Prurigo nodularis (PN) is characterised by an immunological interplay among T helper (Th) 2, Th17, Th22, and Th1 cells, along with dysregulation of fibroblastic biology and neural dysfunction.1 T helper subtypes are assessed by the tissue expression of signal transducer and activator of transcription (STAT) proteins, a part of the Janus kinase (JAK)/STAT signalling pathway. STAT proteins mediate the actions of immune cells, including STAT1 and STAT4 (Th1), STAT5 and STAT6 (Th2), and STAT3 and STAT5 (Th17).2 Notably, cytokines such as IL-4, IL-13, IL-17, IL-22, and IL-31, which are up-regulated in PN, act via the JAK/STAT pathway.3,4
STAT1, STAT3, and STAT6 are all implicated in the pathophysiology of PN, with a dominance of STAT3 and STAT6,5,6 suggesting that the Th2/Th17 pathway and its related cytokines play a significant role in PN. The marked neural proliferation observed in PN is a consequence of certain cytokines, specifically IL-4, IL-13, and IL-31.4,6 The role of STAT receptors in PN, particularly in the context of concurrent use of JAK inhibitors, has not been previously investigated. In this study, we assessed the expression of STAT1, STAT3, and STAT6 in biopsies of PN lesions and evaluated the efficacy of oral tofacitinib.
Methods Settings and ParticipantsThis prospective study was carried out in the Dermatology OPD of a Tertiary Care Hospital from 1st March 2021 to 1st April 2023. Institutional and University Ethics Approval (F. No.TP (MD/MS)(109/2018)/IEC/PGIMER/RMLH1945) were obtained.
All clinically diagnosed cases of PN were assessed to rule out any secondary causes. The study excluded individuals under 18 years of age, immunocompromised patients, pregnant and lactating women, and those receiving active treatment for PN. Patients receiving active systemic treatment were instructed to discontinue all medications for three weeks and were maintained on topical glucocorticosteroids and antihistamines prior to the start of the study.
InvestigationsA complete blood count, erythrocyte sedimentation rate, fasting blood sugar levels, liver and kidney function tests, viral markers, stool for occult blood (to exclude malignancy), chest X-ray (to exclude tuberculosis or other chest infections), and abdominal ultrasound were performed in all patients to rule out secondary causes of PN.
Severity gradingThe PGSS was used to assess the severity of prurigo, which was graded as mild (score 0–5), moderate (score 6–11), and severe (score 12–19).7
Histology and immunohistochemistry (IHC) evaluation for STAT1, 3 and 6All cases underwent histological assessment using immunohistochemical (IHC) markers targeting components of the JAK-STAT cell signaling pathway, which serve as surrogate markers for T helper cells. The IHC markers used included STAT-1, STAT-3, and STAT-6 (Polymer HRP IHC Detection System, Biogenex) to identify Th1, Th17/Th2, and Th2 cells, respectively.
TreatmentTofacitinib was initiated at a dose of 5 mg twice daily in 13 patients and 11 mg (extended-release) once daily in 4 patients. The onset of response, defined as a 50% reduction in pruritus, was recorded. When the PGSS decreased by more than 75% from baseline, the dose was reduced. The dosage was further adjusted to alternate days after the patient achieved remission, defined as the absence of new lesions and a PGSS reduction of less than 75% while on a dose of 5 mg once daily for one month. Treatment was discontinued if remission was maintained on alternate-day therapy for an additional two months (remission off therapy). In the event of a relapse, defined as a 25% increase in PGSS, treatment was restarted at a dose of 5 mg once daily. Concurrent emollients and antihistamines were used as adjunctive therapies.
Statistical methodsData were entered into an MS Excel spreadsheet, and analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 23.0. A p-value of < 0.05 was considered statistically significant.
Results Demographic profile and prior treatmentsThe demographic and clinical details are presented in Table 1. The mean disease duration was 16.6 ± 10 months, and the baseline PGSS was 12.1 ± 2.3.
Table 1: Clinical characteristics and treatment response in patients of prurigo nodularis on tofacitinib
Mean age 37.6±22.7 Gender Male 7 Female 10 PN cases as per PGSS (pre-treatment) Mild 0 Moderate 8 Severe 9 Mean duration of disease 16.6±10 months Baseline PGSS 12.1±2.3 Previous treatmentsThalidomide (n=2), methotrexate (n=3), oral steroids (n=3), topical steroids (n=6), phototherapy (n=1) and ayurvedic (n=1) and homeopathic treatments (n=1).
Combination treatments (n=4)
Of the 17 patients studied, 8 had moderate PN, and 9 had severe PN. Six of these patients had previously been treated with topical steroids, while 3 each had received systemic steroids and methotrexate. Additionally, 2 patients were treated with thalidomide, and one patient each underwent phototherapy, Ayurveda, and homeopathy. Some patients also received combination therapies.
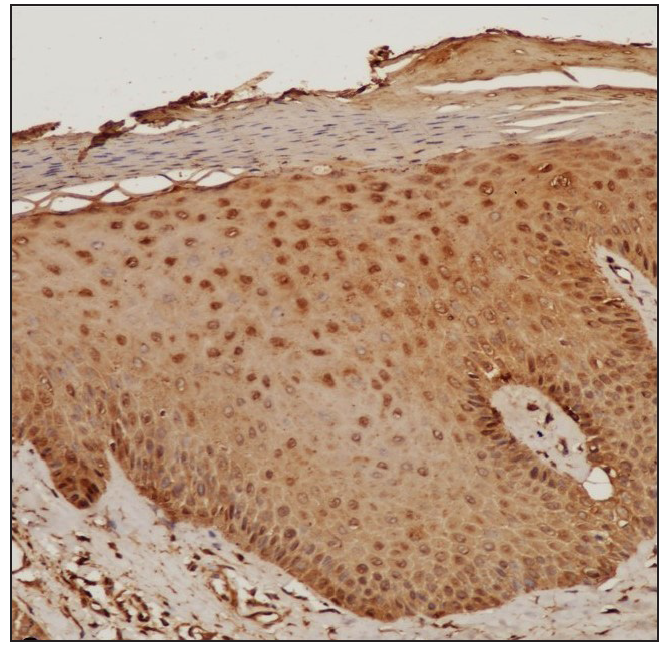
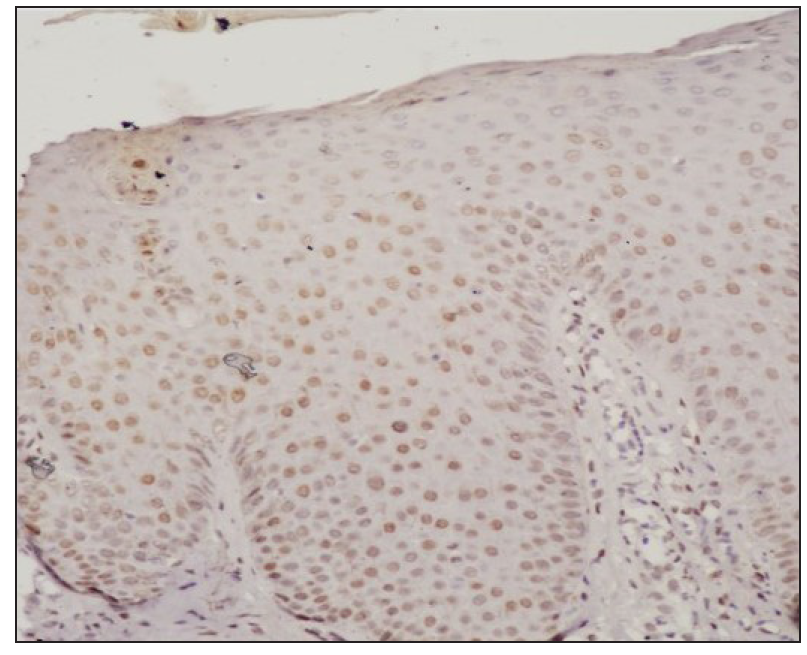
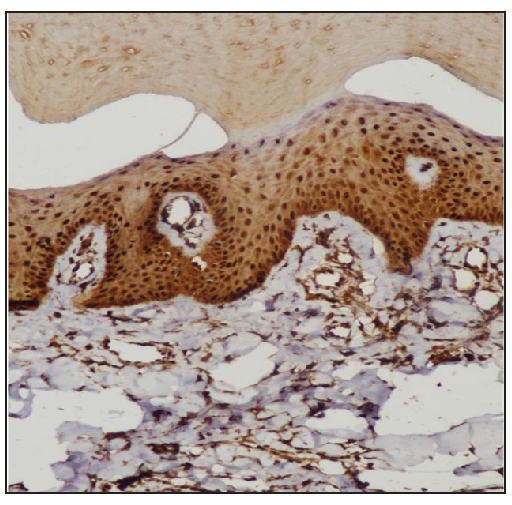
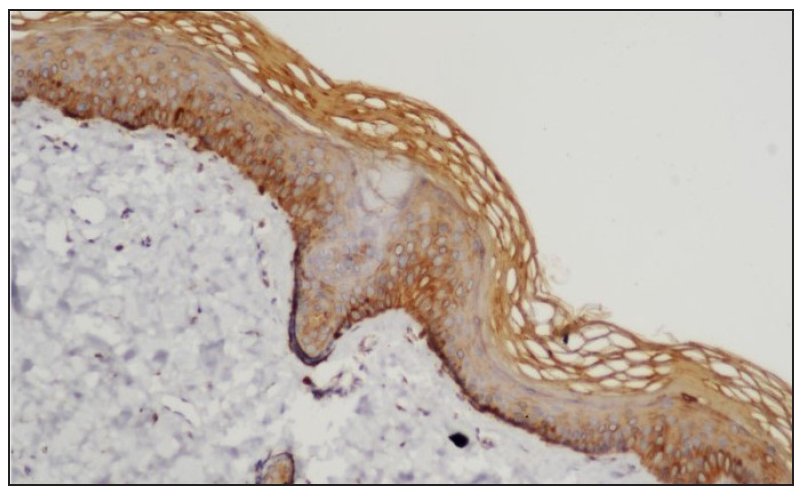
STAT expressionImmunohistochemistry analysis revealed significant expression of STAT6 and STAT3 in 13 and 10 patients, respectively, and STAT1 expression in 4 patients. [Figure 1, Table 2].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
Table 2: STAT1, 3 and 6 expression in prurigo nodularis
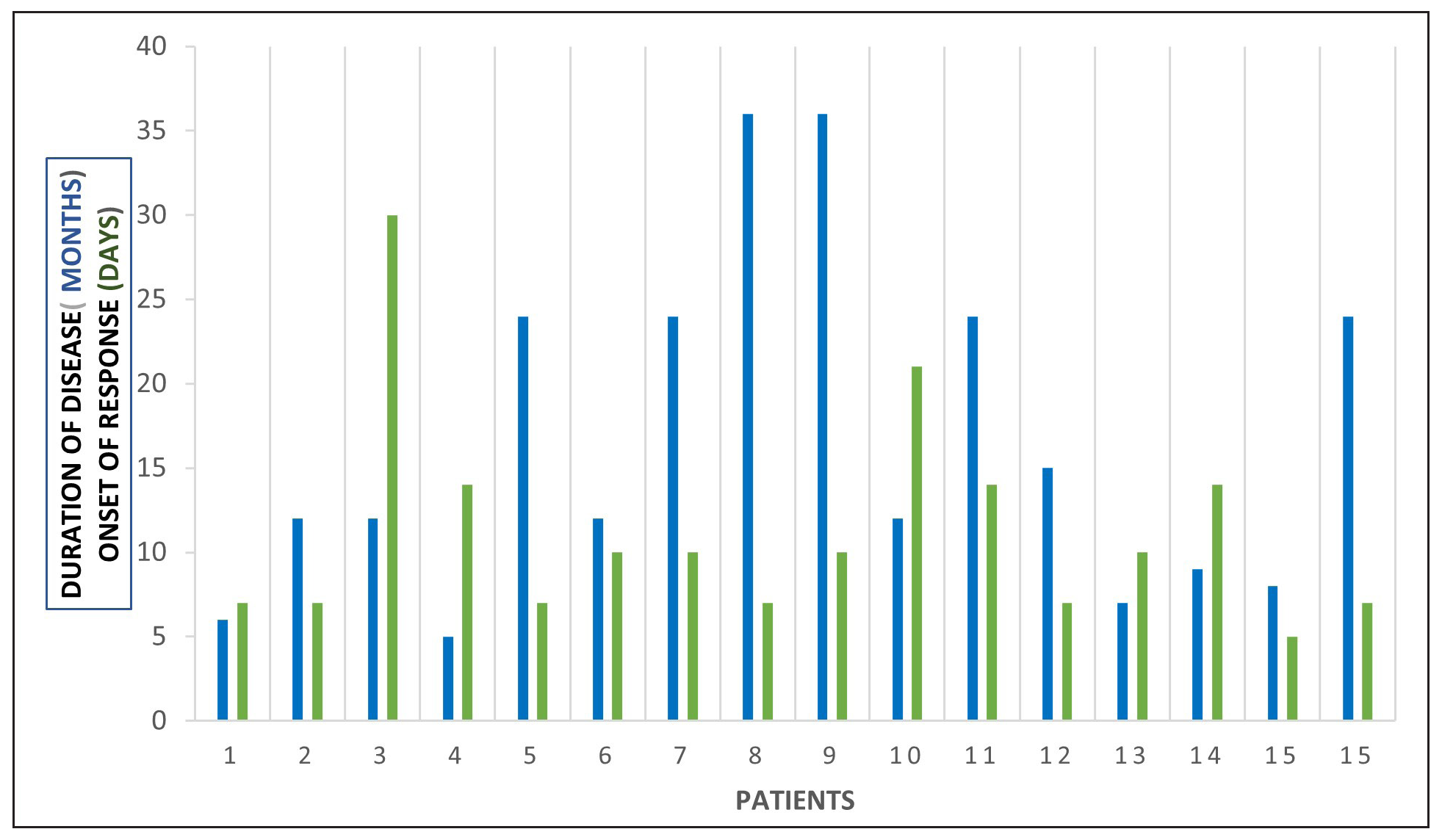
STAT Positive Negative Chi-square test P value* STAT6 13 (8.5) [2.38] 4 (8.5) [2.38] 4.3714 0.036546 STAT1 4 (8.5) [2.38] 13 (8.5) [2.38] STAT3 10 (7) [1.29] 7 (10) [0.9] 9.5294 0 .002022 STAT1 4 (7) [1.29] 13 (10) [0.9] Treatment with Systemic TofacitinibThe mean treatment duration was 5.6 ± 2.2 months, with a 50% reduction in pruritus observed after 11.2 ± 6.44 days [Figure 2]. No significant correlation was found between disease duration and treatment response (p=0.16). The final mean PGSS was 4.1 ± 1.1, reflecting a statistically significant reduction of 66.1% [Table 3, Figure 3]. At the time of data analysis, 14 of the 16 patients were in remission on low-dose therapy, while 2 had completed treatment. One patient who relapsed after discontinuing treatment had therapy restarted [Figure 4].
Export to PPT
Table 3: Efficacy of tofacitinib for the treatment of moderate to severe prurigo nodularis
Mean duration of treatment 5.6±2.2 months Mean onset of action 11.2±6.44 days Mean final PGSS 4.1±1.1 (P value 0.0004) Number of PN cases as per PGSS (post-treatment) Mild 14 Moderate 3 Severe 0 Remission on low-dose therapy 15 Remission off therapy 2 Relapse 1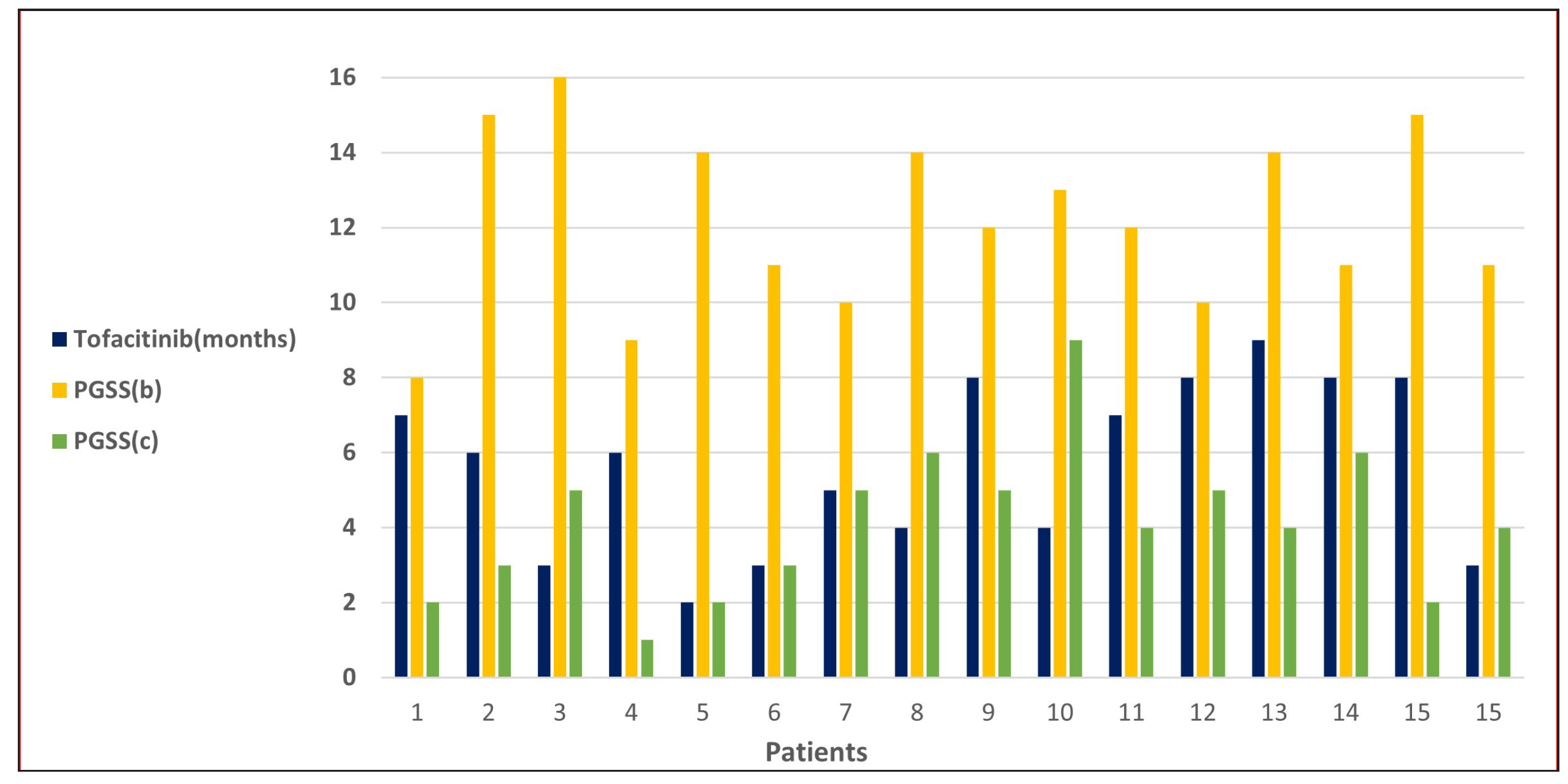
Export to PPT
Export to PPT
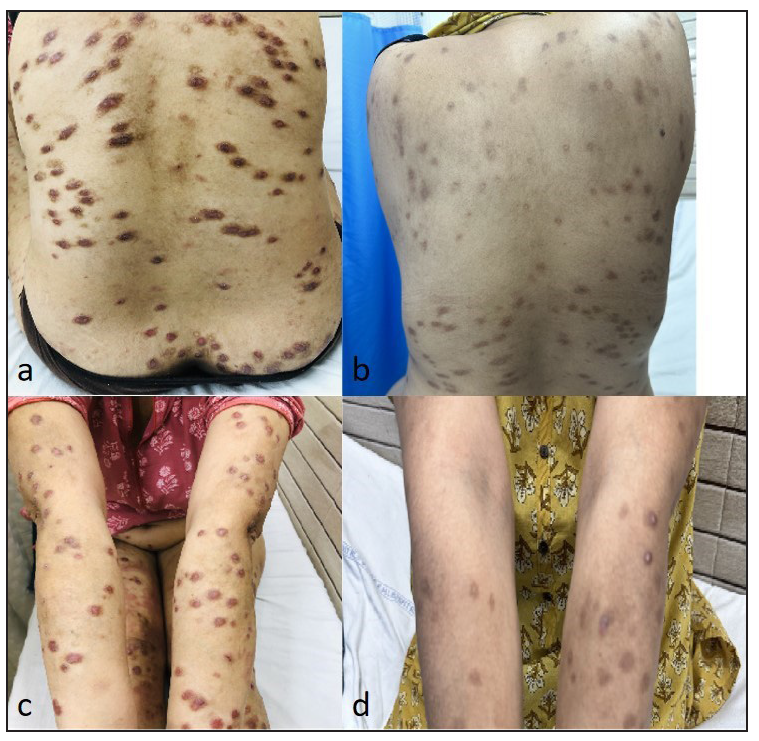
Side effects observed included mild elevations in transaminases (SGOT or SGPT) in two patients and increased triglycerides in one patient and a reduction in lymphocyte/neutrophil counts in another patient. Acneiform eruptions were seen in two patients, and varicella occurred in a single patient. Except for the patient with varicella in whom treatment was temporarily paused for two weeks, no other patient had their treatment discontinued due to side effects.
DiscussionWe observed increased tissue expression of STAT3 and STAT6 [Figure 1, Table 2]. STAT3 is activated by cytokines from the IL-6 and IL-10 families, as well as IL-21, IL-27, G-CSF, leptin, and IFN.8,9 STAT3 regulates the Th17 immune response, while STAT6 is primarily involved in the transduction of IL-4 and IL-13 signals.9,10 IL-4 is a key cytokine that mediates the differentiation of Th2 cells and immunoglobulin isotype switching.11,12 Additionally, STAT6 promotes the proliferation and maturation of B cells, enhances MHC-II and IgE expression, and plays a crucial role in mast cell activation. Our results explain the increased expression of mast cells and neural markers, suggesting STAT6 activation and are consistent with a previous study.1,6
Cytokines mediate their action through T helper cell subtypes, including Th1, Th2, Th17, and Th22, primarily via the JAK-STAT signaling pathway.9 These cytokines interact with cutaneous nerve fibers, keratinocytes, macrophages, mast cells, eosinophils and the JAK-STAT signalling pathway to shape the inflammatory response in PN. Interleukin (IL)-4 and IL-13 directly activate sensory neurons in the skin, driving itch sensation and enhancing the production of pro-inflammatory cytokines, IgE and fibroblasts through STAT6 signaling.11-13 IL-31, a Th2 cytokine, signals via the IL-31 receptor and oncostatin M receptor (OSMR) β to activate itch-sensing neurons, stimulate neuronal growth, and promote inflammatory cell activation.14,15 Additionally, IL-31 encourages fibroblast activity, drives T helper (Th) 2 polarisation and encourages further IL-31 production.16 While Th1, Th17, and Th22 cells play minor roles in inducing inflammation, IL-17 notably induces endothelin-1 (a histamine-independent pruritogen)17 while IL-22 facilitates epidermal differentiation, cutaneous inflammation, and keratinocyte proliferation.18
While a marked Th2 response is characteristic of PN, studies have shown that, unlike atopic dermatitis (AD), PN exhibits a strong fibrotic response in the stromal compartment, accompanied by abnormal activation of keratinocytes. This occurs alongside a relatively dampened type 2 inflammatory response compared to AD.19,20 Thus, the Th2 response in PN is less pronounced than in AD. Blocking IL-31Rα reduces both Th2/IL-13 and Th17/IL-17 responses, thereby stabilizing extracellular matrix remodeling.21
Our data suggest that JAK inhibitors can suppress the inflammatory activity of Th2/Th17 cells (STAT6/STAT3).22 Current treatment options for patients with PN are limited and many patients are dissatisfied with their therapy.21 The use of systemic tofacitinib has been largely speculative, with only a few reports addressing STAT expression.23 The cytokines involved, along with the STAT expression profile in PN, suggest that a treatment targeting multiple cytokines and immune cell pathways, such as JAK inhibitors, could offer a beneficial and potentially cost-effective approach to managing PN.24
Although most of our patients responded favorably to tofacitinib, the majority remained on low doses, suggesting that a maintenance dose may be necessary for patients with PN. A meta-analysis of 45 patients treated with dupilumab found that the mean time to first improvement was 10.15 ± 10.56 weeks, with final improvement achieved at 19.28 ± 13.71 weeks. Complete clearance of pruritus, when observed, occurred approximately four months after initiating dupilumab.23 However, our results suggest that tofacitinib is both more effective and acts more rapidly than dupilumab, with a 50% reduction in pruritus occurring in 11.2 ± 6.44 days and complete remission achieved on a low dose in 5.6 ± 2.2 months.
Published case reports have documented promising results with the use of tofacitinib (a JAK1/3 inhibitor), baricitinib (a JAK1/2 inhibitor), and upadacitinib (a JAK1 inhibitor) in the treatment of PN. Phase 2 clinical trials are currently investigating the efficacy of two other JAK1 inhibitors, abrocitinib (NCT05038982) and povorcitinib (NCT05061693), while a phase 3 trial is underway for ruxolitinib cream (a JAK1/JAK2 inhibitor) (NCT05755438, NCT05764161).25 Based on our findings, a pan-JAK inhibitor, such as tofacitinib, appears to be the most cost effective treatment option for PN.
Limitation and conclusionsOur study confirms the enhanced tissue expression of STAT3 and STAT6, which corresponds to the major cytokines involved in prurigo nodularis and in turn the therapeutic efficacy of a JAK inhibitor. The limitations of our work is that ideally tissue cytokine as well as JAK-STAT expression both prior to and following treatment with JAK inhibitors would be ideal, to validate the impact of tofacitinib on clinical outcomes and immune pathways. Nevertheless, the consistent reduction in itching, marked clinical improvement, and favorable safety profile suggest that tofacitinib holds significant potential for treating PN.
留言 (0)