Progressive right ventricular (RV) failure is a frequent long-term sequela following the complete surgical repair of tetralogy of Fallot (rTOF), contributing to substantial morbidity and mortality as patients move into adolescence and adulthood. The mechanism for this failure was initially presumed to be severe pulmonary valve insufficiency, acquired during the relief of the pulmonary outflow obstruction. The chronic nature of the severe pulmonary insufficiency is then presumed to lead to progressive RV dilation, and eventual RV dysfunction similar to the mechanism of aortic insufficiency leading to left ventricular dysfunction in adults (1). While pulmonary valve replacement to restore pulmonary valve competency in later childhood or early adulthood improves RV dilation, this intervention fails to fully resolve RV dysfunction and decrease mortality (2–4). The persistence of RV dysfunction in the setting of subsequently acquired pulmonary valve competency therefore raises the important question of whether RV dilation is the sole mechanism for dysfunction.
Post-operative right bundle branch block (RBBB) is also a common sequela of rTOF, and its degree of prolongation is independently associated with RV dilation and risk of sudden death (5–7). A growing body of research has noted an association between RV electromechanical dyssynchrony (EMD) in rTOF patients and adverse RV remodeling, worse RV systolic function, decreased exercise capacity, and even atrial arrhythmias independent of the degree of pulmonary valve insufficiency (8–10). The combination of electromechanical dyssynchrony in the setting of coexisting RBBB is present in most adolescent and adult patients after rTOF (9, 11–13). However, it is unclear whether dyssynchrony is present at the onset of development of RBBB in patients with rTOF, or if dyssynchrony develops as its own sequela of chronic RBBB or pulmonary valve insufficiency.
Investigations into left ventricular dyssynchrony using speckle tracking strain analysis in adult patients, specifically identifying and defining classic pattern dyssynchrony (CPD), have proven clinically useful by (1) specifically identifying dyssynchrony due to left bundle branch block; (2) being reproducibly and distinctly different from other uncoordinated strain patterns due to scar, surgical incision, and changes in loading conditions (14); and (3) predicting better response to cardiac resynchronization therapy (CRT) in dyssynchrony-induced heart failure (15, 16). CPD also has been seen in subgroups of patients across the spectrum of congenital and acquired pediatric heart disease (17–20).
Insight into a connection between electrical conduction delay, such as bundle branch block or classic pattern dyssynchrony, and dyssynchrony-induced heart failure has been previously best understood through examining the outcomes of adult patients undergoing transcatheter aortic valve replacement who develop left bundle branch block as an immediate and persistent post-procedural outcome (21). We therefore hypothesize that patients with rTOF will have the similar immediate development of dyssynchrony, but in the right ventricle, in association with the acute acquisition of RBBB during the surgical repair.
Methods Study cohortPre- and post-operative ECGs and transthoracic echocardiograms with RV strain analysis were compared in 20 subjects from a single center. Inclusion criteria were a diagnosis of TOF with prograde pulmonary blood flow and complete surgical repair in infancy. A complete surgical repair was defined as ventricular septal closure without intentional fenestration and relief of pulmonary outflow obstruction with or without a transannular patch or pulmonary arterioplasty. Patients with TOF-pulmonary atresia and TOF-absent pulmonary valve were excluded, as were TOF patients requiring any preceding palliative procedure or unable to be completely repaired in a single surgery. This study was approved by the Duke Institutional Review Board and informed consent was obtained for all subjects.
Electrocardiography protocol12-lead electrocardiograms (ECGs) were performed on all subjects before and after complete repair and reviewed by a pediatric electrophysiologist (ZZS). QRS duration was measured in leads II, V1, and V5, and averaged for the final reported QRS duration. Right bundle branch block was defined as a QRS duration longer than the upper limit of normal for age, with new rightward deviation of the QRS axis, and characteristic QRS morphology in lead V1 (rsR', R, qR, Qr) (22).
Echocardiography protocolTransthoracic echocardiograms and RV strain pattern analyses were performed on all 20 subjects before and after complete surgical repair. All echocardiograms were performed free breathing and without inotropic support. All post-surgical imaging and analyses were performed prospectively on a GE Vivid E9 or E95 (GE, Vingmed Ultrasound, Horten, Norway).
Two-dimensional imaging was optimized for longitudinal 2D speckle tracking strain analysis using a 6 or 12 MHz ultrasound probe from three apical RV views selected to evaluate three different opposing walls for dyssynchrony, analogous to the three standard apical LV views as previously described (23). Examples of these views and the RV walls imaged are shown in Figure 1. These post-operative echocardiograms were compared with pre-operative echocardiograms in all subjects. An identical prospective protocol was used to obtain the pre-operative echocardiograms on subjects enrolled prior to their surgical repair (n = 10). For subjects referred from outside institutions and enrolled at the time of surgical repair (n = 10), retrospective strain analysis was performed on clinically ordered pre-operative echocardiograms. These retrospective echocardiograms were acquired on a Vivid E9 (GE) or a Phillips IE33 or Epiq imaging system (Phillips Medical Systems, Andover, MA) and had a single apical RV view (RV focused four-chamber view) per the standard clinical protocol. The primary goal of the pre-operative studies was to exclude the presence of any RV electromechanical dyssynchrony prior to surgery.
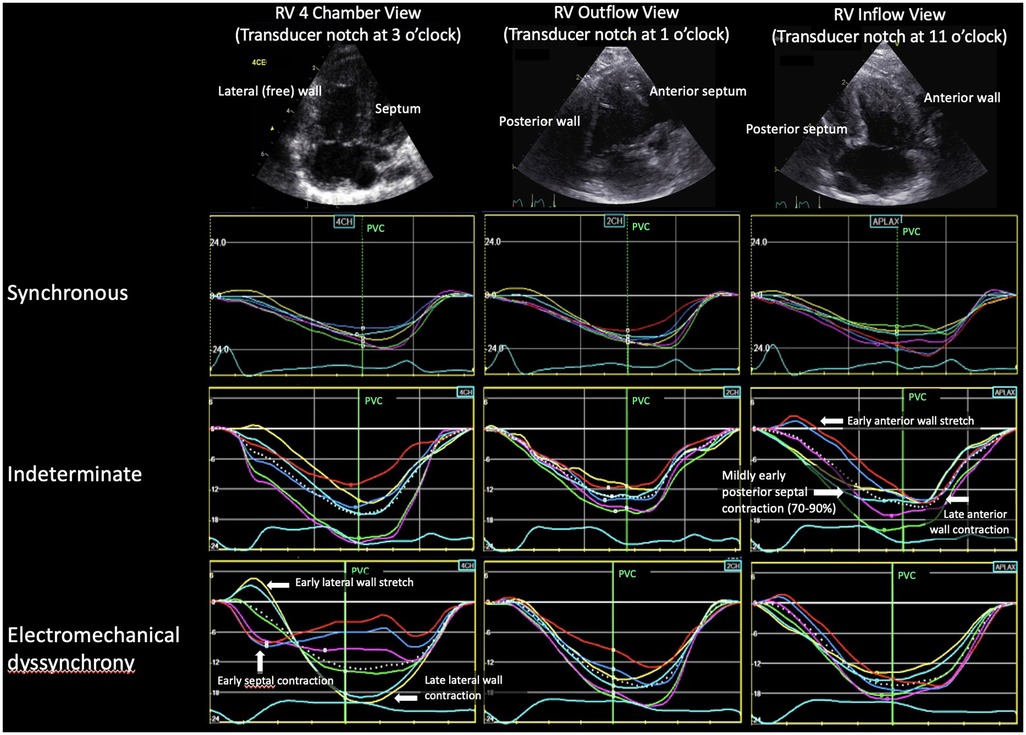
Figure 1. Example of the three strain patterns for each RV view. In the RV 4 chamber and outflow views, the septal strain segments are red, dark blue, purple and the lateral wall strain segments are yellow, light blue, green. Because the notch is at 11 o'clock for the RV inflow view, the colors are reversed. RV, right ventricle; PVC, pulmonary valve closure.
2D RV strain protocolStrain analysis for all prospective studies was performed off-line in GE EchoPac PC version BT13, while retrospective analysis was performed using vendor neutral software (Tomtec, Image Arena 2D Cardiac Performance Analysis version 1.2, Tomtec Imaging Systems, Unterschleissheim, Germany). The endocardial border was traced in end-systole from the lateral tricuspid valve annulus to the tip of the septal crest (excluding the VSD patch region), and the region of interest was adjusted to exclude the pericardium, papillary muscles, and chordal apparatus. The integrity of speckle-tracking was evaluated by the software and visually confirmed by the reader. In the case of poor tracking, the tracing and shape of the region of interest were adjusted by the reader. Mid-myocardial RV global longitudinal peak strain (RV GLS) was calculated as the average of the GLS from each of the RV views (except for the retrospective cases which were calculated using the four-chamber RV view only). Segments without adequate tracking on repeated attempts were rejected and views with more than two rejected segments were excluded from the analysis.
Strain pattern analysisRV longitudinal strain pattern analysis from all studies was analyzed independently by two experienced investigators (DF, JK). For analysis timing, tracings were set to start at the onset of the QRS. In Figure 1, pulmonary valve closure (PVC) relative to the QRS complex was defined on a spectral Doppler tracing of pulmonary outflow. The definitions for strain pattern analysis categories (synchronous, electromechanical dyssynchrony, and indeterminate) are presented in Table 1.
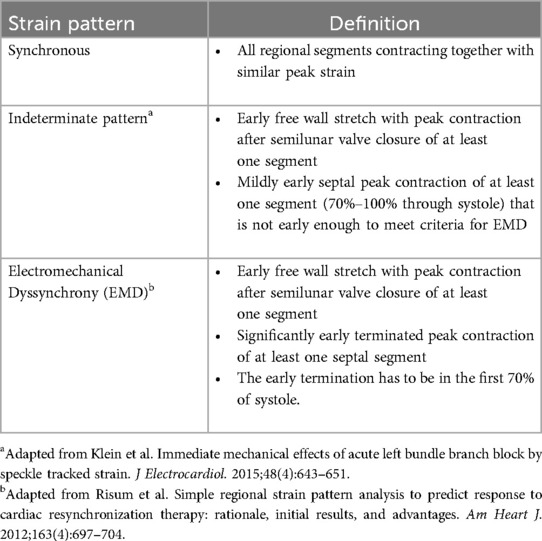
Table 1. Definitions of RV strain pattern analysis.
Statistical analysisNonprobability consecutive sampling was used for cohort selection. Standard summary statistics were used to compile data including medians with interquartile range or means with standard deviations for continuous variables and percentages for discrete variables. The cohort was summarized by clinical, electrocardiographic, and echocardiographic variables. Observed frequencies and continuous variables were assessed using bivariate testing including t-tests and Fisher's exact tests. Comparisons between three groups were analyzed with one-way analysis of variance (ANOVA) test.
ResultsThe median age at time of surgery for the 20 TOF subjects was 87 days (IQR 51–150 days). One of two surgeons performed all procedures. All VSDs were repaired via transatrial approach with mostly a bovine pericardial patch (15 of 20; remainder were synthetic polyester fiber patches). Clinical characteristics are summarized in Table 2 with pre- and post-operative strain and ECG measurements summarized in Table 3. Prior to surgery, all subjects had a normal QRS duration, normal RV function and synchronous RV strain patterns. The majority of subjects (15/20) underwent complete TOF surgical repair with a transannular patch; the remainder (5/20) had TOF repair with a valve sparing technique and muscle bundle resection.
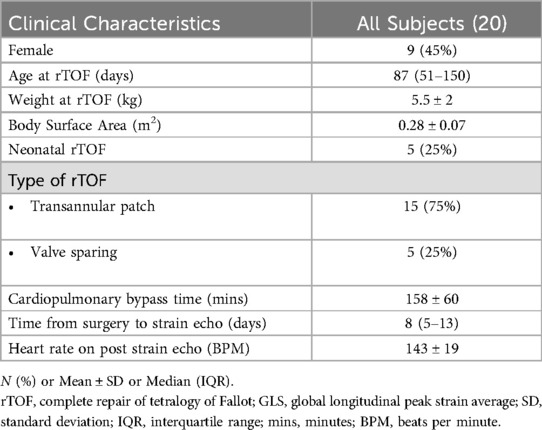
Table 2. Clinical characteristics.
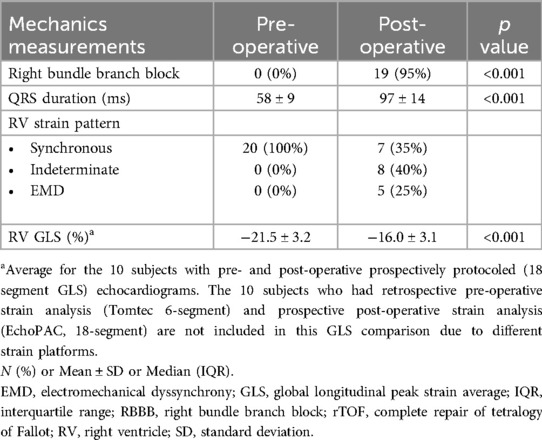
Table 3. Pre- and post-operative measures.
Electrocardiograms performed immediately following surgery (postoperative day 0) were abnormal in almost all subjects (19/20, 95%) with a prolongation in QRS duration meeting criteria for RBBB. The final subject met criteria for RBBB with QRS prolongation by 3 months after surgery. Post-operative echocardiograms were performed a median of 8 days (IQR 5–13 days) following surgery. Strain patterns remained synchronous in 7 subjects, newly met criteria for EMD in 5 subjects, or shifted to an indeterminate pattern in 8 subjects as shown in Table 4. The post-rTOF RV GLS was diminished across the entire cohort (RV GLS −16 ± 3.1%) with the most preserved RV GLS present in subjects who maintained synchronous RV mechanics (−17.7 ± 2.9%), followed by the indeterminate group (−16.5 ± 2.4%), and the EMD group (−12.9 ± 2.4%). When comparing the EMD vs. non-EMD groups, EMD was associated with lower RV GLS (p = 0.006) while there was no difference in age, weight, surgical repair type, post-rTOF QRS duration, and pre-rTOF RV GLS. No RV views were excluded from analysis due to poor tracking. Both readers independently agreed on the strain pattern in all subjects.
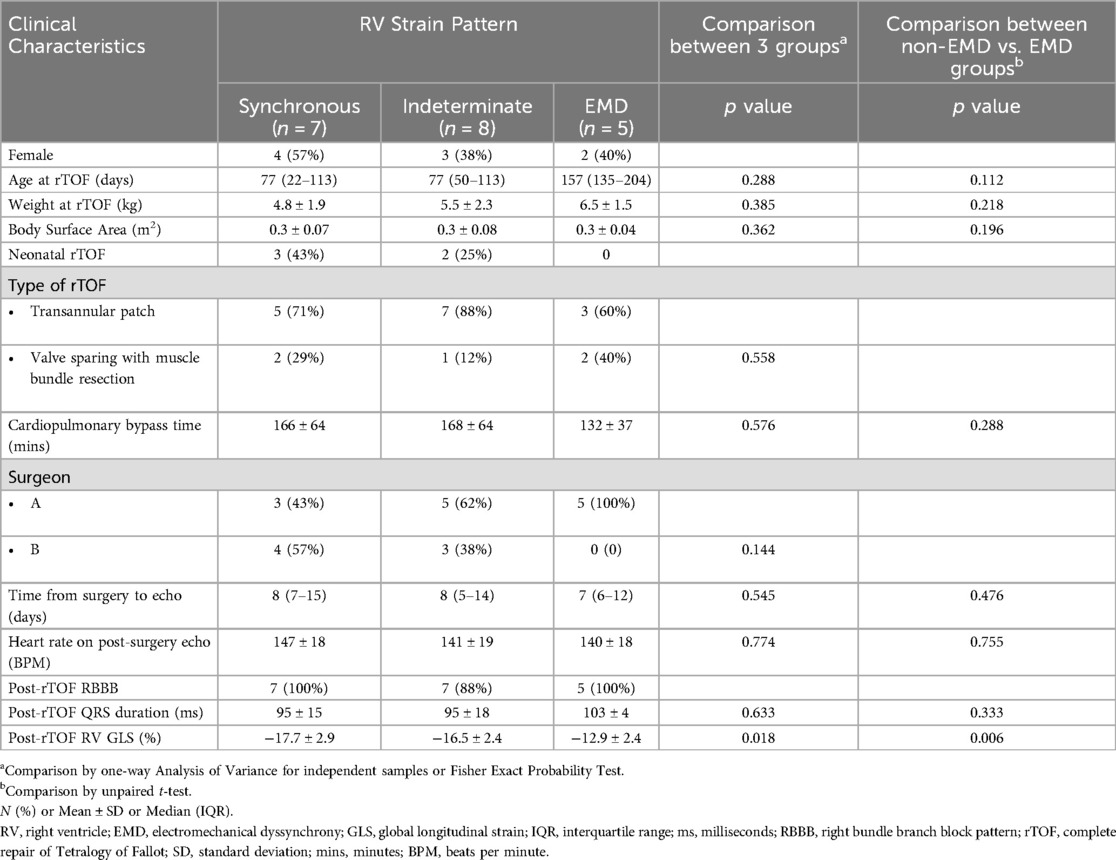
Table 4. Characteristics by RV strain pattern.
Evidence of dyssynchrony (EMD or indeterminate patterns) was most frequently seen in the RV 4-chamber and inflow views (Figure 1). Among the five subjects with EMD, the pattern was seen in both the RV 4-chamber and inflow views in two subjects, only the 4-chamber view in two subjects, and only the inflow view in one subject. Among the eight subjects who developed an indeterminate pattern, dyssynchrony was seen in both the RV 4-chamber and inflow views in six subjects and only in the inflow view in two subjects. Dyssynchrony was not seen in the outflow view.
Discussion SummaryDespite minimal difference in RBBB pattern or QRS duration on surface ECGs, different phenotypes of RV conduction were present immediately following surgical repair of TOF in infants with normal RV synchrony prior to surgery. 25% of subjects met criteria for EMD, with diminished RV function by GLS compared to the rest of the cohort. 40% of subjects demonstrated abnormal RV mechanical activation but failed to meet criteria for EMD. Finally, 35% of subjects maintained synchronous mechanical activation of the right ventricle, though ECG still demonstrated the delayed electrical activation of RBBB. In light of recent literature suggesting that the risk of RV failure in late childhood or early adulthood is more dependent on the presence of RV electromechanical dyssynchrony than pulmonary regurgitation in rTOF, the presence of these distinct patterns, from synchronous to EMD, in subjects immediately following surgical repair provides novel insight into the etiology of this process (8).
Variations in RV conduction pathwaysIn electromechanical dyssynchrony, an initial electrical activation delay progresses to electromechanical dyssynchrony over time, and the location of right bundle branch injury may affect this progression. Previous histological analyses of the conduction system in specimens with TOF have identified a variety of RV conduction phenotypes, depicted in Figure 2, which vary in their pathways around the ventricular septal defect and through different muscle bundles (24). These variations in conduction patterns as well as in surgical approach for rTOF may result in different levels of injury to the right ventricular conduction system, giving way to different patterns of RV electromechanical activation. Supporting this hypothesis, work from the 1970s and 1980s with epicardial and endocardial mapping of pre- and post-surgical repair of ventricular septal defects (mostly TOF) demonstrated that different sites of electrical block lead to variation in RV activation, despite a similar RBBB pattern on surface ECGs (25, 26).
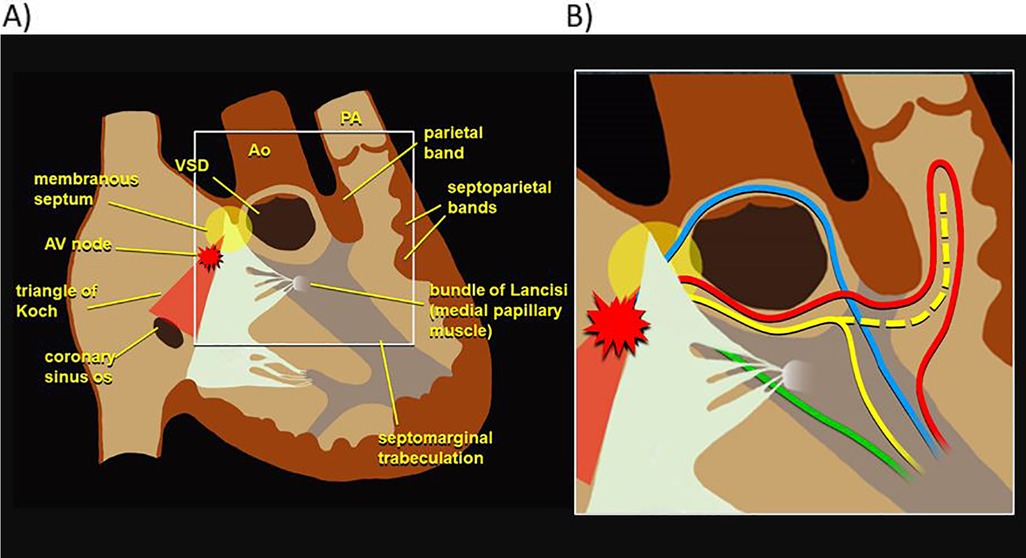
Figure 2. Phenotypic variation in RV electrophysiology. (A) Diagram of right ventricular anatomy. (B) Various RV conduction phenotypes, with each color depicting a different possible pathway of the right bundle as reported in previous histological analyses of specimens with tetralogy of Fallot (24). RV, right ventricle.
Location of right bundle branch injuryRecognizing the variation in right bundle branch patterns as well as surgical techniques may yield insight into the different electromechanical phenotypes seen postoperatively. A more proximal injury to the right bundle, presumably near the site of the ventricular septal defect may produce a delayed but more synchronous mechanical contraction as the ventricle is activated through cell-to-cell activation. In contrast, preservation of the proximal right bundle with injury to a distal ramification of the bundle could lead to differential timing of ventricular activation and thus mechanical dyssynchrony. Previous work has shown that injury in a portion of the terminal ramifications of the right bundle through outflow tract muscle bundle resection and/or ventriculotomy would result in delay in segments of RV free wall with intact and unchanged condition to the apex and septum (27–30). All subjects in this study had either right ventriculotomies or significant muscle bundle resection, but electrical mapping was not performed, so they cannot be differentiated based on our data. However, differences in the site of the bundle injury may explain why some subjects retained a synchronous contraction pattern in the setting of electrical delay while others clearly met criteria for EMD. A future study combining electrical mapping of the right bundle branch with echocardiographic strain analysis of RV mechanics in infants undergoing TOF repair will provide important additional insights into this process.
Effects of electromechanical dyssynchronyRV prestretch duration and basal lateral-midseptal delay have been shown to be independently associated with RV dysfunction in rTOF patients, and those same maladaptive contraction patterns, which are also present in patients with Ebstein's anomaly, have correlated with worse RV function and exercise tolerance (9, 31). This suggests that the activation patterns identified in this study may hold predictive value for clinical outcomes in this patient population. Moreover, ventricular-ventricular interactions must be considered when analyzing the effects of RV dyssynchrony, as RV dysfunction leads to LV functional abnormalities, emphasizing the importance of preserving synchronous mechanical contraction even in the setting of global electrical delay in rTOF (10, 32, 33). Although post-operative ECGs showed comparable patterns of RBBB in 95% of subjects in this study, the variable timecourse of progression to electromechanical dyssynchrony is demonstrated with only 25% of subjects meeting criteria for EMD on the post-operative study. In fact, mechanically synchronous contraction patterns were maintained post-operatively in 35% of subjects, despite clear electrical delay on ECG. The remaining 40% of subjects demonstrated altered mechanical contraction patterns on strain analysis after surgical repair that did not fully meet criteria for EMD. These “indeterminate” patterns may represent a transitional stage during which time the RBBB starts to impact RV mechanics, but the septal contraction is not yet completely terminated by the late freewall contraction. Alternatively, this pattern could represent transient RV dysfunction and dispersion related to perioperative factors and requires further study. Duration from RBBB to complete EMD may vary as it does for LBBB in adults with normal anatomy. With this in mind, perhaps the surgical techniques for repairing TOF ought to be reevaluated, since they have remained relatively unchanged for the last fifty years despite TOF persisting as the most common cause of cyanotic heart disease in children. For example, intraoperative electrophysiologic mapping could be used to inform surgical planning when repairing TOF. If interference into the RV conduction system is unavoidable during surgical repair, perhaps transecting the conduction pathway proximally, inducing delayed but near simultaneous mechanical activation, would preserve RV synchronous contraction patterns. However, even with RV synchronous mechanical contraction, electrical delay of the RV with respect to the LV may prove to be consequential. A longitudinal study analyzing biventricular synchrony and function in these patients would be needed to better understand the progressive development of electromechanical dyssynchrony. If avoidance of RBBB is not possible, resynchronization therapy may be a useful intervention for RV electromechanical dyssynchrony, as it has demonstrated consistent improvements in left ventricular ejection fraction and QRS duration in patients with rTOF (34).
LimitationsIn this study, the relatively small sample size did not provide adequate power to detect differences that may have clinical and predictive importance for these infants. Not all pre-operative echocardiograms were performed with a prospective protocol on the GE platform. As the primary goal for the pre-operative echocardiograms was to confirm RV synchrony on the strain pattern analysis prior to surgery, this does not impact the study's aim, and all post-repair studies were prospectively protocoled studies. However, this did limit our ability to compare pre- and post-rTOF GLS; any report of GLS data directly comparing pre- and post-repair values are confined to the 10 subjects enrolled prior to surgical repair who had prospectively protocoled studies at both time points (Table 3). Further, this study relied on pattern analysis from experienced imagers. Time to peak data to assess mechanical dispersion was not captured, but should be considered for future investigations into similar cohorts. Additionally, in the subjects who appeared to maintain synchronous contraction patterns on RV strain analysis, it is possible that there is dyssynchrony present that was simply not well visualized from these views. Lastly, as there was only a single post-operative time point, we were not able to comment on the evolution of the patterns over time or the association of EMDwith progressive RV failure and clinical symptoms seen in older TOF patients.
ConclusionsThough electrocardiographically similar, various patterns of RV mechanical activation were seen immediately following TOF repair. These activation patterns may reflect right bundle variation as well as surgical technique. Given the known progression to RV dysfunction and failure in TOF patients with electromechanical dyssynchrony, renewed investigation is needed into the preservation of the right bundle or its prescriptive transection.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Duke Health Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributionsAM: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. SC: Visualization, Writing – original draft, Writing – review & editing. ZS: Data curation, Formal Analysis, Investigation, Writing – review & editing. PK: Data curation, Investigation, Writing – review & editing. PB: Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing. JK: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. DF: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a research grant from Medtronic (Minneapolis, Minnesota).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Henry WL, Bonow RO, Rosing DR, Epstein SE. Observations on the optimum time for operative intervention for aortic regurgitation. II. Serial echocardiographic evaluation of asymptomatic patients. Circulation. (1980) 61(3):484–92. doi: 10.1161/01.cir.61.3.484
PubMed Abstract | Crossref Full Text | Google Scholar
2. Ferraz Cavalcanti PE, Sá MP, Santos CA, Esmeraldo IM, Escobar RR, Menezes AM, et al. Pulmonary valve replacement after operative repair of tetralogy of Fallot. J Am Coll Cardiol. (2013) 62(23):2227–43. doi: 10.1016/j.jacc.2013.04.107
PubMed Abstract | Crossref Full Text | Google Scholar
3. Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, et al. Randomized trial of pulmonary valve replacement with and without right ventricular remodeling surgery. Circulation. (2010) 122(11_suppl_1):S201–8. doi: 10.1161/circulationaha.110.951178
PubMed Abstract | Crossref Full Text | Google Scholar
4. Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, et al. Pulmonary valve replacement in tetralogy of Fallot. Circulation. (2009) 119(3):445–51. doi: 10.1161/circulationaha.108.775221
PubMed Abstract | Crossref Full Text | Google Scholar
5. Gelband H, Waldo AL, Kaiser GA, Bowman FO, Malm JR, Hoffman BF. Etiology of right bundle-branch block in patients undergoing total correction of tetralogy of Fallot. Circulation. (1971) 44(6):1022–33. doi: 10.1161/01.cir.44.6.1022
PubMed Abstract | Crossref Full Text | Google Scholar
6. Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. (2000) 356(9234):975–81. doi: 10.1016/s0140-6736(00)02714-8
PubMed Abstract | Crossref Full Text | Google Scholar
8. Lumens J, Fan CS, Walmsley J, Yim D, Manlhiot C, Dragulescu A, et al. Relative impact of right ventricular electromechanical dyssynchrony versus pulmonary regurgitation on right ventricular dysfunction and exercise intolerance in patients after repair of tetralogy of Fallot. J Am Heart Assoc. (2019) 8(2):e010903. doi: 10.1161/jaha.118.010903
PubMed Abstract | Crossref Full Text | Google Scholar
9. Yim D, Hui W, Larios G, Dragulescu A, Grosse-Wortmann L, Bijnens B, et al. Quantification of right ventricular electromechanical dyssynchrony in relation to right ventricular function and clinical outcomes in children with repaired tetralogy of Fallot. J Am Soc Echocardiogr. (2018) 31(7):822–30. doi: 10.1016/j.echo.2018.03.012
PubMed Abstract | Crossref Full Text | Google Scholar
10. Bitterman Y, Hui W, Fan C-PS, Kiss A, Mertens L, Wald RM, et al. Electromechanical dyssynchrony is associated with right ventricular remodeling and dysfunction independently of pulmonary regurgitation late after tetralogy of Fallot repair. J Am Soc Echocardiogr. (2023) 36(12):1315–23. doi: 10.1016/j.echo.2023.06.010
PubMed Abstract | Crossref Full Text | Google Scholar
11. Hui W, Slorach C, Dragulescu A, Mertens L, Bijnens B, Friedberg MK. Mechanisms of right ventricular electromechanical dyssynchrony and mechanical inefficiency in children after repair of tetralogy of Fallot. Circ Cardiovasc Imaging. (2014) 7(4):610–8. doi: 10.1161/circimaging.113.001483
PubMed Abstract | Crossref Full Text | Google Scholar
12. Scherptong RW, Mollema SA, Blom NA, Kroft LJ, de Roos A, Vliegen HW, et al. Right ventricular peak systolic longitudinal strain is a sensitive marker for right ventricular deterioration in adult patients with tetralogy of Fallot. Int J Cardiovasc Imaging. (2009) 25(7):669–76. doi: 10.1007/s10554-009-9477-7
PubMed Abstract | Crossref Full Text | Google Scholar
13. Weidemann F, Eyskens B, Mertens L, Dommke C, Kowalski M, Simmons L, et al. Quantification of regional right and left ventricular function by ultrasonic strain rate and strain indexes after surgical repair of tetralogy of Fallot. Am J Cardiol. (2002) 90(2):133–8. doi: 10.1016/s0002-9149(02)02435-9
PubMed Abstract | Crossref Full Text | Google Scholar
14. Forsha D, Slorach C, Chen CK, Sherman A, Mertens L, Barker P, et al. Patterns of mechanical inefficiency in pediatric dilated cardiomyopathy and their relation to left ventricular function and clinical outcomes. J Am Soc Echocardiogr. (2016) 29(3):226–36. doi: 10.1016/j.echo.2015.11.011
PubMed Abstract | Crossref Full Text | Google Scholar
15. Risum N, Jons C, Olsen NT, Fritz-Hansen T, Bruun NE, Hojgaard MV, et al. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: rationale, initial results, and advantages. Am Heart J. (2012) 163(4):697–704. doi: 10.1016/j.ahj.2012.01.025
PubMed Abstract | Crossref Full Text | Google Scholar
16. Risum N, Tayal B, Hansen TF, Bruun NE, Jensen MT, Lauridsen TK, et al. Identification of typical left bundle branch block contraction by strain echocardiography is additive to electrocardiography in prediction of long-term outcome after cardiac resynchronization therapy. J Am Coll Cardiol. (2015) 66(6):631–41. doi: 10.1016/j.jacc.2015.06.020
PubMed Abstract | Crossref Full Text | Google Scholar
17. Forsha D, Risum N, Smith B, Kanter RJ, Samad Z, Barker P, et al. Frequent activation delay-induced mechanical dyssynchrony and dysfunction in the systemic right ventricle. J Am Soc Echocardiog. (2016) 29(11):1074–83. doi: 10.1016/j.echo.2016.08.002
PubMed Abstract | Crossref Full Text | Google Scholar
18. Forsha D, Slorach C, Chen CK, Stephenson EA, Risum N, Hornik C, et al. Classic-pattern dyssynchrony and electrical activation delays in pediatric dilated cardiomyopathy. J Am Soc Echocardiogr. (2014) 27(9):956–64. doi: 10.1016/j.echo.2014.06.014
PubMed Abstract | Crossref Full Text | Google Scholar
19. Forsha D, Risum N, Barker P. Activation delay-induced mechanical dyssynchrony in single-ventricle heart disease. Cardiol Young. (2017) 27(7):1390–1. doi: 10.1017/S1047951117001123
PubMed Abstract | Crossref Full Text | Google Scholar
20. Forsha D, Gamboa DG, Risum N, Kropf PA, Hornik CP, Barker PCA, et al. Electromechanical dyssynchrony and clinically silent ventricular dysfunction in young subjects with ventricular pacing for congenital and early acquired Av block. Biomed J Sci Tech Res. (2019) 20(2):14942–50. doi: 10.26717/bjstr.2019.20.003435
Crossref Full Text | Google Scholar
21. Klein MR, Sundh F, Simlund J, Harrison JK, Jackson KP, Hughes GC, et al. Immediate mechanical effects of acute left bundle branch block by speckle tracked strain. J Electrocardiol. (2015) 48(4):643–51. doi: 10.1016/j.jelectrocard.2015.05.005
PubMed Abstract | Crossref Full Text | Google Scholar
22. Surawicz B, Knilans T, Gering L, Tavel M. Chou’s Electrocardiography in Clinical Practice. Philadelphia: Saunders Elsevier (2008).
23. Forsha D, Risum N, Kropf PA, Rajagopal S, Smith PB, Kanter RJ, et al. Right ventricular mechanics using a novel comprehensive three-view echocardiographic strain analysis in a normal population. J Am Soc Echocardiogr. (2014) 27(4):413–22. doi: 10.1016/j.echo.2013.12.018
PubMed Abstract | Crossref Full Text | Google Scholar
24. Lev M. The architecture of the conduction system in congenital heart disease. II. Tetralogy of Fallot. AMA Arch Pathol. (1959) 67(5):572–87.13636638
PubMed Abstract | Google Scholar
25. Horowitz LN, Alexander JA, Edmunds LH Jr. Postoperative right bundle branch block: identification of three levels of block. Circulation. (1980) 62(2):319–28. doi: 10.1161/01.circ.62.2.319
PubMed Abstract | Crossref Full Text | Google Scholar
26. Okoroma EO, Guller B, Maloney JD, Weidman WH. Etiology of right bundle-branch block pattern after surgical closure of ventricular-septal defects. Am Heart J. (1975) 90(1):14–8. doi: 10.1016/0002-8703(75)90251-3
PubMed Abstract | Crossref Full Text | Google Scholar
27. Krongrad E, Bharati S, Steinfeld L, Lev M. Histologic observations of the cardiac conduction system in a patient with postoperative bilateral bundle branch block. Am J Cardiol. (1977) 40(4):635–40. doi: 10.1016/0002-9149(77)90083-2
PubMed Abstract | Crossref Full Text | Google Scholar
28. Krongrad E, Hefler SE, Bowman FO Jr, Malm JR, Hoffman BF. Further observations on the etiology of the right bundle branch block pattern following right ventriculotomy. Circulation. (1974) 50(6):1105–13. doi: 10.1161/01.cir.50.6.1105
PubMed Abstract | Crossref Full Text | Google Scholar
29. Horowitz LN, Simson MB, Spear JF, Josephson ME, Moore EN, Alexander JA, et al. The mechanism of apparent right bundle branch block after transatrial repair of tetralogy of Fallot. Circulation. (1979) 59(6):1241–52. doi: 10.1161/01.cir.59.6.1241
PubMed Abstract | Crossref Full Text | Google Scholar
30. Coggin C, Warcham E, Selvester R. Post ventriculotomy right bundle branch block: its etiology. Circulation. (1960) 22(4):734.
31. Akazawa Y, Fujioka T, Yazaki K, Strbad M, Hörer J, Kühn A, et al. Right ventricular electromechanical dyssynchrony and its relation to right ventricular remodeling, dysfunction, and exercise capacity in ebstein anomaly. J Am Soc Echocardiogr. (2023) 36(6):634–43. doi: 10.1016/j.echo.2023.02.013
PubMed Abstract | Crossref Full Text | Google Scholar
32. Dragulescu A, Friedberg MK. A tale of two ventricles: ventricular-ventricular interactions and LV dysfunction after surgical repair of tetralogy of Fallot. Eur Heart J Cardiovasc Imaging. (2014) 15(5):498–9. doi: 10.1093/ehjci/jet260
PubMed Abstract | Crossref Full Text | Google Scholar
33. Menting ME, Eindhoven JA, van den Bosch AE, Cuypers JA, Ruys TP, van Dalen BM, et al. Abnormal left ventricular rotation and twist in adult patients with corrected tetralogy of Fallot. Eur Heart J Cardiovasc Imaging. (2014) 15(5):566–74. doi: 10.1093/ehjci/jet244
PubMed Abstract | Crossref Full Text | Google Scholar
34. Ramdat Misier NL, Moore JP, Nguyen HH, Lloyd MS, Dubin AM, Mah DY, et al. Long-term outcomes of cardiac resynchronization therapy in patients with repaired tetralogy of Fallot: a multicenter study. Circ Arrhythm Electrophysiol. (2024) 17(3):e012363. doi: 10.1161/circep.123.012363
留言 (0)