 Andrea Mancini1†
Andrea Mancini1† Alessandro Colapietro1†
Alessandro Colapietro1† Loredana Cristiano2
Loredana Cristiano2 Alessandra Rossetti1
Alessandra Rossetti1 Vincenzo Mattei3
Vincenzo Mattei3 Giovanni Luca Gravina1,4
Giovanni Luca Gravina1,4 Héctor Perez-Montoyo5
Héctor Perez-Montoyo5 Marc Yeste-Velasco5
Marc Yeste-Velasco5 Jose Alfon5
Jose Alfon5 Carles Domenech5
Carles Domenech5 Claudio Festuccia1*1Laboratory of Radiobiology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy2Department of Clinical Medicine, Public Health, Life Sciences, University of L’Aquila, L’Aquila, Italy3Biomedicine and Advanced Technologies Rieti Center, “Sabina Universitas”, Rieti, Italy4Division of Radiation Oncology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy5R&D Department, Ability Pharmaceuticals, Parc Tecnològic del Vallès, Cerdanyola del Vallès, Barcelona, Spain
Claudio Festuccia1*1Laboratory of Radiobiology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy2Department of Clinical Medicine, Public Health, Life Sciences, University of L’Aquila, L’Aquila, Italy3Biomedicine and Advanced Technologies Rieti Center, “Sabina Universitas”, Rieti, Italy4Division of Radiation Oncology, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy5R&D Department, Ability Pharmaceuticals, Parc Tecnològic del Vallès, Cerdanyola del Vallès, Barcelona, SpainIn the published article, the authors have identified some errors that happened during the cropping and placing of the IHC images. All authors of the original paper agree to the request for these changes. This has resulted in the incorrect uploading of specific images in Figure 2C (confocal images of ABTL 0812 at 20 µM) and 2D (control image of the invasion assay) as well as in the panel C of Figure 6 that affects images depicting the expression status of pAKT thr 308, tunnel, CD34 and caspase 3 (for control, ABTL 0812 240 mg/kg and Everolimus 5 mg/kg). Similarly, in the panel D of Figure 6 the affected images include those for pAKT ser 473 (control only), tunnel, caspase 3 and HIF-1 alpha (for control, ABTL 0812 240 mg/kg and Everolimus 5 mg/kg). The authors have rectified these mistakes in the revised Figures 2 and 6 and the images have been replaced by the correct ones. The corrected Figure 2 and Figure 6 and their captions appear below.
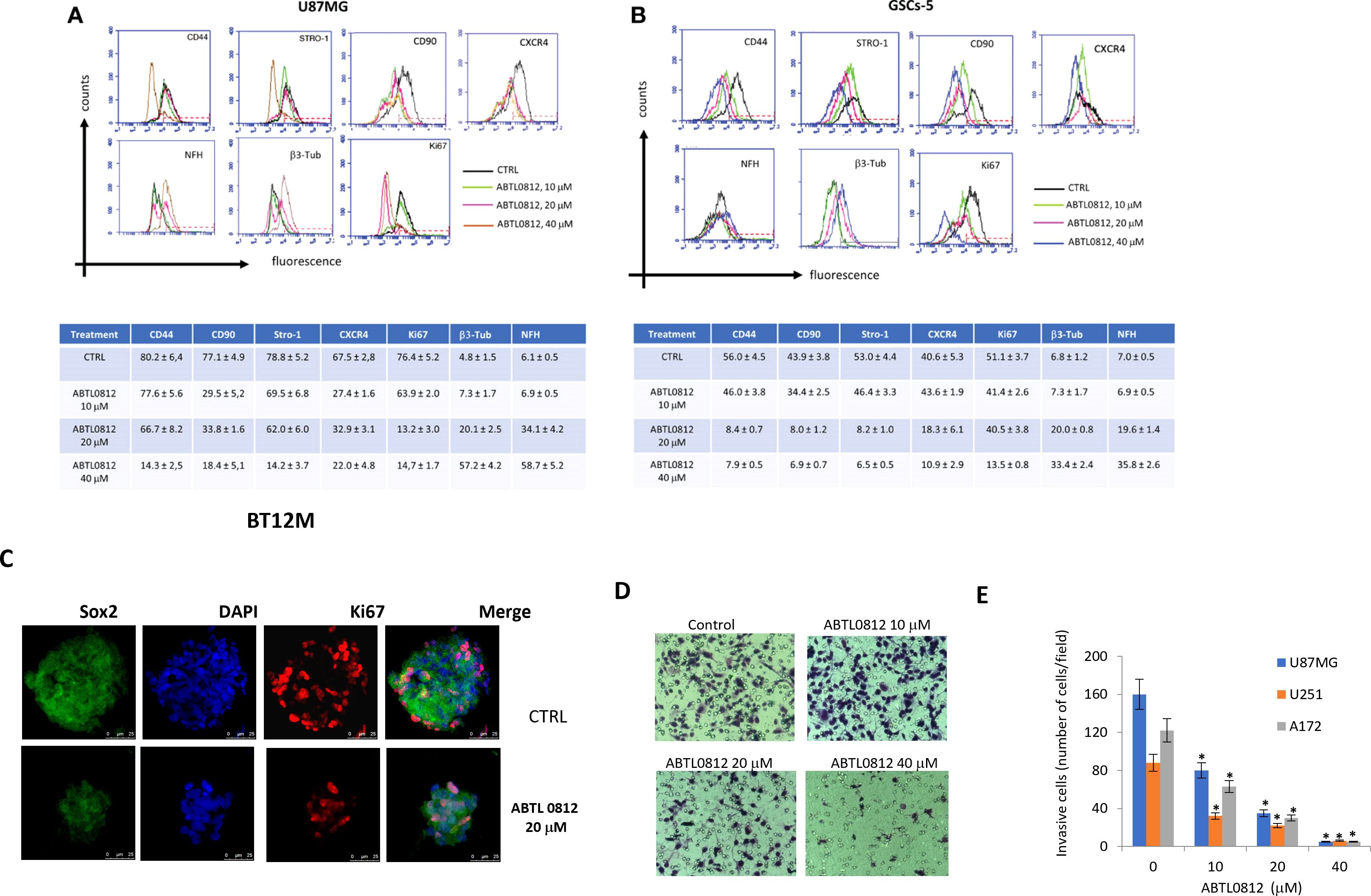
Figure 2. ABTL0812 induces glioblastoma and GSCs differentiation and reverts proneural to mesenchymal transition. (A) Representative FACS expression profiles of mesenchymal (CD44, Stro1 and CD90), stemness (CXCR4), neural (βIII tubulin, NFH and GAP43) and proliferation (Ki67) markers and table and histograms showing the percentage of cells expressing the markers analyzed by FACS in glioblastoma cells U87MG treated with ABTL0812 for 48 hours (B) and in glioblastoma stem cells GSCs-5 treated for 48 hours with ABTL0812 (C) Representative confocal images of BT12M cells stained with Sox2, βIII tubulin and Ki67. Cell nuclei were stained with DAPI. (D) Representative images from Boyden chamber assays showing invasive U87MG cells after a 6-hour assay that were pretreated with ABTL0812 for 48 hours (E) Quantification of invasive cells from a matrigel invasion assays performed in U87MG, U251 and A172 cells treated with ABTL0812. CTRL, control vehicle-treated cells. * p<0.01 vs vehicle basal.
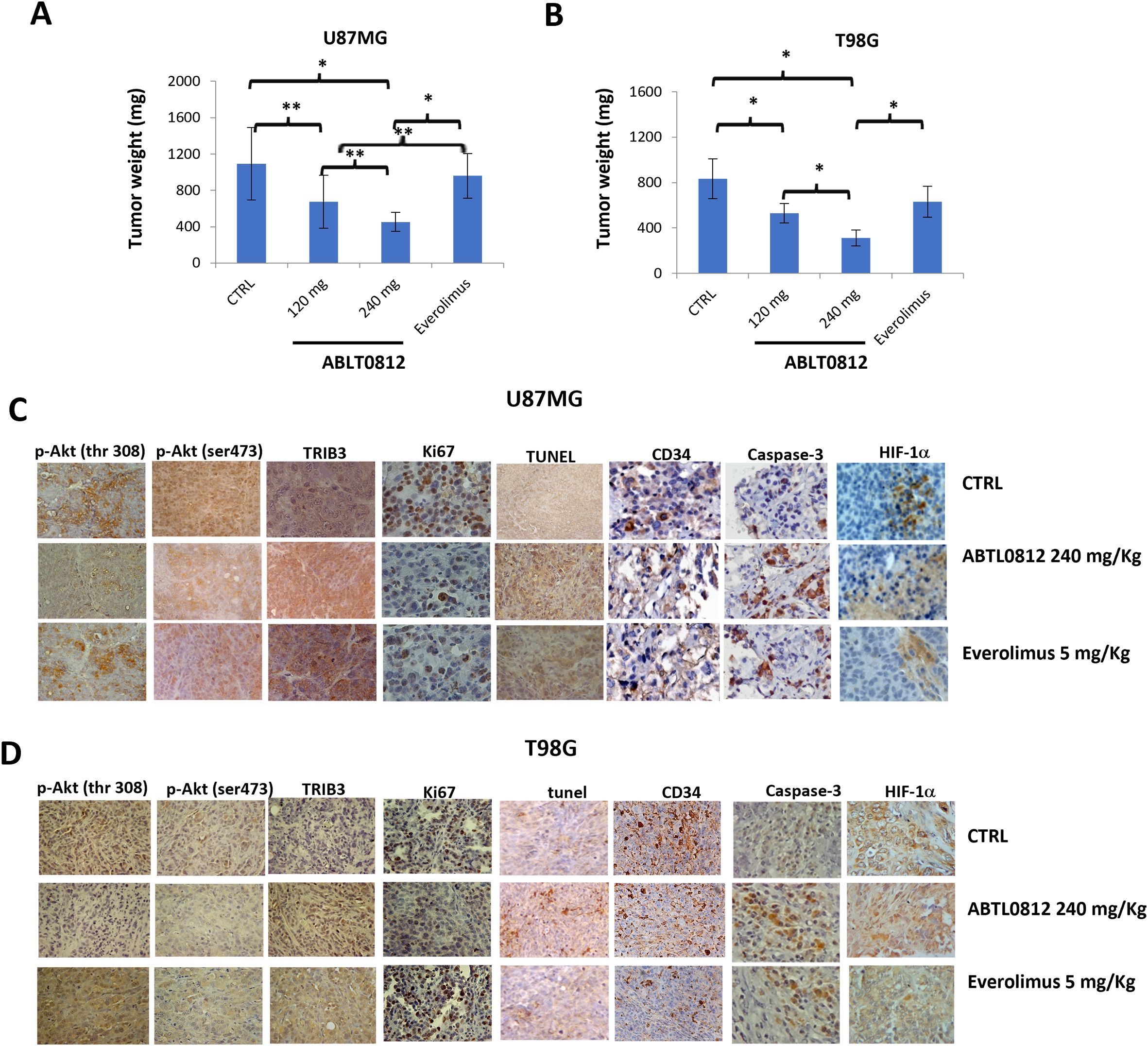
Figure 6. ABTL0812 impairs tumor growth in glioblastoma subcutaneous xenograft models. U87MG and T98G cells were injected subcutaneously in athymic female cd1 nu/nu mice (N=10 each group). Mice were treated daily with vehicle or ABTL0812 at 120 or 240 mg/Kg by oral administration. The mTORC inhibitor everolimus (ve) was used as a comparator for antitumor activity and was administered orally at a dose of 5 mg/kg/2 days per week (A, B) Weight of U87MG and T98G cells-derived xenograft tumors removed from nude mice. (C, D) Representative immunohistochemistry images from U87MG (C) and T98G (D) xenograft tumors stained with Akt-mTORC axis markers (TRIB3, p-Akt Ser473 and p-Akt Thr308); the cell proliferation marker Ki67; the endothelial cell marker CD34; the apoptosis marker caspase3; the hypoxia marker HIF-1α; and TUNEL staining to measure apoptosis (Magnification 400X). Statistical significance levels: *p<0.05, **p<0.01 and n =10. CTRL, control vehicle-treated cells.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: ABTL0812, glioblastoma, TRIB3, Akt, mTOR, ER stress, UPR, autophagy
Citation: Mancini A, Colapietro A, Cristiano L, Rossetti A, Mattei V, Gravina GL, Perez-Montoyo H, Yeste-Velasco M, Alfon J, Domenech C and Festuccia C (2025) Corrigendum: Anticancer effects of ABTL0812, a clinical stage drug inducer of autophagy-mediated cancer cell death, in glioblastoma models. Front. Oncol. 15:1538834. doi: 10.3389/fonc.2025.1538834
Received: 03 December 2024; Accepted: 06 January 2025;
Published: 22 January 2025.
Edited and Reviewed by:
Robert Clarke, University of Minnesota Twin Cities, United StatesCopyright © 2025 Mancini, Colapietro, Cristiano, Rossetti, Mattei, Gravina, Perez-Montoyo, Yeste-Velasco, Alfon, Domenech and Festuccia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Festuccia, Y2xhdWRpby5mZXN0dWNjaWFAdW5pdmFxLml0
†These authors have contributed equally to this work
留言 (0)