 patients who received radiosurgery alone survived a median of 13 months and 16 patients who received both resection and radiosurgery survived a median of 8 months (36). Based on these results, resection was recommended for patients with multiple BM. In our study, 72.1% of the patients survived for more than 6 months, and 15.2% survived for more than 3 years. This survival outcome was better than the survival time expected for LUAD BM, suggesting that resection should be considered as an option, even in the case of patients with multiple BM, in order to improve their survival and QOL.
In addition, of the 190 patients (91.3%) who were successfully followed up, only 72 patients (34.7%) received post-operative treatment. The use of biomarker-matched target therapies has been considered solely responsible for improving population-level lung cancer-specific mortality between 2013 and 2016 (37). In 2 published retrospective studies of NSCLC patients with BM and EGFR mutations, patients who received an EGFR TKI at any time after diagnosis of BM survived longer than those who did not (38, 39). Both the Gow et al. and Eichler et al. series suggested that EGFR TKI therapy after the initial diagnosis of BM may provide such a substantial benefit in duration of central nervous system response that survival is ultimately impacted. Similarly, in our study, patients who received molecular targeted therapy after craniotomy showed improved overall survival compared with patients who did not, and multivariate analysis revealed molecular targeted therapy after craniotomy as an independent factor affecting the survival time of patients. Taken together, these data suggest that LUAD patients with BM could benefit from molecular targeted therapy after BM resection.
The reasons for most patients not receiving post-operative treatment were as follows: first, some patients could not tolerate the systemic treatment because of the treatment side effects; second poor KPS after surgery; and third economic considerations. In addition, when the patients knew that the pathological diagnosis was malignant tumor, most of them chose to give up the postoperative treatment.
Our study has some limitations. First, it is a retrospective study at a single center and the sample size is limited, which may have led to a bias in patient selection and limited firm conclusions. Therefore, our results may not apply to other populations and other centers. Further multicenter prospective studies are thus recommended. Second, the clinical data is limited; we were unable to collect hematology examination and cerebrospinal fluid examination, which may be important factors affecting the survival time of patients. Third, the study was focused on LUAD BM who underwent craniotomy at our center, and most patients did not receive treatment post-operatively. No patients received SRS which is a recommendation of NCCN for low tumor volume, both to resection cavity and any other non-resected BM. Our data did not fully represent the overall status of all lung cancer patients with BM. Despite these limitations, to the best of our knowledge, it is the largest published cohort that clarifies the clinical characteristics and prognosis factors of LUAD BM treated with surgical resection, which may be relevant to similar patient cohorts. In the follow-up study, we will carry out a multi-center study, further expand the sample size, and improve the clinical data by incorporating tumor genetics, hematology and cerebrospinal fluid to determine the factors affecting the survival of patients undergoing craniotomy. Meanwhile, we will collect all the data of patients with lung cancer BM as much as possible to present the comprehensive status of the patients in an effort to improve the prognosis of patients.
5 ConclusionIn summary, our study demonstrates the clinical characteristics and prognostic factors of patients undergoing craniotomy for LUAD BM. The intracranial location of BM was mostly in the frontal lobe and the metastatic lesions were mostly single; the metastatic tumor size was mostly between 2-5 cm. Moreover, nearly half of the patients had EGFR mutations and most had Ki67 > 30% and positive PD-L1. Few patients received lung tumor treatment before craniotomy, and only a minor subset continued tumor therapy after craniotomy. Although the 3-year survival was poor, surgical resection for patients who had no-smoking history, Ki67 percentage ≤30%, KPS≥80 after craniotomy, and molecular targeted therapy after craniotomy can improve the prognosis and prolong the survival time.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Ethics Committee of Zhengzhou University People’s Hospital, Henan province, China (Approval No.H2023-07-025). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsML: Data curation, Writing – original draft. ZL: Data curation, Methodology, Writing – original draft. HZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. HW: Formal analysis, Investigation, Writing – review & editing. LS: Investigation, Methodology, Writing – original draft. TW: Conceptualization, Resources, Writing – original draft. SZ: Funding acquisition, Visualization, Writing – review & editing. LZ: Funding acquisition, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. (2011) 8:344–56. doi: 10.1038/nrclinonc.2011.58
PubMed Abstract | Crossref Full Text | Google Scholar
3. Zhang Q, Abdo R, Iosef C, Kaneko T, Cecchini M, Han VK, et al. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat Commun. (2022) 13:5983. doi: 10.1038/s41467-022-33365-y
PubMed Abstract | Crossref Full Text | Google Scholar
5. You M, Fu M, Shen Z, Feng Y, Zhang L, Zhu X, et al. HIF2A mediates lineage transition to aggressive phenotype of cancer-associated fibroblasts in lung cancer brain metastasis. Oncoimmunology. (2024) 13:2356942. doi: 10.1080/2162402X.2024.2356942
PubMed Abstract | Crossref Full Text | Google Scholar
7. Zhou K, Cai X, Wang X, Lan X, Zhang X. Efficacy and safety of WBRT+EGFR-TKI versus WBRT only in the treatment of NSCLC patients with brain metastasis: An updated meta-analysis. Thorac Cancer. (2022) 13:563–70. doi: 10.1111/1759-7714.14299
PubMed Abstract | Crossref Full Text | Google Scholar
8. Berger A, Mullen R, Bernstein K, Alzate JD, Silverman JS, Sulman EP, et al. Extended survival in patients with non-small-cell lung cancer-associated brain metastases in the modern era. Neurosurgery. (2023) 93:50–9. doi: 10.1227/neu.0000000000002372
PubMed Abstract | Crossref Full Text | Google Scholar
9. Lehrer EJ, McGee HM, Peterson JL, Vallow L, Ruiz-Garcia H, Zaorsky NG, et al. Stereotactic radiosurgery and immune checkpoint inhibitors in the management of brain metastases. Int J Mol Sci. (2018) 19:3054. doi: 10.3390/ijms19103054
PubMed Abstract | Crossref Full Text | Google Scholar
10. Page S, Milner-Watts C, Perna M, Janzic U, Vidal N, Kaudeer N, et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer. (2020) 132:187–98. doi: 10.1016/j.ejca.2020.03.006
PubMed Abstract | Crossref Full Text | Google Scholar
11. Perng PS, Hsu HP, Lee PH, Huang CC, Lin CC, Lee JS. Correlation of EGFR mutation subtypes and survival in surgically treated brain metastasis from non-small-cell lung cancer. Asian J Surg. (2023) 46:269–76. doi: 10.1016/j.asjsur.2022.03.076
PubMed Abstract | Crossref Full Text | Google Scholar
12. Wang B, Guo H, Xu H, Yu H, Chen Y, Zhao G. Research progress and challenges in the treatment of central nervous system metastasis of non-small cell lung cancer. Cells. (2021) 10:2620. doi: 10.3390/cells10102620
PubMed Abstract | Crossref Full Text | Google Scholar
13. Chen M, Li H, Xu X, Bao X, Xue L, Ai X, et al. Identification of RAC1 in promoting brain metastasis of lung adenocarcinoma using single-cell transcriptome sequencing. Cell Death Dis. (2023) 14:330. doi: 10.1038/s41419-023-05823-y
PubMed Abstract | Crossref Full Text | Google Scholar
14. Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic Malignancy: a population-based study. Neuro Oncol. (2017) 19:1511–21. doi: 10.1093/neuonc/nox077
PubMed Abstract | Crossref Full Text | Google Scholar
17. Shen H, Deng G, Chen Q, Qian J. The incidence, risk factors and predictive nomograms for early death of lung cancer with synchronous brain metastasis: a retrospective study in the SEER database. BMC Cancer. (2021) 21:825. doi: 10.1186/s12885-021-08490-4
PubMed Abstract | Crossref Full Text | Google Scholar
18. Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer. (2018) 19:e373–9. doi: 10.1016/j.cllc.2018.01.007
PubMed Abstract | Crossref Full Text | Google Scholar
19. Shi W, Wang Y, Xia W, Liu B, Ni M, Shen J, et al. Brain metastases from small cell lung cancer and non-small cell lung cancer: comparison of spatial distribution and identification of metastatic risk regions. J Neurooncol. (2023) 161:97–105. doi: 10.1007/s11060-022-04211-4
PubMed Abstract | Crossref Full Text | Google Scholar
22. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. (2002) 359:114–7. doi: 10.1016/S0140-6736(02)07369-5
PubMed Abstract | Crossref Full Text | Google Scholar
23. Videtic GM, Stitt LW, Dar AR, Kocha WI, Tomiak AT, Truong PT, et al. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol. (2003) 21:1544–9. doi: 10.1200/JCO.2003.10.089
PubMed Abstract | Crossref Full Text | Google Scholar
24. Baser S, Shannon VR, Eapen GA, Jimenez CA, Onn A, Lin E, et al. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest. (2006) 130:1784–90. doi: 10.1378/chest.130.6.1784
PubMed Abstract | Crossref Full Text | Google Scholar
26. Shenker RF, McTyre ER, Ruiz J, Weaver KE, Cramer C, Alphonse-Sullivan NK, et al. The Effects of smoking status and smoking history on patients with brain metastases from lung cancer. Cancer Med. (2017) 6:944–52. doi: 10.1002/cam4.1058
PubMed Abstract | Crossref Full Text | Google Scholar
28. Berghoff AS, Ilhan-Mutlu A, Wöhrer A, Hackl M, Widhalm G, Hainfellner JA, et al. Prognostic significance of Ki67 proliferation index, HIF1 alpha index and microvascular density in patients with non-small cell lung cancer brain metastases. Strahlenther Onkol. (2014) 190:676–85. doi: 10.1007/s00066-014-0639-8
PubMed Abstract | Crossref Full Text | Google Scholar
29. Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. (1984) 53:2002–7. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w
PubMed Abstract | Crossref Full Text | Google Scholar
30. Liang L, Wen L, Qin S, He Z, Lu J, Cui R, et al. Poor Karnofsky performance status is not a contraindication for neurosurgical resection in patients with lung cancer brain metastases: a multicenter, retrospective PSM-IPTW cohort study. J Neurooncol. (2023) 162:327–35. doi: 10.1007/s11060-023-04293-8
PubMed Abstract | Crossref Full Text | Google Scholar
31. Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. (2014) 11:203–22. doi: 10.1038/nrclinonc.2014.25
PubMed Abstract | Crossref Full Text | Google Scholar
32. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. (1990) 322:494–500. doi: 10.1056/NEJM199002223220802
PubMed Abstract | Crossref Full Text | Google Scholar
33. Swift PS, Phillips T, Martz K, Wara W, Mohiuddin M, Chang CH, et al. CT characteristics of patients with brain metastases treated in RTOG study 79-16. Int J Radiat Oncol Biol Phys. (1993) 25:209–14. doi: 10.1016/0360-3016(93)90341-r
PubMed Abstract | Crossref Full Text | Google Scholar
34. Sankey EW, Tsvankin V, Grabowski MM, Nayar G, Batich KA, Risman A, et al. Operative and peri-operative considerations in the management of brain metastasis. Cancer Med. (2019) 8:6809–31. doi: 10.1002/cam4.2577
PubMed Abstract | Crossref Full Text | Google Scholar
35. Sauvageot S, Mollevi C, Thomas QD, Charissoux M, Darlix A, Rigau V, et al. Prognostic impact of the number and total tumor burden of secondary cerebral lesions in patients with resected brain metastases of non-small cell lung cancers. J Neurosurg. (2024) 141:89–99. doi: 10.3171/2023.11.JNS231923
PubMed Abstract | Crossref Full Text | Google Scholar
36. Pollock BE, Brown PD, Foote RL, Stafford SL, Schomberg PJ. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J Neurooncol. (2003) 61:73–80. doi: 10.1023/a:1021262218151
PubMed Abstract | Crossref Full Text | Google Scholar
37. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. (2020) 383:640–9. doi: 10.1056/NEJMoa1916623
PubMed Abstract | Crossref Full Text | Google Scholar
38. Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. (2010) 12:1193–9. doi: 10.1093/neuonc/noq076
PubMed Abstract | Crossref Full Text | Google Scholar
39. Gow CH, Chien CR, Chang YL, Chiu YH, Kuo SH, Shih JY, et al. Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res. (2008) 14:162–8. doi: 10.1158/1078-0432.CCR-07-1468
留言 (0)