Acne vulgaris affects approximately 9.4% of the global population, making it a significant public health concern.1 The primary pathogen associated with acne vulgaris is Cutibacterium acnes, a bacterium that plays a pivotal role in maintaining the skin’s microbiota balance, ultimately leading to the development of this chronic inflammatory skin disease.2–4
Over the years, antibiotics, both in topical and oral forms, have been the cornerstone of acne management. However, the extended and often indiscriminate use of antibiotics has led to the emergence of antibiotic-resistant C. acnes strains, posing a formidable challenge to the current treatment protocols. Recent research has shed light on the potential role of biofilms (the structured aggregates of bacterial cells) in conferring antibiotic resistance to C. acnes.5,6 Biofilm formation may act as a protective shield, limiting the penetration of antibiotics and exacerbating resistance issues.
In light of this concern, our study aims to investigate the ability of C. acnes to generate biofilms and elucidate their potential connection to antibiograms. By doing so, we seek to contribute to a broader understanding of the dynamics of antibiotic resistance in acne vulgaris and pave the way for innovative strategies that address C. acnes biofilms and their implications for treatment.
MethodsPatients with clinically diagnosed acne vulgaris satisfying the inclusion and exclusion criteria, and attending the dermatology outpatient department for 18 months from November 2020 to May 2022 at a Tertiary Hospital in South India, were considered for the study. Approval from the institution’s scientific and ethics council was taken.
Study design: Descriptive Cross-sectional study.
Selection criteriaPatients over the age of 12 years with acne vulgaris were considered for the study. Patients who had not been treated with topical or oral antibiotics in any form for at least four weeks were enrolled. Patients who were using oral or topical isotretinoin, hormonal medication, or any complementary and alternative medicine (CAM) therapy were excluded; patients diagnosed with hormonal acne were also excluded.
Each patient was subjected to a comprehensive general physical, systemic, and cutaneous examination, and data were recorded on a preformatted proforma.
A clinical diagnosis of acne vulgaris was made. Using the Investigator’s Global Assessment Scale System (IGA), the disease severity was defined and categorised as Grade 0 to 4.
Grade 0: Clear; Grade 1: Few comedones with few papule; Grade 2: Less than half the face is involved with many visible comedones and pustules; Grade 3: Involvement of more than half the face with visible comedones, papules, pustules and one nodule; Grade 4: The entire face is involved with comedones, papules pustules, nodules and cyst.
Sample collection and processingThe skin surface was cleaned with ethanol. The sample was collected according to the type of lesion. Comedones/pustules were extracted/expressed from the lesion using a sterile needle and placed in a thioglycolate transport medium. The sample was mixed for 30 seconds in a vortex mixer to disperse the bacteria. The vortex sample was inoculated on Propionibacterium selective agar, placed in a Becton and Dickinson (BD) GasPak EZ Sachet (Germany) (anaerobic gas generating pouch system with indicator) and incubated at 37ᵒC for 48 hours to isolate Cutibacterium. After incubation, the characteristic large white to yellow dry colonies were identified as Cutibacterium species based on Gram’s staining, colony morphology, and Matrix Assisted Laser Desorption Ionization – Time of Flight Mass Spectrometry (MALDI-TOF MS) (Bruker, Germany).
Upon completion of Cutibacterium species identification, only C. acnes colonies were subjected to antimicrobial susceptibility testing on Brucella Blood Agar in an anaerobic environment using a Becton and Dickinson (BD) GasPak EZ sachet by disc diffusion technique.
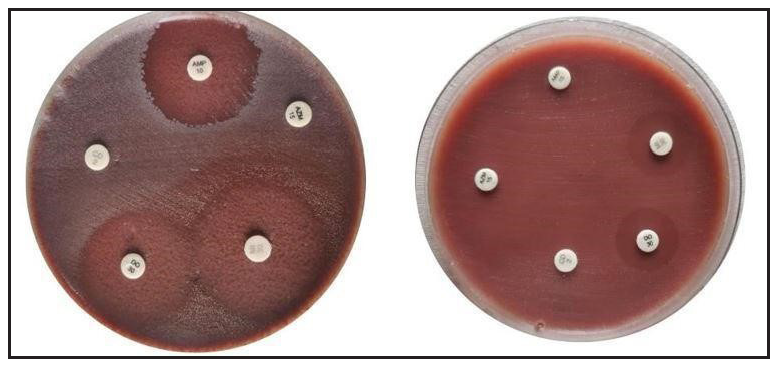
For susceptibility testing, the following antimicrobial agents were used: doxycycline (30 mcg/disc), minocycline (30 mcg/disc), azithromycin (15 mcg/disc), ampicillin (10 mcg/disc) and, clindamycin (10 mcg/disc) [Figure 1].
Export to PPT
Due to its lack of lipophilic properties, ampicillin is not routinely used to treat acne vulgaris. However, it has been used for antibiotic sensitivity testing in C. acnes strains because it is a commonly used beta-lactam antibiotic that can effectively target gram-positive bacteria, including some C. acnes strains. In addition, using a well-known and widely used antibiotic facilitates the comparison of results across studies.
Antibiogram – disc diffusion techniqueC. acnes were grown in Viande Levure (VL) broth, and the turbidity was adjusted to 0.5 McFarland before being applied to Brucella blood agar. On Brucella blood agar plates, the aforementioned antimicrobial discs were placed. After anaerobic incubation, the zones of inhibition on the plates were measured and compared with the Kirby-Bauer chart as established by the Clinical Laboratory Standards Institute (CLSI).7
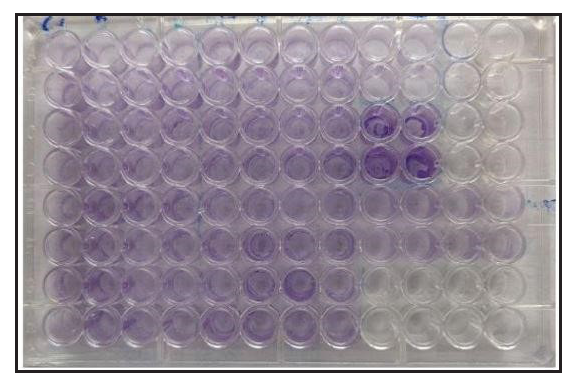
Biofilms – using microtitre plate assayStock isolates frozen at ‒80°C in glycerol (50%) were revived in Bacto Brain Heart Infusion broth supplemented with glucose (0.5%) in the Don Whitley Anaerobic Chamber for 48 hours at 37°C. Quantification of biofilm formation was performed using a microtiter plate assay with modifications based on the study by Holmberg et al.6
C. acnes was grown for 48 hours in the Brain Heart Infusion broth supplemented with glucose, and 50 μL of this culture was added to the wells of a sterile 96-well flat-bottomed plastic culture plate containing 150 μL of the medium and incubated anaerobically at 37°C for 48 hours. A biofilm-producing Staphylococcus epidermidis was used as a positive control on each plate, and the medium alone served as a negative control. Each isolate was tested in duplicate. The medium was removed, followed by gentle washing (three times) with 200 μL of phosphate-buffered saline (PBS), by pipetting.
The remaining biofilm was fixed in 200 μL of methanol for ten minutes and then stained in 160 μL of crystal violet (1% [w/v] in water, gram staining for microscopy) for 4 min. The wells were destained by pipetting 200 μL of PBS three times and extracted with 200 μL of acetone-ethanol (20:80 [v/v]). In an enzyme-linked immunosorbent assay (ELISA) reader, the absorbance was measured at 550 nm [Figure 2]. The cut off OD values for the study were as follows:
1.
>0.240 was considered Strong Positive.
2.
>0.120 to <0.240 was considered Moderate positive
3.
<0.120 was considered to be Weak positive.
Export to PPT
ResultsFifty-one per cent of patients belonged to the 21–30-year age group, while 45.5% were less than 20, with a mean age of 21 ± 4.45 years. Women constituted 71.6% of the patient population, while only 28.4% of patients were men. The female-to-male ratio was 2.5:1. The duration of acne varied from five months to six years.
The most frequently encountered severity of acne among the patients was Grade 3 (43.2%), followed by Grade 2 (31.8%) and Grade 4 (13.6%). The most prevalent skin lesions observed in these patients were pustules. C. acnes were isolated from 43.1% of the patients (N = 38).
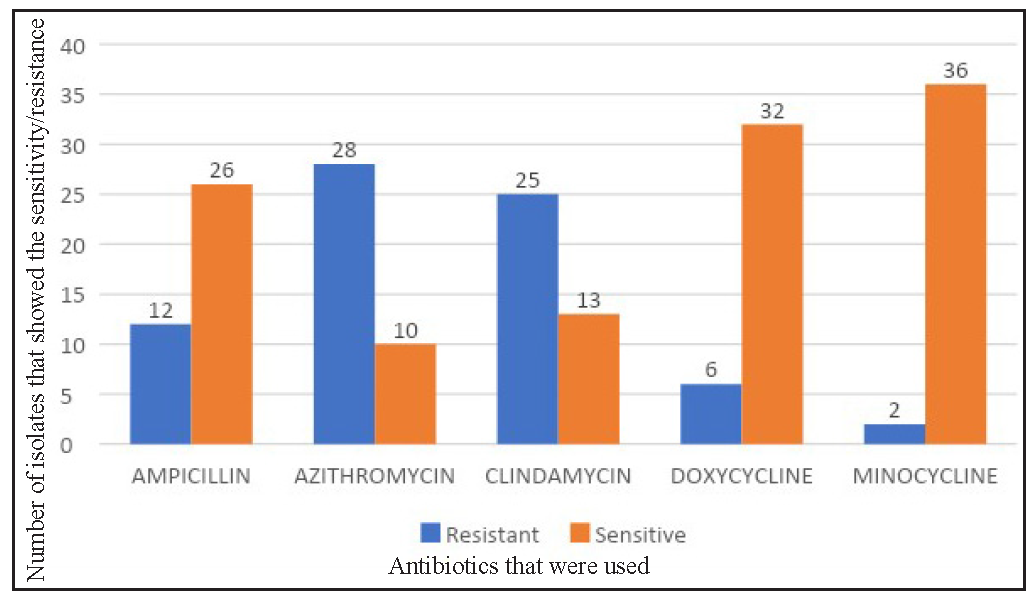
Seventy-four per cent of the isolates showed resistance to azithromycin. The second most common antibiotic to show resistance was clindamycin (65.8%), followed by ampicillin (31.6%), doxycycline (15.8%), and minocycline (5.3%). Minocycline showed the most sensitivity (94.78%) followed by doxycycline (84.2%). Only two isolates showed resistance to minocycline [Figure 3, Table 1].
Export to PPT
Table 1: Antibiotic susceptibility pattern of all antibiotics. This table highlights the sensitivity and resistance pattern of each antibiotic for 38 isolates
Antibiotic Sensitive Resistance Number Percentage Number Percentage Azithromycin (N=38) 10 26.3 28 73.7 Doxycycline (N=38) 32 84.2 6 15.8 Minocycline (N=38) 36 94.7 2 5.3 Ampicillin (N=38) 26 68.4 12 31.6 Clindamycin (N=38) 13 34.2 25 65.8An analysis of antibiotic susceptibility across different age groups, a distinct pattern was revealed for azithromycin. While most antibiotics exhibited comparable susceptibility profiles, azithromycin demonstrated significantly higher resistance (73.9%) in the 20 years and older age group compared to the under 20 group (73% susceptibility).
In our study, out of 38 isolates, 68.4 % of the isolates were resistant to two or more types of antibiotics, while 13.2% were resistant to only one antibiotic. Only 18 % of the samples were susceptible to all the antibiotics [Table 2 and Table 3].
Table 2: Representation of the number of isolates showing multi-antibiotic resistance on antibiotic naïve patients. (Total number of isolates = 38)
Number of antibiotics to which resistance was present Number of isolates showing antibiotic resistance Percentage distribution 0 7 18.4% 1 5 13.2 % 2 14 36.8% 3 8 21.1% 4 4 10.5% Total Isolates: 38Table 3: Highlighting the various combinations of antibiotics that the isolates had shown the resistance
Antibiotics No of Strains Percentage Azithromycin, Clindamycin 11 29% No Antibiotics 7 18% Ampicillin, Azithromycin, Clindamycin 5 13% Ampicillin, Azithromycin 3 8% Azithromycin, Clindamycin, Doxycycline 3 8% Clindamycin 2 5% Azithromycin 2 5% Ampicillin, Azithromycin, Clindamycin, Doxycycline 2 5% Ampicillin, Azithromycin, Clindamycin, Minocycline 1 3% Ampicillin 1 3% Azithromycin, Clindamycin, Doxycycline, Minocycline 1 3% Grand Total 38 100%The biofilm-forming capacity of the isolates was evaluated and compared to that of S. epidermidis, which served as a positive control.
The isolates were graded based on their capacity to produce biofilms as strong (S), moderate (M), and weak (W).
Sixty-three percent of C. acnes was found to have weak biofilm formation capability. As depicted in Table 4, only 37% of the isolates possessed a moderate ability to form biofilms which was much less than the percentage showing antibiotic resistance Thus we could conclude that the capacity to form biofilms is not necessarily indicative of the antibiotic resistance.
Table 4: Frequency distribution of biofilm capacity of C. acnes isolates
Biofilm capacity Number of isolates Percentage distribution (%) Moderate 14 36.8 Weak 24 63.2 Total 38 100More than 60% of the samples had resistance to more than two types of antibiotics and a weak capacity to form biofilms. There was no statistical significance between the biofilm-forming capacity and the number of antibiotics an isolate was resistant to (P = 0.761) [Tables 2 and 4]. Consequently, this study indicates that the capacity for biofilm formation is not necessarily proportional to the number of antibiotics to which a sample is resistant.
DiscussionAntibiotics have been the cornerstone of acne treatment for decades, working primarily in two ways: first, as an anti-inflammatory agent and second, as an antimicrobial agent.8 Antibiotics must possess lipophilic qualities to penetrate the lipid-rich sebaceous gland.
Global reports have indicated shifts in antibiotic susceptibility patterns, with the prescribing practices of medical professionals significantly shaping the landscape of antibiotic resistance. Incorrect dosages, inadequate treatment durations, and prescription practices can contribute to the emergence of antibiotic resistance.
In our study, we found that minocycline and doxycycline were the antibiotics with the most sensitivity followed by ampicillin, clindamycin, and azithromycin.
An intriguing pattern emerged in our investigation: 73.7% of bacteria were resistant to Azithromycin, 65.8% were resistant to clindamycin and nearly 29% were resistant to both. Research by Ross et al. (2003) across Europe revealed that Spain had the highest levels of resistance to clindamycin and erythromycin.9 In 2016, a study conducted by Sardana et al, reported 100% resistance to azithromycin, 98% to erythromycin, 90.4% to clindamycin, and 44.2% resistance to doxycycline, with minocycline showing the least resistance.10 In Malta, a study by Mercieca et al. (2020) discovered 47.4% population had resistance to azithromycin.11 Notably, in both domestic and international studies, minocycline regularly emerges as the most sensitive drug [Table 5]. Numerous antibiotic susceptibility studies indicate an evolving global pattern of antibiotic resistance. However, analysing these researches necessitates consideration of limitations, such as the difficulty in comparing data due to the variations in methodologies used for susceptibility studies.
Table 5: Prevalence of antibiotic resistance in acne – worldwide. Comparing the present study with the antibiotic resistance pattern published globally
Prevalence of antibiotic resistance in acne – world-wide comparison (%) Study Location Clin Doxy Mino Azi Amp Mercieca et al (2020)11 Malta 42.1 5.3 0 47.4 NS Rodriguez-Cavallini et al. (2004)22 Costa Rica 23 NS NS NS 27 Schafer et al. (2013)23 Chile 7.5 0 NS NS NS Mendoza et al. (2013)24 Colombia 15 9 1 NS NS Galvan Perez et al. (2002)25 Spain 51.8 NS 2.3 NS NS Ross et al. (2003)9 Spain 90 NS 0 NS NS Greece 75 NS NS NS NS Hungary 40 NS NS NS NS Italy 58 NS NS NS NS UK 50 NS NS NS NS Sweden 45 NS NS NS NS Bettoli et al. (2006)26 Italy 37.6 NS 1.1 NS NS Dumont-Wallon et al. (2010)27 France NS 100 NS NS NS Tan et al. (2007)1 Singapore 7.5 3.4 1.7 NS NS Ishida et al. (2008)28 Japan 8.3 NS 0 NS NS Zandi et al. (2011)29 Iran 43 NS NS NS NS Luk et al. (2013)30 Hong Kong 53.5 16.3 16.3 NS NS Sardana et al. (2016)10 India 90.4 44.2 1.9 100 NS Nakase et al. (2017)31 Japan 38.6 0 0 44.3 NS Zhu et al. (2019)32 China 55.5 1.3 NS 58.6 NS Our Study (2024) India 65.8 15.8 5.3 73.7 31.6The sampling techniques used in different investigations vary greatly, with some using expressed material and others using surface swabs. Lack of standardized data collection protocols, sampling methods, and antibiotic susceptibility testing, make cross-study comparisons difficult.
While hyperseborrhoea, hyperkeratinisation, sebaceous gland follicle occlusion, and C. acnes colonisation have historically been viewed as pivotal in acne pathogenesis, recent years have systematically challenged this notion.12
Acne vulgaris is increasingly being connected to the interaction of skin bacteria and host immunity.13 Despite the lack of a direct link between C. acnes and acne, addressing C. acnes remains a key component of acne treatments. Antibiotics have been a mainstay due to their combination of bactericidal and anti-inflammatory effects. Antibiotics, both topical and systemic, are routinely utilised and are normally prescribed for three to four months.14–15
Antibiotic overuse has resulted in therapeutic failures, treatment-resistant acne, impaired skin microbiome, and increased opportunistic infection risks. In the 1970s, Leyden et al. addressed antibiotic resistance concerns in acne vulgaris.16
Antibiotic resistance in acne must be considered when experiencing diminished responses, absence of response, or relapses. Early detection of antibiotic failure is critical for reducing needless and excessive antibiotic use.
Recent research attributes C. acnes antibiotic resistance primarily to biofilm formation and genetic/biochemical plasticity.
Biofilms are protective matrices made up of extracellular polysaccharides, proteins, lipids, and DNA that allow bacteria to survive in harsh environments.17
Burkhart and Burkhart established the concept of biofilms in acne in 2007.18–20 Subsequent research revealed that C. acnes biofilms stimulate proliferation, improve follicular adherence and virulence and increase pro-inflammatory and lipase activity. However, evidence of its clinical application remains limited.21
In our study, we assessed isolates’ antibiotic susceptibility alongside their biofilm-forming capacity. Comparatively, only 63.2% displayed weak biofilm-forming ability against S. epidermidis biofilm forming ability as control. Holmberg et al. and Mongaret et al separately demonstrated deeper infections to possess greater biofilm-forming potential than commensal acne-derived isolates.6,22
Interestingly, four isolates demonstrated resistance to four distinct antibiotic classes. Of these, two isolates exhibited weak biofilm-forming capabilities, while the remaining two displayed moderate biofilm formation capacity. Correlation analysis between antibiotic resistance and biofilm formation capacity revealed no statistically significant association.
LimitationsThe study is a single centre study and it is limited by its sample size. Long-term follow-up was not done; hence the correlation between the antibiotic resistance and treatment outcomes has not been identified. The antibiogram performed in this study is only for C. acnes. This data is not representative for other species, such as S. epidermidis.
ConclusionPatients with acne vulgaris have historically relied on antibiotics as their primary therapeutic choice. Antibiotic resistance has emerged as a result of its prolonged and irrational usage.
It is concerning that the global pattern of antibiotic resistance is constantly evolving. The absence of standardized sample collection and isolation protocols can further contribute to the heterogeneity between research.
A significant amount of research has been done on antibiotic resistance in acne. One of the possibilities that were proposed centred on the formation of a biofilm by C. acnes. The potential of C. acnes to generate biofilms has been demonstrated; however, our findings suggest that its role in acne is not significant.
Due to the restricted ability of the cutaneous C. acnes to form biofilms, its contribution to antimicrobial resistance in acne vulgaris is likely to be modest or limited. It is important to consider alternative mechanisms such as genetic or biochemical plasticity that may contribute to antibiotic resistance.
留言 (0)