Lung cancer is the most common cancer worldwide and the leading cause of cancer-related death (1). Surgery is the treatment of choice for early non-small-cell lung cancer (NSCLC) (2, 3). The evaluation of lymph node metastasis (LNM) status prior to surgery is important because it determines subsequent treatment strategies (4, 5).
Lobectomy has been considered the standard surgical treatment for early NSCLC since its landmark trial (3). One condition for this surgical modality is that patients do not have mediastinal LNM (2). However, with ongoing research progress, the extent of surgical resection has become more refined, while LNM status requirements have become more stringent. In 2022, the Japan Clinical Oncology Group (JCOG) 0802 study reported that segmentectomy could be considered as a standard surgical procedure for peripheral NSCLC patients with a tumor diameter ≤ 2.0 cm and a consolidation-to-tumor ratio (CTR) > 0.5 (6). One of the conditions for this approach is that patients do not have LNM.
However, current examination methods in clinical practice for preoperative LNM status still have shortcomings, such as poor diagnostic performance (7–14), anatomical location-dependent issues, high associated risks, and expense (8, 15–17). Moreover, few predictive models have been developed for the specific patient population in the JCOG0802 study.
Therefore, we developed and validated a clinically useful predictive model to assess LNM probability in peripheral NSCLC patients with a tumor diameter ≤ 2.0 cm and CTR > 0.5, which could help clinicians develop clinical decisions and formulate individualized surgical protocols.
2 Materials and methodsWe recruited a retrospective cohort of consecutive 435 patients with NSCLC who underwent surgical treatment between January 1, 2018 and October 31, 2022. The study was approved by the Institutional Review Board of our hospital.
2.1 Study populationThe patient recruitment process is outlined (Figure 1). We recruited 268 cases from Yuncheng Central Hospital as the development cohort, and 167 cases from Peking University Cancer Hospital as the validation cohort.
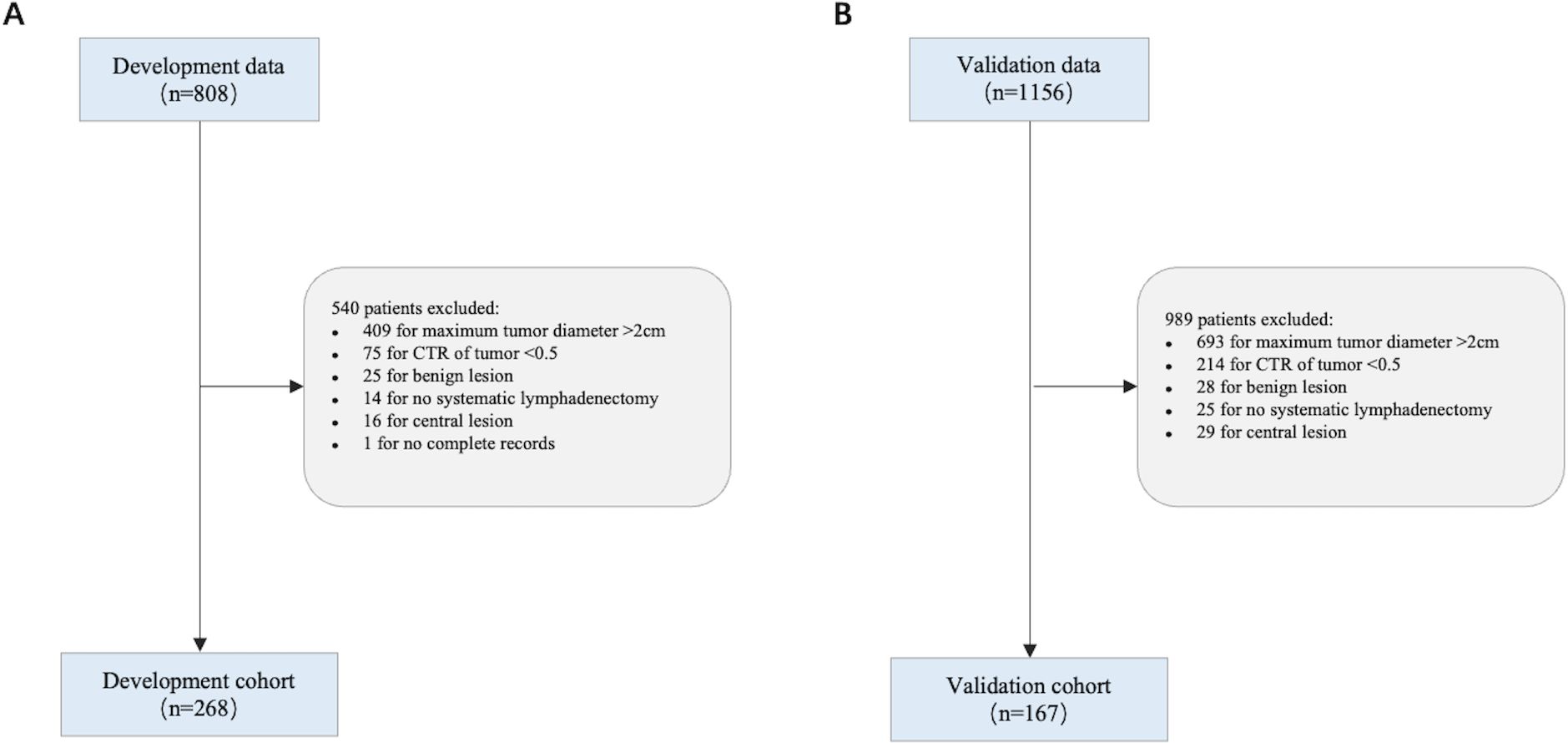
Figure 1. Flowchart showing the study population. (A) Development cohort; (B) Validation cohort.
2.1.1 Inclusion criteria included(I) patients who underwent a lobectomy or segmentectomy with systemic lymph node dissection or sampling; (II) a postoperative pathological diagnosis of primary NSCLC; (III) patients who underwent an enhanced thin layer thoracic computed tomography (CT) scan within 60 days of the operation; (IV) CT fulfilled the following criteria: single tumor, tumor center located in the outer one-third of the lung parenchyma, tumor diameter ≤ 2.0 cm, and CTR > 0.5; and (V) an age range of 20–85 years old.
2.1.2 Exclusion criteria included(I) patients having received radiotherapy, chemotherapy or a combination of both before surgery; (II) patients with simultaneous or metachronous (within the past 5 years) double cancers; (III) patients with active bacterial or fungal infections; and (IV) patients with severe pulmonary fibrosis or emphysema.
2.2 OutcomesThe main outcome measure was LNM-positivity as determined by pathological examination - pathological lymph node involvement (N1 or N2 lymph nodes), and vice versa for LNM-negative.
2.3 Predictive factorsWe selected the following predictive factors that potentially influenced LNM: (I) gender (male/female); (II) age (continuous variable); (III) body mass index (BMI) (continuous variable measured in kg/m2); (IV) smoking history (yes/no); (V) tumor size measured by CT (≤ 1.5 cm/1.5 cm); (VI) tumor location in bronchi (fourth-order/fifth-order or higher bronchi); (VII) tumor location in the lobe (right upper lobe/right middle lobe/right lower lobe/left upper lobe/left lower lobe); (VIII) CTR (continuous variable); (IX) pleural indentation (presence/absence); and (X) carcinoembryonic antigen (CEA) value (≤ 5.0 ng/ml/> 5.0 ng/ml).
2.4 Surgical procedureAll surgery was performed by thoracic surgeons at respective centers. For patients with poor lung function reserve or comorbidities, and were considered to have poor surgical tolerance, segmentectomy was performed. For all other patients, lobectomy was performed. The operational criteria for systematic lymph node dissection conformed to the International Association for the Study of Lung Cancer definition of adequate lymph node dissection (18).
2.5 Imaging evaluationsCT images were evaluated by two independent physicians (15 and 20 years of experience, respectively) who were blinded to all patient clinical information. Tumor size, its solid component, and the presence/absence of pleural indentations, were assessed in lung window setting (window width, 1200 HU; window position, -600 HU) (19). Tumor size was defined as the largest tumor diameter, including the solid component and surrounding ground glass component on axial images. Solid component size was defined as the largest diameter of the solid tumor component on axial images. The solid component was defined as an opaque area with locally increased density such that it completely obscured underlying vascular and bronchiolar markings. The ground glass component was defined as an area with locally increased density that did not completely obscure underlying vascular structures. CTR was calculated by dividing the solid component size by the tumor size (19). Pleural indentation was defined as a visually sharp, linear indentation or cord-like linear shadow on lung tumors by CT (20, 21) (Figure 2).
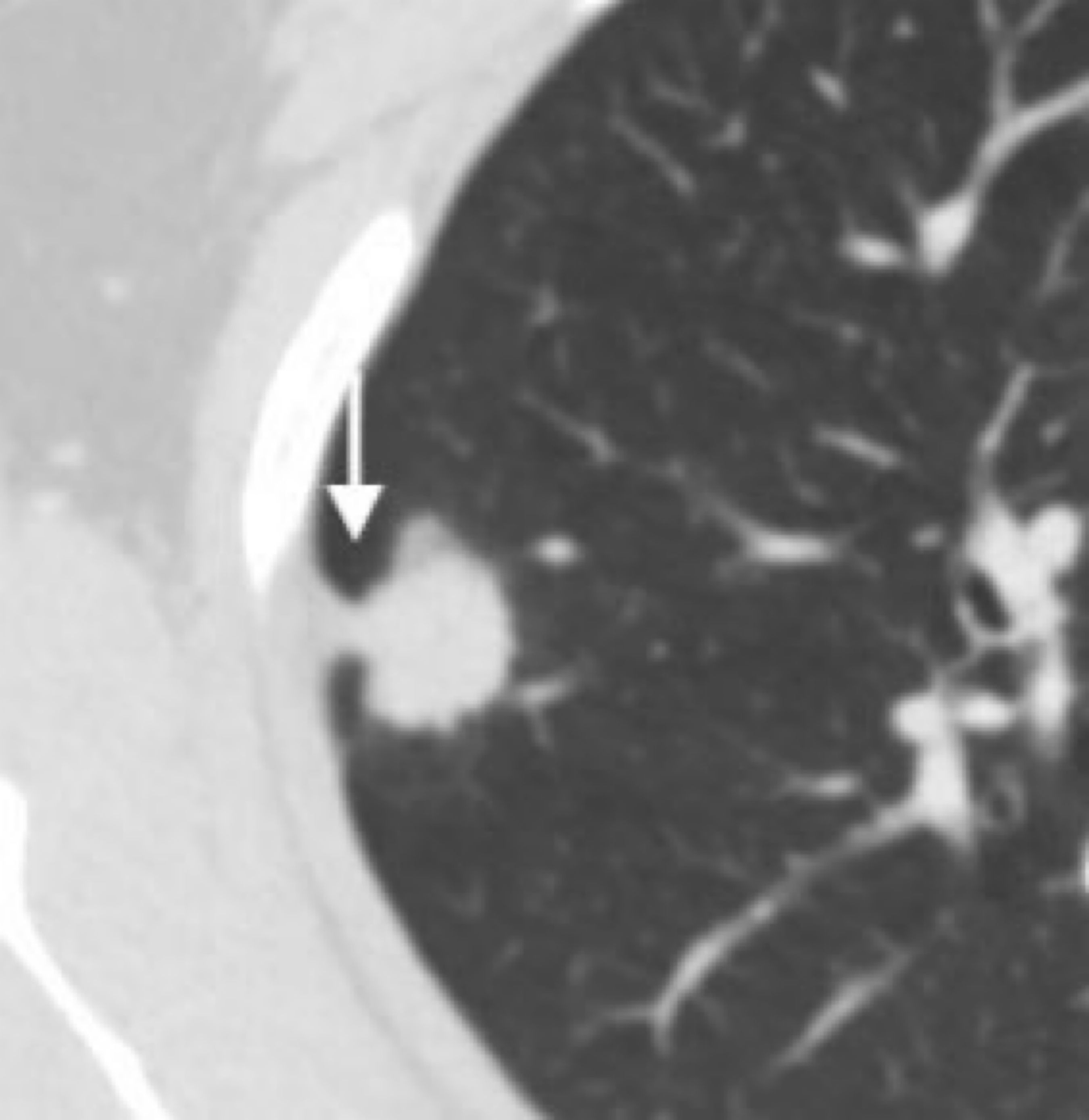
Figure 2. Pleural indentation (arrow).
2.6 Model development and validationA predictive model was developed using development cohort data. Logistic regression analysis was performed using candidate predictors as independent variables and LNM status as a dependent variable, and correlation strength was estimated using odds ratio (OR) with 95% confidence interval (CI) values. Variables with P values < 0.1 in univariate analysis were included as candidate predictors in multivariate regression analysis, and screened using the stepwise backward method. Finally, predictors identified after screening were used to build the model, after which it was validated using validation cohort data.
Model performance was assessed by discrimination and calibration. The area under the curve (AUC) in receiver operating characteristic (ROC) analysis was used to measure discrimination, while calibration was assessed using Hosmer–Lemeshow (HL) tests and a calibration curve. Additionally, we used Brier scores to assess overall model performance, and used clinical decision curve analysis (DCA) to quantify net model benefits to patients at different threshold probabilities, thus assessing model value in clinical settings.
2.7 Risk stratification strategies based on clinical guideline recommendations and model performanceThe relationship between the risk the stratification strategy and a pathological diagnosis is shown (Table 1). If a patient was pathologically diagnosed as LNM-negative but was considered high-risk by stratification, this was considered “over-screening” (Group B, Table 1). On the other hand, if a patient had a pathological LNM-positive diagnosis but was considered low-risk by stratification, this was considered “under-screening” (Group C, Table 1). From this, the stratification strategy had a sensitivity of A/(A+C); a false positive rate of B/(B+D); a specificity of D/(B+D); and a false negative rate of C/(A+C). Missed diagnoses in LNM-positive patients can have serious consequences such as tumor recurrence, so priority was given to avoid under-screening. Under this premise, a balance was sought where false positive rates were as low as possible.
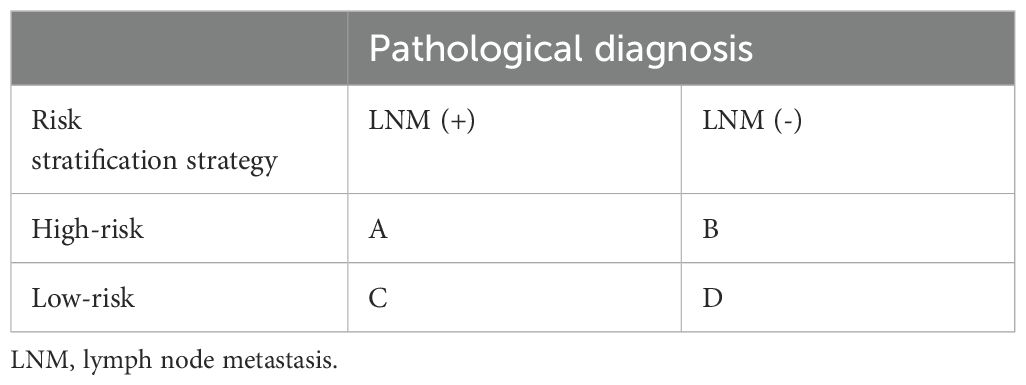
Table 1. The relationship between the risk stratification strategy and a pathological diagnosis.
According to American College of Chest Physicians (8) and National Comprehensive Cancer Network guidelines (2), invasive mediastinal staging is generally indicated in the following circumstances: tumor size > 3.0 cm, centrally located tumor, and the presence of lymph node disease (hilar/mediastinal lymph node enlargement, or elevated deoxyglucose uptake in lymph nodes) by imaging tests. Patients with any of these conditions are considered high-risk using these guidelines.
After modeling, we assessed the diagnostic performance of the model by setting a LNM-positive cutoff probability. We categorized patients as high-risk when their LNM-positive probability was above the cutoff probability, and vice versa for low-risk. Based on the criteria for setting the cutoff probability with different emphasis, we designed three risk stratification strategies. Strategy 1: Cutoff probability that facilitated model sensitivity to approximately that of the guideline strategy; Strategy 2: The optimal cutoff probability corresponded to the maximum Youden index in ROC analysis; and Strategy 3: Cutoff probability that maximized sensitivity (> 90%).
2.8 Statistical analysisInterobserver agreement was assessed for continuous variables, categorical variables using the intraclass correlation co-efficient (ICC), and Kappa consistency tests (22). Baseline characteristics of the study population were described by mean valued ± standard deviation (normal distribution), median values with interquartile ranges (non-normal distribution) for continuous variables, and absolute and relative frequencies for categorical variables. Differences in continuous variables were compared using Mann–Whitney or two-tailed Student’s t tests. Differences in categorical variable distributions were compared using Pearson chi-square tests. Two-sided P values < 0.05 were considered statistically significant. All statistical analyses were performed in R version 4.1.1 (The R Foundation for Statistical Computing; http://www.R-project.org/).
3 Results3.1 Interobserver agreementInterobserver agreements were good for CT imaging features: tumor size (ICC = 0.95), solid component size (ICC = 0.94), and pleural depression (kappa = 0.92).
3.2 General patient characteristicsIn total, 435 patients met inclusion criteria, including 184 (42.3%) males and 251 (57.7%) females, with a mean age of 59.8 years (range = 31–82 years). We observed that 59 (13.6%) patients were LNM-positive and 376 (86.4%) were LNM-negative. There were 249 (57.2%) patients with tumor sizes ≤ 1.5 cm, and 186 (42.8%) with tumor sizes > 1.5 cm. CTR was ≤ 0.75 in 153 (5.2%) patients and > 0.75 in 282 (64.8%). The demographic and clinical characteristics of both cohorts are shown (Table 2). The development cohort included 268 (61.6%) patients and the validation cohort included 167 (38.4%) patients. The LNM-positive rate was 14.2% (38/268) in the development cohort and 12.6% (21/167) in the validation cohort. Significant differences were observed between cohorts in terms of gender and tumor size. When compared with the development cohort, the validation cohort had a larger proportion of females (65.9% versus 52.6%, P value = 0.009) and a larger proportion of patients with tumor sizes ≤ 1.5 cm (67.1% versus 51.1%, P value = 0.002), which were possibly related to the fact that regions where validation centers were located were more economically developed, and populations may have had a better awareness of medical checkups, which helped earlier disease detection.
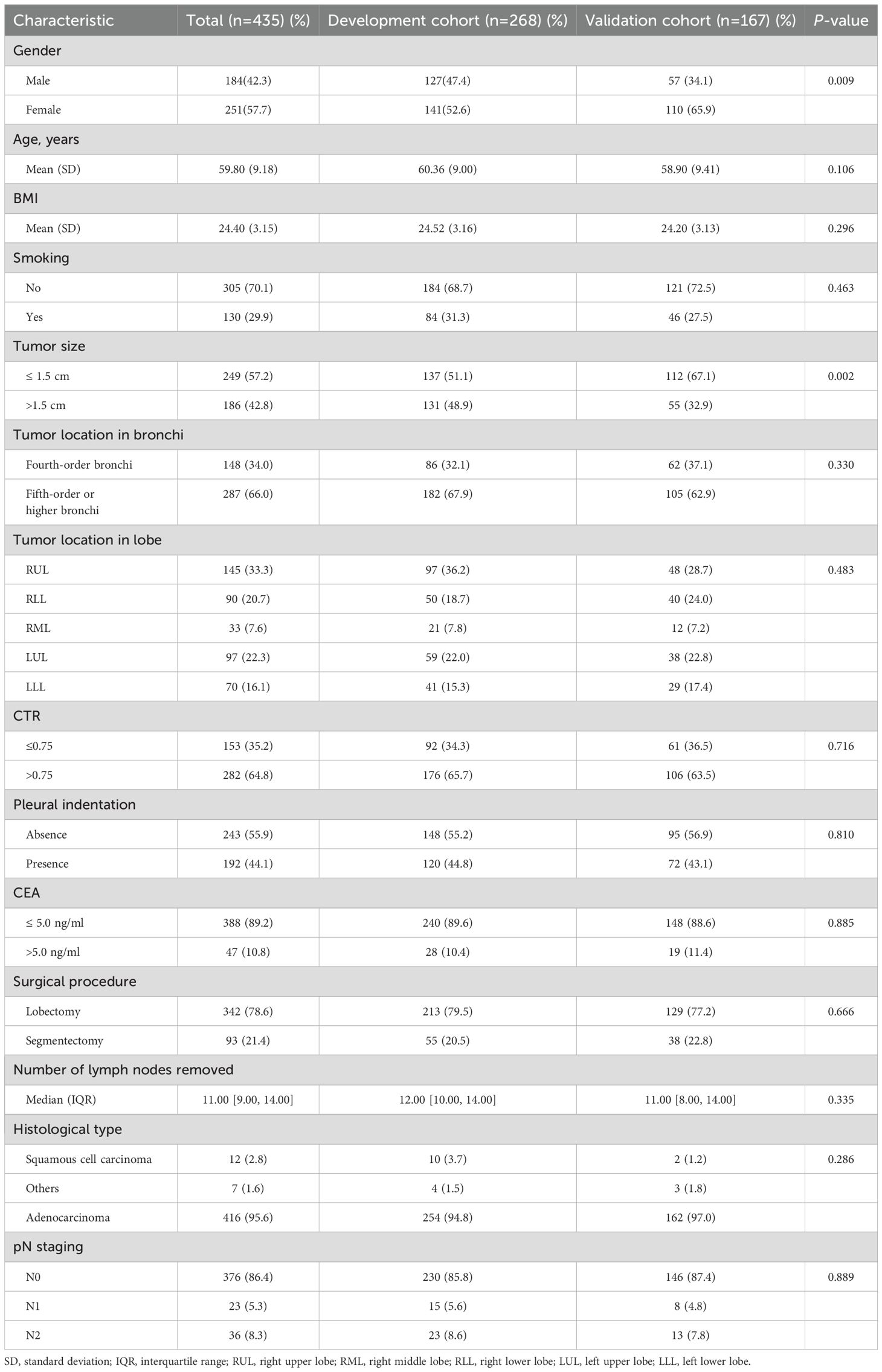
Table 2. Baseline patient characteristics.
3.3 Model developmentUnivariate analyses showed that tumor size, CTR, pleural indentation, and CEA values were significantly associated with LNM-positive tumors (P value < 0.05, Table 3). We then included these variables in multivariate analysis. Additionally, although the tumor location in the bronchi predictor was not statistically significant in univariate analysis, we included this in multivariate analysis due to its clinical significance (9, 23).
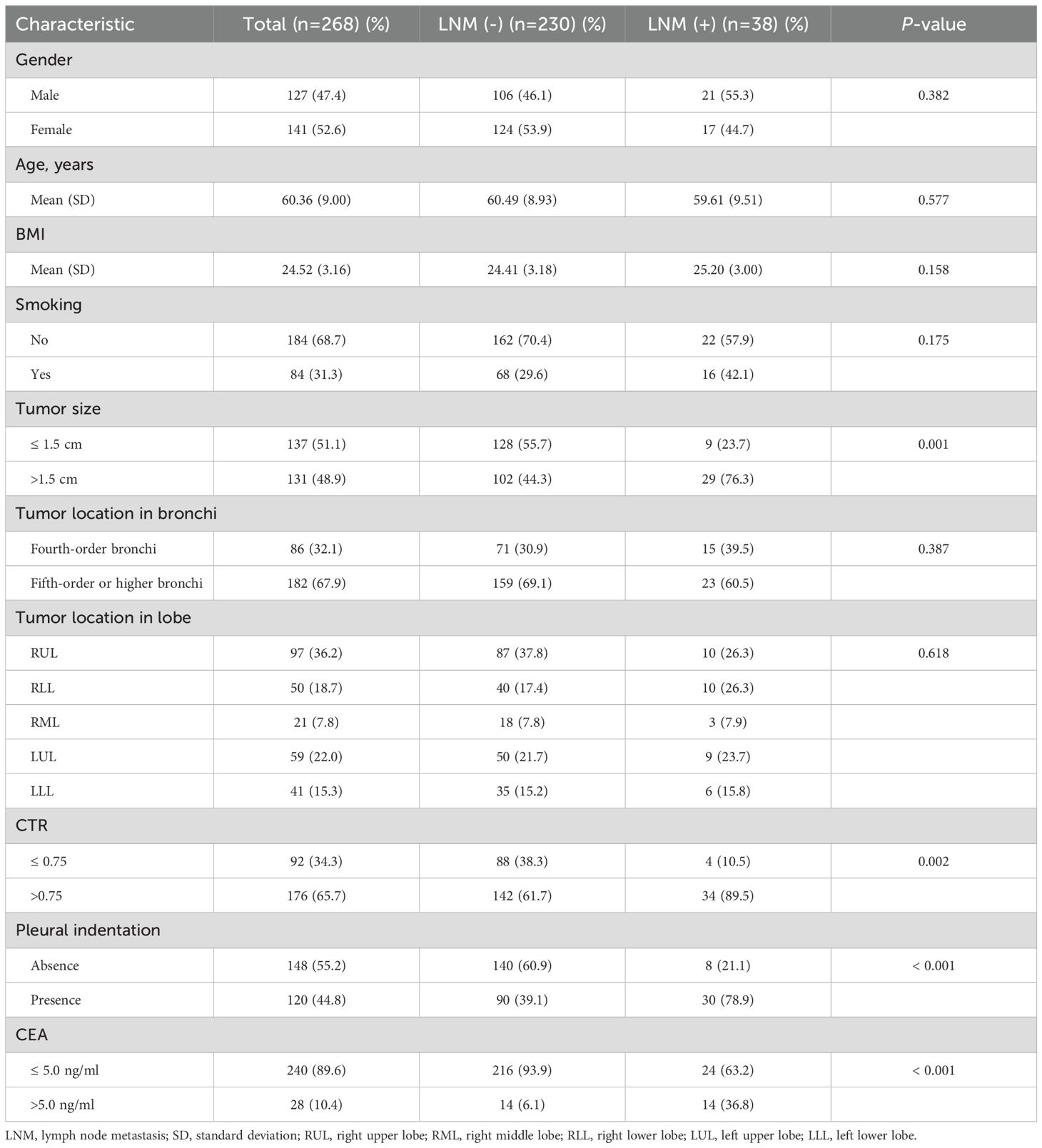
Table 3. Univariate analysis of lymph node metastasis (LNM) risk.
From multivariate logistic regression analysis, we identified four independent LNM-positive predictors: tumor size (OR = 2.613; 95% CI: 1.130–6.468; P value = 0.029), CTR (OR = 3.428; 95% CI: 1.196–12.540; P value = 0.035), pleural indentation (OR = 4.441; 95% CI: 1.899–11.499; P value = 0.001), and CEA values (OR = 6.521; 95% CI: 2.537–17.196; P value < 0.001) (Table 4). The model indicated that patients with a tumor size > 1.5 cm, a CTR > 0.75, a pleural indentation around the tumor by CT imaging, and a CEA value > 5.0 ng/ml had a greater risk of being LNM-positive. Based on this model, we developed a nomogram to assess the probability of a patient presenting with LNM-positive status (Figure 3).
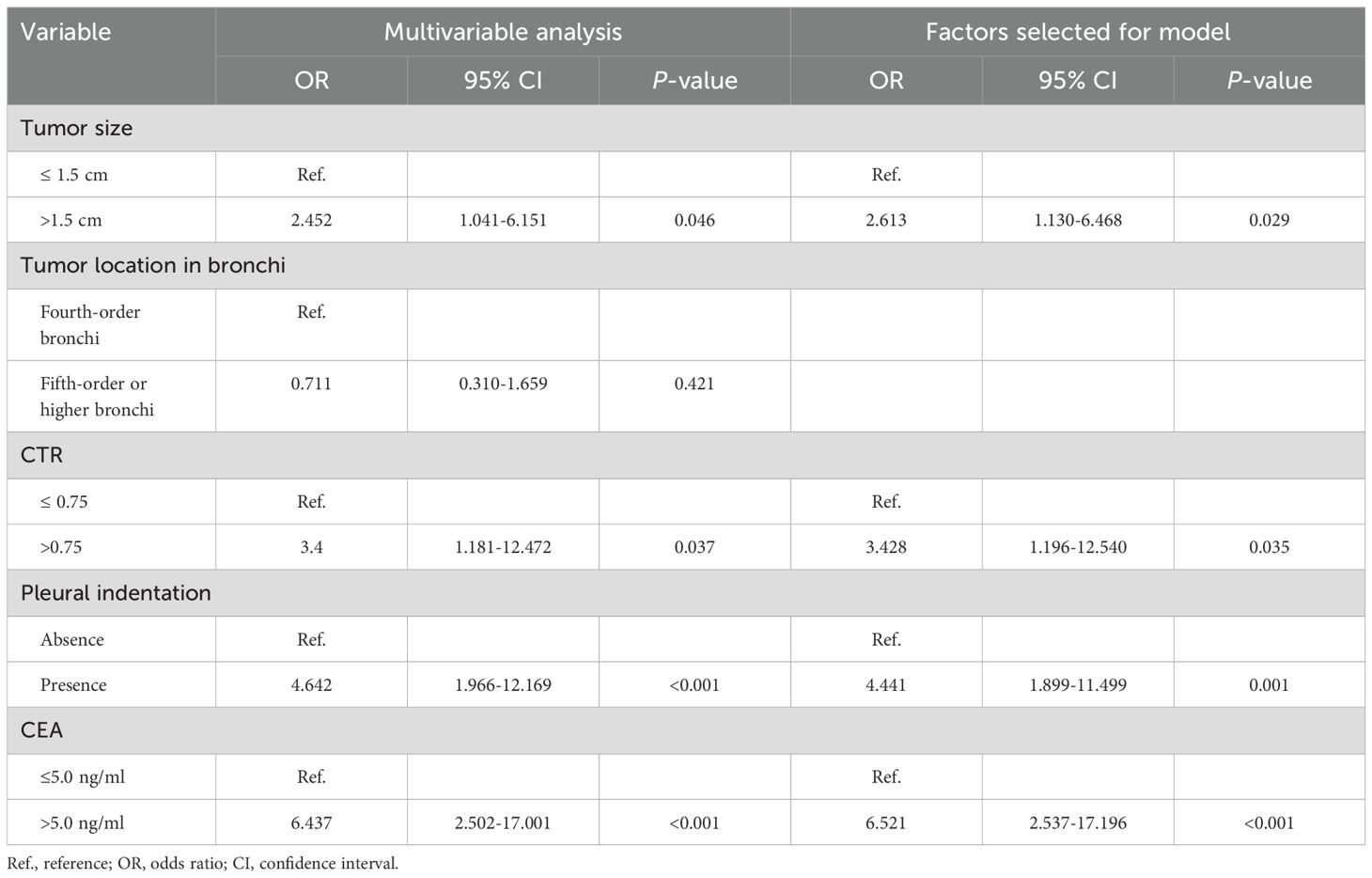
Table 4. Variables identified by logistic multivariable regression analysis.
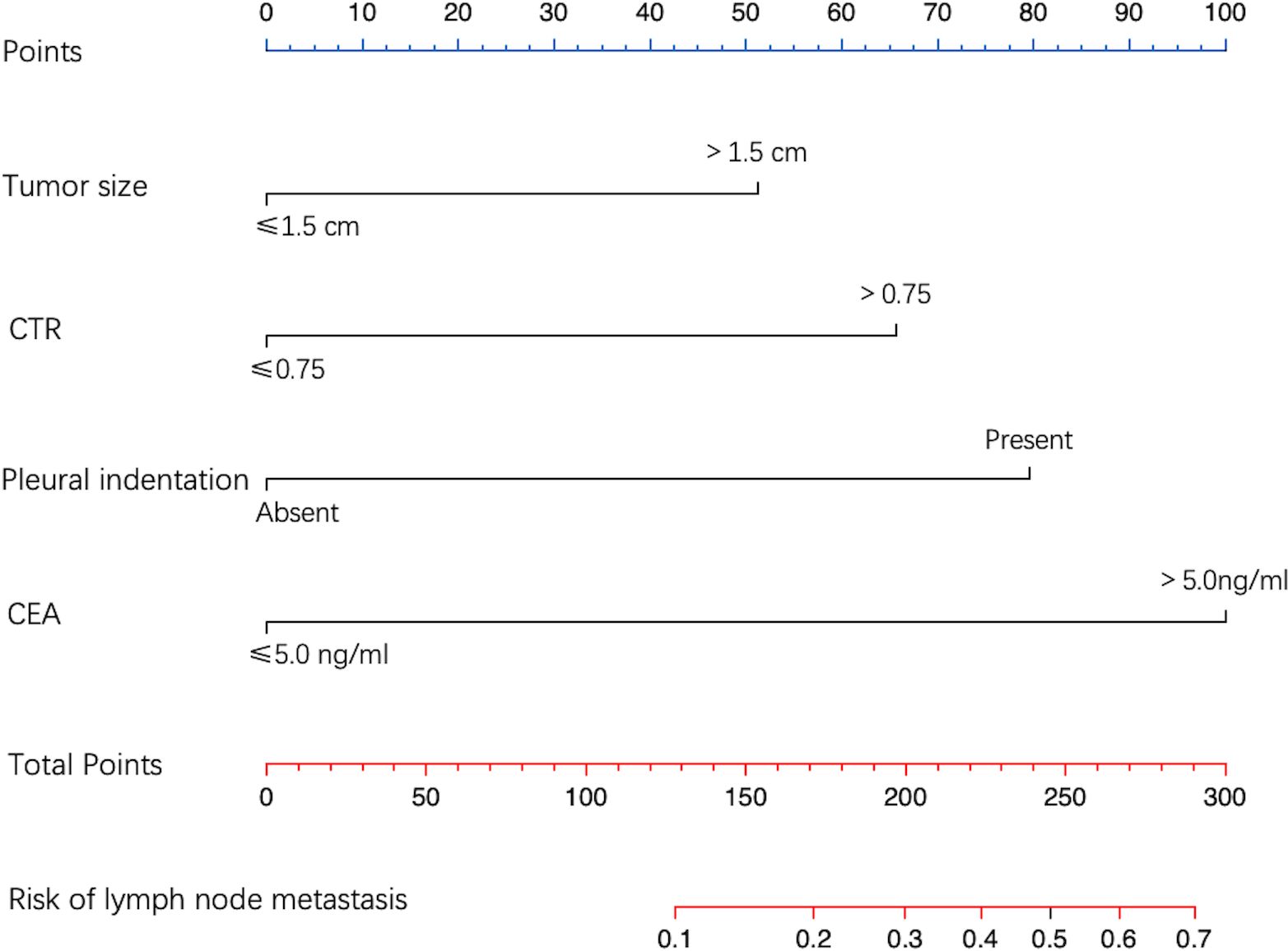
Figure 3. Nomogram for predicting LNM probability. The value of each predictive factor was given a score on the point scale axis. A total score was calculated by adding every single score, and by projecting the total score to the lower total point scale, LNM probability was estimated.
3.4 Model validationIn the development cohort, the model AUC was 0.834 (95% CI: 0.773–0.896) (Figure 4A), and when LNM-positive probability (11.9%) corresponding to the maximum Youden index (0.49) was used as a cutoff probability, model sensitivity was 81.6% (95% CI: 69.3%–93.9%) and specificity was 67.4% (95% CI: 61.3%–73.4%). HL tests showed that the model was well calibrated (P value = 0.921). The calibration curve also showed good agreement between predicted and actual results (Figure 5A). The model Brier score was 0.095.
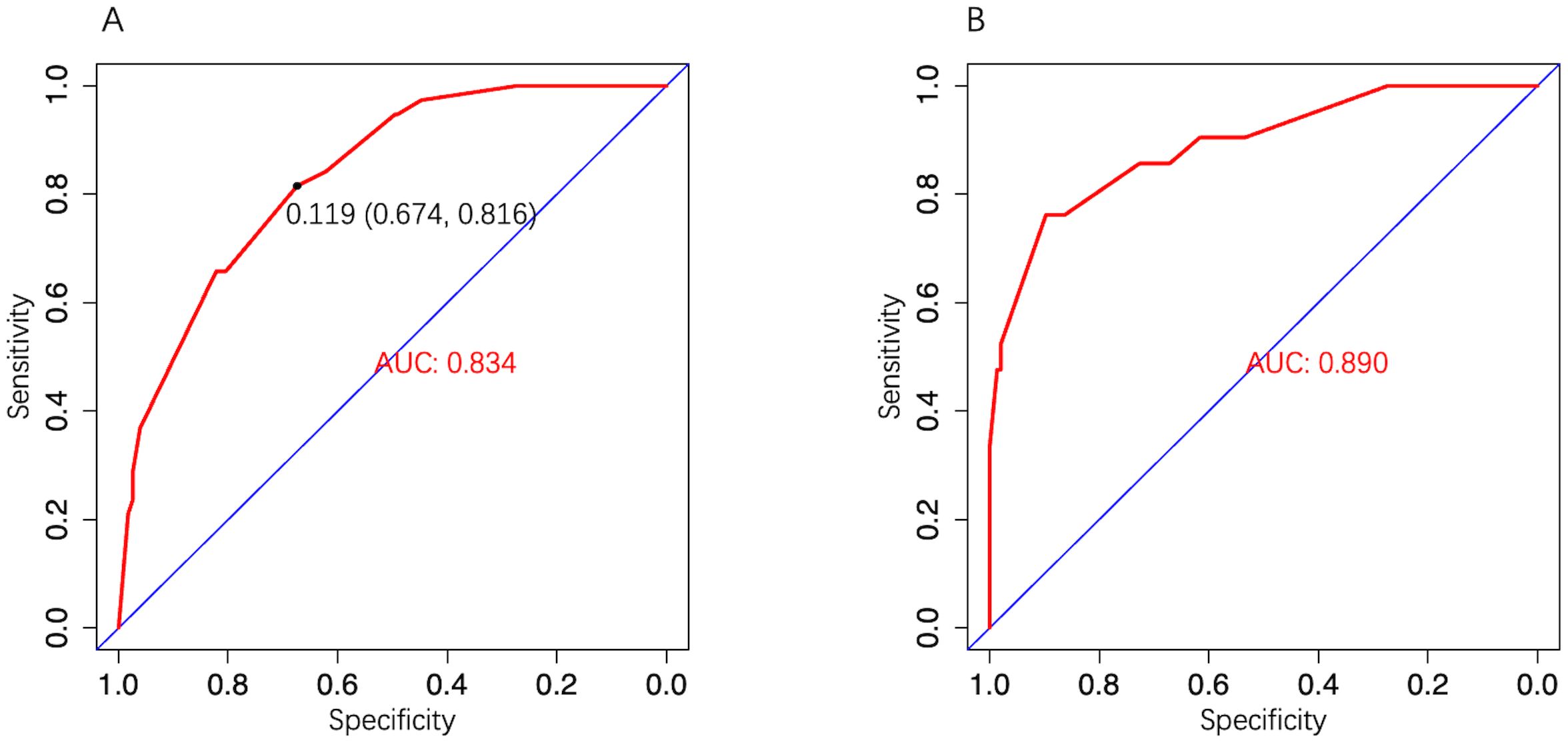
Figure 4. The model ROC curve. (A) Development cohort; (B) Validation cohort.
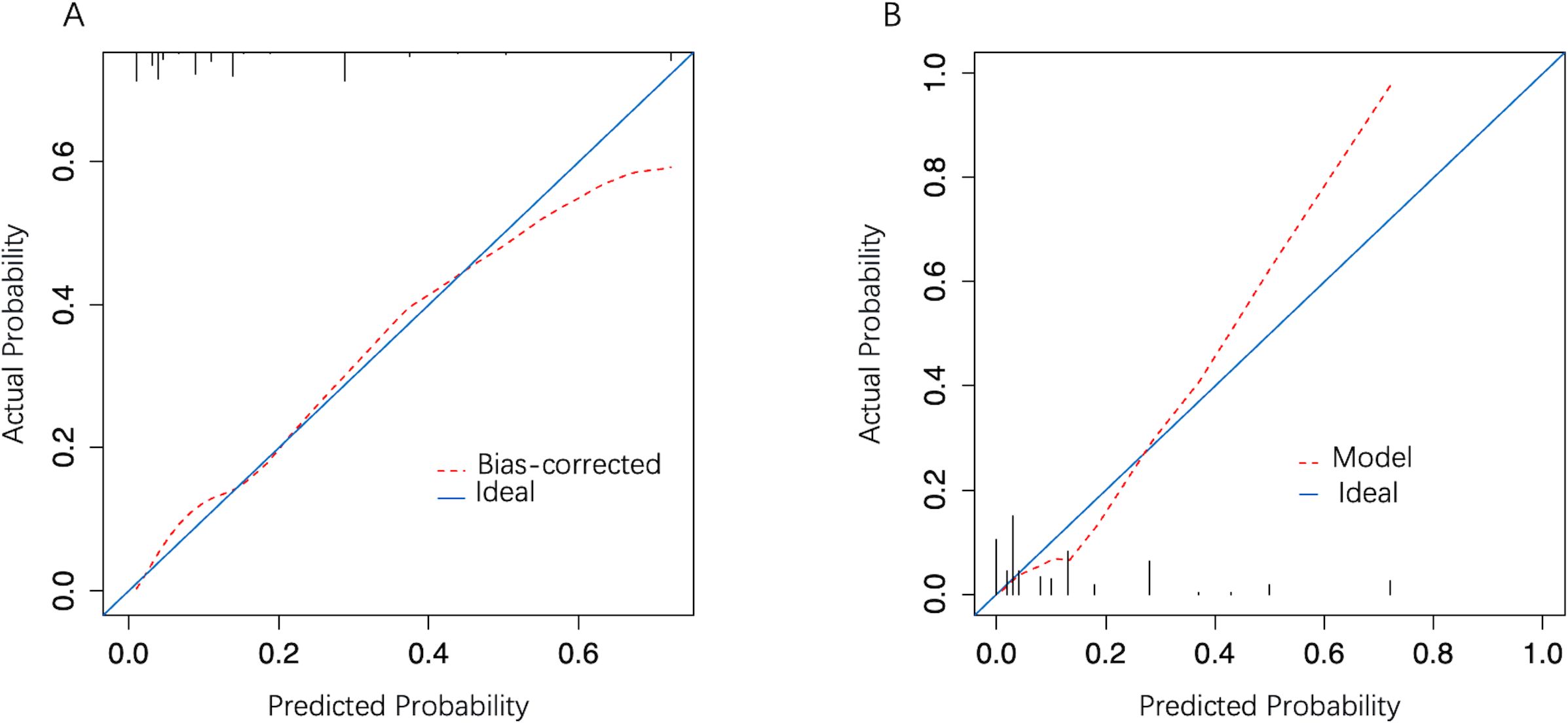
Figure 5. The model calibration curve. (A) Development cohort; (B) Validation cohort. The x-axis represents the predicted probability and the y-axis represents actual LNM probability. A perfect prediction corresponds to the diagonal solid blue line. The red dashed line represents model performance, of which, a closer fit to the diagonal line represents a better prediction.
In the external validation cohort, model AUC was 0.890 (95% CI: 0.808–0.972), demonstrating robust discriminatory ability (Figure 4B). When 11.9% was used as the model cutoff probability, corresponding sensitivity was 85.7% (95% CI: 71.4%–100.0%) and specificity was 72.6% (95% CI: 65.1%–80.1%). HL tests demonstrated good calibration (P value = 0.680). Although the calibration curve showed that agreement between predicted and actual results decreased when compared with the development cohort, it was still acceptable (Figure 5B), further illustrating the reliability of the calibration. The model Brier score in the external validation cohort was 0.065, which indicated an overall good performance. DCA showed that when using the model to predict LNM-positive probability, it delivered significantly more net benefits than other diagnostic options (Figure 6).
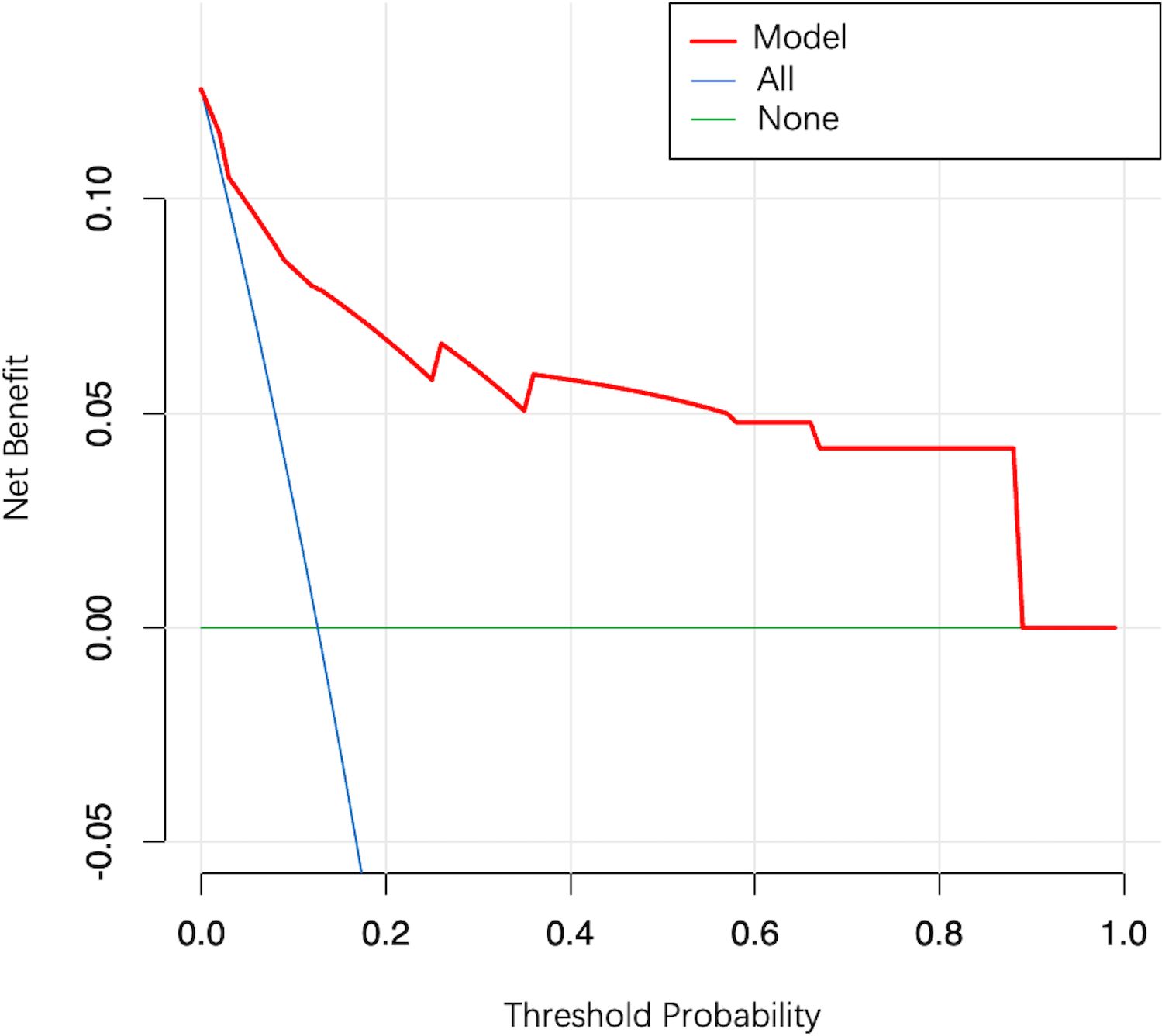
Figure 6. Decision curve analysis for the model. The y-axis measures the net clinical benefit. The red line represents the model. The blue line represents the assumption that all patients have LNM. The green line represents the assumption that all patients have no LNM.
3.5 Risk stratification strategies based on clinical guideline recommendations and model performanceBased on high-risk characteristics as recommended by guidelines, we identified nine high-risk patients (5.4%) in the validation cohort. Of these, five were LMN-positive, resulting in a sensitivity of 23.8% and a specificity of 97.3% for the guideline strategy (Table 5). The diagnostic performance of different model strategies was as follows (Table 5); Strategy 1: Minimum model sensitivity was 33.3% (95% CI: 14.3%–57.1%), corresponding to a cutoff probability of 61.2% and a specificity of 100.0% (95% CI: 100.0%–100.0%); Strategy 2: The cutoff probability corresponding to the maximum Youden index was 11.9%, and corresponding model sensitivity was 85.7% (95% CI: 71.4%–100.0%) with a specificity of 72.6% (95% CI: 65.1%–80.1%); Strategy 3: When model sensitivity was elevated to > 90.5% (95% CI: 76.2%–100.0%), the corresponding specificity was 61.6% (95% CI: 53.2%–69.9%).
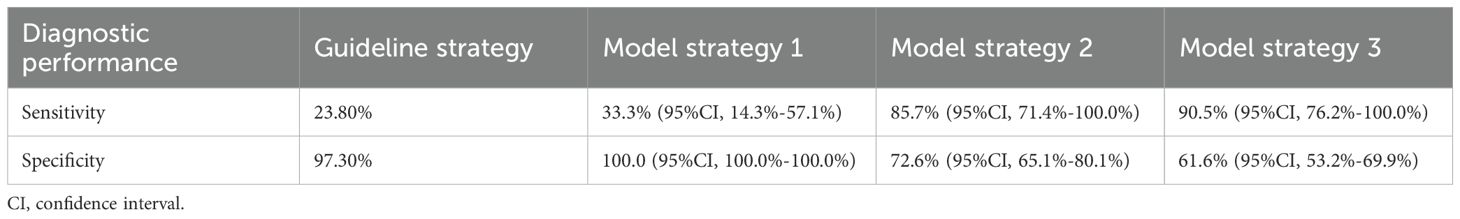
Table 5. The diagnostic performance of different strategies.
4 DiscussionIn this retrospective study, we developed and validated a predictive model to assess LNM-positive risk in peripheral NSCLC patients with a tumor size ≤ 2.0 cm and CTR > 0.5. The model indicated that patients with a tumor size > 1.5 cm, a CTR > 0.75, the presence of a pleural indentation, and a CEA value > 5.0 ng/ml had a greater risk of being LNM-positive.
Preoperative LNM status assessment is crucial for appropriate surgical choices in early-stage NSCLC patients. The much-anticipated JCOG0802 study (2022) required that patients with potential segmentectomy indications fulfilled applicable LNM-negative conditions (6). The CALGB140503 study (2023) (24), which was another heavyweight study, further side-stepped the importance of being LNM-negative for patients with indications for sublobar resection while obtaining similar results to the JCOG0802 study. Based on such clinical needs, we conducted this study with specific patient types similar to the JCOG0802 study and identified results that differed somewhat to the previous literature.
We found that tumor size was an independent predictor, consistent with most studies (25–29). Asamura and colleagues (26) reported that mediastinal LNM incidences in peripheral NSCLC patients increased from 19.5% for tumors ≤ 2.0 cm to 32.5% for tumors of 2.0–3.0 cm. Yang et al. (27) also reported that the probability of being LNM-negative was 70.8%, 58.88%, 48.03%, 47.55%, and 33.33% when tumor diameters were ≤ 2.0 cm, 2.1–3.0 cm, 3.1–5.0 cm, 5.1–7.0 cm, and > 7.0 cm, respectively. From further analyses, we found no interactions between tumor size and CTR (P value = 0.97).
Our results suggested that a higher CTR was an independent risk factor. A Japanese study reported that only 2% of adenocarcinoma patients with no solid component in their tumor had LNM, whereas 14% with a solid tumor component had LNM (30). Pathology-based studies also suggested that solid and sub-solid components roughly corresponded to invasive and non-invasive tumor components, respectively (31, 32). Since a higher CTR reflected a higher percentage of invasive components in adenocarcinoma (31, 32), and the majority of patients (95.6%) in our study had a pathological adenocarcinoma type, the association between higher CTR and higher LNM risk is reasonable.
Previous studies have been controversial with respect to pleural indentation as a radiographic indicator of tumor infiltration (20, 33, 34). Although some studies concluded that pleural indentation was associated with visceral layer invasion (20) and LNM (34), other studies concluded the opposite (33). It is possible that such contradictions were caused by subjective differences between researchers in determining pleural indentation. For this reason, we arranged for two specialists to carefully interpret CT images and examine interobserver agreements on result assessments to minimize errors. The findings conclusively suggested that the presence of a pleural indentation increased the risk of being LNM-positive, even for tumors ≤ 2.0 cm. Thus, these observations must be seriously considered by clinicians.
We observed no correlation between age and LNM. Previous studies reported that younger patients were more likely to have mutations in target genes (35), and that genotypic mutations were associated with LNM (36, 37), which may explain why a younger age is a risk factor for LNM in many studies (11, 25, 38). However, in the literature, any correlation between age and LNM was weakened or disappeared with decreasing tumor size when the study population was limited to NSCLC patients with tumors ≤ 3.0 cm (21, 33, 39), which we corroborated. Thus, we posited several plausible reasons for this: (I) a significant correlation between genotypic mutations and age no longer exists in small-sized tumors due to tumor heterogeneity and (II) interactions may occur between age and tumor size in predicting LNM risk. Further studies are required to clarify these reasons.
Many studies have demonstrated that central tumors have higher LNM rates than peripheral tumors (9, 23), which is consistent with the clinical logic that the closer the tumor is to the center, the more likely it is to invade lymph nodes in the drainage area. However, as no uniform standard exists for defining central versus peripheral lung cancer, study criteria often vary (2, 29, 40–42). Also, due to tumor heterogeneity (43), it is worth exploring whether the relationship between tumor location and LNM status is still applicable in small-sized peripheral tumors. Therefore, we attempted to define the tumor location based on the bronchial level at which the tumor is located and classified it with fourth-order bronchi (subsegmental bronchi) and fifth-order or higher bronchi (beyond the subsegmental bronchi). Such a definition could objectively reflect lesion proximity to the center, while at the same time refining classification and possibly detecting correlations at more subtle levels. We observed that patients with tumors at lower order bronchi were more likely to be LNM-positive (development cohort: 39.5% versus 30.9%, and validation cohort: 52.4% versus 34.9%), although this difference was not statistically significant (P value > 0.05). Thus, tumor location may not significantly affect LNM probability in these patients.
Our study had the following advantages over previous studies: (I) We validated our model using external data, thus model performance was more accurately evaluated; (II) We analyzed relationships between tumor location and LNM at more detailed levels based on the bronchial level and obtained the results; and (III) Our model is one of the few current models based on JCOG0802 study patients, and has better applicability when compared to other models.
Our study also has limitations. Firstly, an inherent retrospective bias may have occurred but we improved this using multivariate analysis. Secondly, we did not include maximum standardized uptake positron emission tomography-computed tomography (PET-CT) values as predictors because PET-CT is not a routine examination for small-sized NSCLC in clinical practice, and variables with more missing values are unsuitable for analysis. Finally, we were unable to enter N1/N2 LNM predictions separately due to our limited sample size. In future studies, we will improve this by collecting more samples.
In conclusion, we developed and externally validated a parsimonious clinical prediction model for calculating LNM-positive probability in patients with peripheral NSCLC, with a tumor diameter ≤ 2.0 cm and CTR > 0.5. Patients with a tumor size > 1.5 cm, a CTR > 0.75, a pleural indentation around the tumor on CT imaging, and a CEA value > 5.0 ng/ml were associated with an increased probability of being LNM-positive. The model showed good performance and has great potential in assisting clinicians making individualized clinical decisions.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Yuncheng Central Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsDL: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation. SL: Conceptualization, Data curation, Writing – review & editing. HZ: Data curation, Formal analysis, Writing – review & editing. CX: Data curation, Writing – review & editing. XN: Data curation, Writing – review & editing. HL: Formal analysis, Writing – review & editing. JC: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Beijing Nova Program (Z201100006820092) from Beijing Municipal Science & Technology Commission.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. (1995) 60:615–23. doi: 10.1001/jamaoncol.2017.2993
PubMed Abstract | Crossref Full Text | Google Scholar
4. Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of Pathologic Nodal Staging Quality with Survival among Patients with Non–Small Cell Lung Cancer after Resection with Curative Intent. JAMA Oncol. (2018) 4:80. doi: 10.1001/jamaoncol.2017.2993
PubMed Abstract | Crossref Full Text | Google Scholar
5. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. The international association for the study of lung cancer lung cancer staging project. J Thorac Oncol. (2015) 10:1675–84. doi: 10.1097/JTO.0000000000000678
PubMed Abstract | Crossref Full Text | Google Scholar
6. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. (2022) 399:1607–17. doi: 10.1016/S0140-6736(21)02333-3
PubMed Abstract | Crossref Full Text | Google Scholar
7. Herth FJF, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. (2008) 133:887–91. doi: 10.1378/chest.07-2535
PubMed Abstract | Crossref Full Text | Google Scholar
8. Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer. Chest. (2013) 143:e211S–50S. doi: 10.1378/chest.12-2355
PubMed Abstract | Crossref Full Text | Google Scholar
9. Decaluwé H, Stanzi A, Dooms C, Fieuws S, Coosemans W, Depypere L, et al. Central tumour location should be considered when comparing N1 upstaging between thoracoscopic and open surgery for clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. (2016) 50:110–7. doi: 10.1093/ejcts/ezv489
PubMed Abstract | Crossref Full Text | Google Scholar
10. Mattes MD, Weber WA, Foster A, Moshchinsky AB, Ahsanuddin S, Zhang Z, et al. A predictive model for lymph node involvement with Malignancy on PET/CT in non–small-cell lung cancer. J Thorac Oncol. (2015) 10:1207–12. doi: 10.1097/JTO.0000000000000601
PubMed Abstract | Crossref Full Text | Google Scholar
11. Lv X, Wu Z, Cao J, Hu Y, Liu K, Dai X, et al. A nomogram for predicting the risk of lymph node metastasis in T1–2 non-small-cell lung cancer based on PET/CT and clinical characteristics. Transl Lung Cancer Res. (2021) 10:430–8. doi: 10.21037/tlcr-20-1026
PubMed Abstract | Crossref Full Text | Google Scholar
12. The American Thoracic Society and The European Respiratory Society. Pretreatment evaluation of non–small-cell lung cancer. Am J Respir Crit Care Med. (1997) 156:320–32. doi: 10.1164/ajrccm.156.1.ats156.1
PubMed Abstract | Crossref Full Text | Google Scholar
13. Billé A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography☆. Eur J Cardiothorac Surg. (2009) 36:440–5. doi: 10.1016/j.ejcts.2009.04.003
PubMed Abstract | Crossref Full Text | Google Scholar
14. Herth FJF, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. (2006) 28:910–4. doi: 10.1183/09031936.06.00124905
PubMed Abstract | Crossref Full Text | Google Scholar
15. Eapen GA, Shah AM, Lei X, Jimenez CA, Morice RC, Yarmus L, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration. Chest. (2013) 143:1044–53. doi: 10.1378/chest.12-0350
PubMed Abstract | Crossref Full Text | Google Scholar
16. Truong MT, Viswanathan C, Erasmus JJ. Positron emission tomography/computed tomography in lung cancer staging, prognosis, and assessment of therapeutic response. J Thorac Imaging. (2011) 26:132–46. doi: 10.1097/RTI.0b013e3182128704
PubMed Abstract | Crossref Full Text | Google Scholar
17. Lemaire A, Nikolic I, Petersen T, Haney JC, Toloza EM, Harpole DH, et al. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg. (2006) 82:1185–90. doi: 10.1016/j.athoracsur.2006.05.023
PubMed Abstract | Crossref Full Text | Google Scholar
18. Goldstraw P. Staging Manual in Thoracic Oncology. Editorial Rx Press, Orange Park, FL (2009).
19. Suzuki K, Koike T, Asakawa T, Kusumoto M, Asamura H, Nagai K, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan clinical oncology group 0201). J Thorac Oncol. (2011) 6:751–6. doi: 10.1097/JTO.0b013e31821038ab
PubMed Abstract | Crossref Full Text | Google Scholar
20. Kim HJ, Cho JY, Lee YJ, Park JS, Cho YJ, Yoon HI, et al. Clinical significance of pleural attachment and indentation of subsolid nodule lung cancer. Cancer Res Treat. (2019) 51:1540–8. doi: 10.4143/crt.2019.057
PubMed Abstract | Crossref Full Text | Google Scholar
21. Aokage K, Suzuki K, Wakabayashi M, Mizutani T, Hattori A, Fukuda H, et al. Predicting pathological lymph node status in clinical stage IA peripheral lung adenocarcinoma. Eur J Cardiothorac Surg. (2021) 60:64–71. doi: 10.1093/ejcts/ezaa478
PubMed Abstract | Crossref Full Text | Google Scholar
22. Lardinois D, Deleyn P, Vanschil P, Porta R, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer☆. Eur J Cardiothorac Surg. (2006) 30:787–92. doi: 10.1016/j.ejcts.2006.08.008
留言 (0)