Epstein-Barr virus (EBV), a member of the γ-herpesviruses, may cause persistent, lifelong, latent infections (1). Children with primary EBV infection are usually asymptomatic, but they sometimes develop infectious mononucleosis (IM), which resolves spontaneously after the emergence of EBV-specific immunity. EBV infection in children is considered a self-limiting disease with a favorable prognosis (2). However, patients with an immune deficiency or impairment may develop an aggressive EBV infection that can result in chronic active EBV (CAEBV) disease, hemophagocytic lymphohistiocytosis (HLH), and tumors. CAEBV is characterized by an unusual pattern of anti-EBV antibodies and by chronic or recurrent symptoms mimicking an infectious mononucleosis that persist for a long time. Peripheral blood samples of patients with CAEBV have high virus loads. CAEBV is a high-mortality, high-morbidity disease with life-threatening complications. The pathogenesis of CAEBV involves the clonal expansion of EBV-infected T cells and NK cells (3). EBV can infect lymphocytes and other tissues leading to cardiovascular complications. Approximately 8.5% of patients with CAEBV present with coronary artery lesions (CALs) (4). In contrast, the incidence of CALs in Kawasaki disease is only 4% after intravenous immunoglobulin (IVIG) treatment (5). In addition to CALs, EBV infection may also cause myocarditis, valvular heart disease, aortic lesions, heart failure, and pulmonary artery hypertension (PAH) (6). However, we found only two reports of patients with CAEBV presenting with Valsalva sinus involvement at the onset of their disease (7, 8).
In here, we report the uncommon case of a pediatric patient with EBV infection, whose disease progressed to CAEBV and EBV-HLH with fatal cardiovascular involvement seven years after the infection onset. The cardiovascular findings included a giant coronary artery aneurysm and thrombosis, giant Valsalva sinus aneurysm, and a dilated ascending aorta.
Case presentationA previously healthy 3-year-old girl (Chinese, Han ethnicity) was admitted to the local hospital with a 10-day fever of unknown origin. The physical examination revealed the presence of rash, oral changes, and cervical lymphadenopathy. Laboratory test results showed elevated levels of C-reaction protein (CRP) at 14 mg/L (normal reference value: <8 mg/L), ALT at 226 U/L (normal range < 49 U/L), and AST at 230 U/L (normal range < 40 U/L), as well as an abnormal erythrocyte sedimentation rate (ESR) at 34 mm/h (normal reference value: 0–26 mm/h). The blood routine examination showed normal level of white blood cells (WBC, 5.1 × 109/L, normal range: 4.3–11.3 × 109/L), neutrophils (3.9 × 109/L, normal range: 0.86–6.03 × 109/L), lymphocytes (1.26 × 109/L, normal range: 1.47–5.35 × 109/L), platelets (PLT, 203 × 109/L, normal range: 128–420 × 109/L), hemoglobin (HB, 116 g/L, normal range: 108–144 g/L), and no atypical lymphocytes were detected. Echocardiography images revealed a left coronary artery aneurysm (LCA, 3.7 mm; z score, 4.60) and left anterior descending (LAD, 2.6 mm; z score, 3.07), left circumflex branch (LCX, 1.8 mm; z score, 1.29), right coronary artery (RCA, 3.4 mm; z score, 5.32) aneurysms. A primary diagnosis of incomplete Kawasaki disease (iKD) was proposed based on these findings. On the 11th day after the onset of the fever, she received IVIG (2 g/kg) as a single intravenous infusion and aspirin (30 mg/kg/day) treatment. However, the fever and cervical lymphadenopathy persisted beyond 48 h after the IVIG infusion. The patient was transferred to our hospital on the 14th day after the fever onset. The physical examination upon arrival revealed the presence of fever, tonsillitis with membrane formation, cervical lymphadenopathy, splenomegaly, and hepatomegaly. Symptoms such as coughing, headaches, vomiting, diarrhea, or shivers were not present. A detailed auxiliary examination was positive for Epstein–Barr virus (EBV) DNA with 1.86 × 103 copies/ml. EBV-specific antibody testing was positive for immunoglobulin M-viral capsid antigen (IgM-VCA), IgG-VCA, and the diffuse staining component of early antigen (EA-D). The levels of ALT (193 U/L) and AST (228 U/L) were elevated. The WBC, neutrophil, and PLT counts were normal, as well as the HB levels, and atypical lymphocytes were absent from the peripheral blood. Tests for common respiratory viruses were all negative. Moreover, tests for cytomegalovirus, Toxoplasma gondii, adenovirus, viral hepatitis, HIV, rubella virus, mycoplasma IgM, TPPA, fungal G test, GM test, bacterial cultures of blood and pharyngeal secretions, urine and stool examinations were all negative. We ruled out the possibility of tuberculosis or parasitic infections due to the lack of contact history, a BCG scar, and negative results for parasite-specific antibodies, PPD test, and T-SPOT. Tests for C-reaction protein (CRP), autoantibody, rheumatoid factor, anti-cyclic citrullinated peptide antibody, antineutrophil cytoplasmic antibodies (ANCAs, including p-ANCA and c-ANCA) and HLA-B27 were negative. Marrow cytology inspection did not suggest hemophagocytosis or other common blood system diseases. No other significant laboratory abnormalities were found after testing the renal, myocardial, and coagulation functions; in addition, blood gas analysis, and levels of blood glucose, blood ammonia, and blood lipids were normal. Echocardiography images showed aneurysms of the LCA (3.9 mm; z score, 5.01), LAD (2.4 mm; z score, 2.55), LCX (1.6 mm; z score, 0.69), and RCA (3.8 mm; z score, 6.37). We diagnosed the patient as having a primary EBV infectious mononucleosis based on her clinical features, the high loads of EBV-DNA, and the high levels of EBV-specific antibody, ALT, and AST. We also ruled out the possibility of KD after the lack of diagnostic criteria (9). Given that we also ruled out a coronary-pulmonary artery fistula and the presence of systemic lupus erythematosus (SLE), we speculated that the EBV infection had caused the CALs (4, 10). The patient initiated a therapy with acyclovir and aspirin (3 mg/kg/day), bed rest, and symptomatic treatments. No glucocorticoids was not proposed since the clinical symptoms gradually resolved with negative EBV-DNA, normal liver function, and normal blood examination results one week later. The patient was discharged home with aspirin as her CALs remained to be unchanged by the repeated echocardiographic examination. Unfortunately, she did not have regular follow-ups in the outpatient clinic.
Seven years later, the same girl, now 10 years old, was admitted to the local hospital with complaints of prolonged fever and fatigue. Her echocardiographic findings included giant left and right CAAs [LCA 9.0 mm (z score, 11.0), LAD 5.0 mm (z score, 5.91), LCX 5.0 mm (z-score, 6.1), RCA 16.3 mm (z score, 23.5)], a giant Valsalva sinus aneurysm, and an ascending aorta dilation. The patient was immediately admitted to our hospital again. Upon arrival, the physical examination revealed persistent fever, facial pallor, fatigue, ecchymosis can be seen in bilateral cubital fossa and abdominal wall, scattered petechiae can be seen on the skin of limbs, splenomegaly (line I, 10 cm; line Ⅱ, 10 cm; line Ⅲ, 0 cm), and hepatomegaly (6.5 cm below the costal margin), and no positive results were found in cardiac auscultation. The auxiliary examination showed 4.56 × 105 copies/ml of Epstein–Barr virus (EBV) DNA. The patient had abnormal counts of WBCs (0.5 × 109/L; normal range, 4.3–11.3 × 109/L), neutrophils (0.35 × 109/L; normal range, 1.6–7.8 × 109/L), and lymphocytes (0.01 × 109/L; normal range, 1.5–4.6 × 109/L), and PLTs (10 × 109/L; normal range, 100–450 × 109/L) a low HGB level (73 g/L, normal range, 110–146 g/L), and high levels of CRP (53.78 mg/L), ALT (98 U/L), AST (159 U/L), and ferritin (11,276.20 ng/ml, normal range 10–291 ng/ml). Coagulation tests revealed 38.4 s for the activated partial thromboplastin time (APTT), 4.04 g/L of fibrinogen (Fg), and 1 mg/L of D-dimer (DDI). The levels of inflammatory cytokines including IL-6 (27.15 pg/ml; normal range < 20 pg/ml), IL-10 (79.12 pg/ml; normal range < 5.9 pg/ml), IL-2R (1,084 U/ml; normal range < 710 U/ml), IFN-a (28.65 pg/ml; normal range < 5.5 pg/ml), and sCD25 (19,534 pg/ml; normal range < 6,400 pg/ml) were elevated. The analysis of lymphocyte subgroups showed abnormally low numbers of T lymphocytes (CD3+, 0.01 × 109/L), B lymphocytes (CD3−CD19+, <0.01 × 109/L), and NK cells (CD3−CD56+CD16+, <0.01 × 109/L). The triglyceride level (5.5 mmol/L; normal range < 1.7 mmol/L) was increased. The bone marrow biopsy revealed a hyperplasic bone marrow with hemophagocytosis. We ruled out other infectious diseases, autoimmune diseases, immunodeficiency, and tumors after obtaining negative results for tests on cytomegalovirus, T. gondii, adenovirus, viral hepatitis, HIV, mycoplasma IgM, TPPA, fungal G and GM, bacterial hemocultures, autoantibodies, humoral and cellular immunity, and tumor markers, as well as negative urine and stool examinations, and chest and abdomen CT images. The fundus and electrocardiographic examinations were also negative. This patient' medical history denied the EBV infection during her perinatal stage, organs transplantation, and blood products transfusion. This virus was also negative in her parents. In light of our findings (EBV infection, fever, splenomegaly, bicytopenia, hypertriglyceridemia, hypofibrinogenemia, hemophagocytosis, ferritin ≥ 500 µg/L, low NK-cell activity, and soluble CD25 ≥ 2,400 U/m), we consulted with an expert in infectious diseases and an expert in blood diseases to diagnose a chronic active EB virus infection (CAEBV) complicated with hemophagocytic lymphohistiocytosis (HLH), on the basis of the diagnostic criteria on the guideline for diagnosis and treatment of CAEBV in children and the HLH 2004 clinical trial (11–13). We ruled out the possibility of familial HLH and inherited connective tissue disease with a negative whole exome sequencing and the existence of an unaffected sibling. Unfortunately, the patient's guardian abandoned the standard therapy of EBV-HLH due to economic difficulties and agreed only to a treatment with IVIG, corticosteroid, acyclovir, antiplatelet, and anticoagulant. A high dose of IVIG (2 g/kg as a single dose) and the methylprednisolone (20 mg/kg/day) prescription were initiated, with gradual tapering of the methylprednisolone to oral prednisone (2 mg/kg/day) after the patient's fever resolved and the levels of WBC, HB, PLT, and inflammatory cytokines became normal. An echocardiographic examination revealed an enlarged atrium and ventricle (left atrium 44 × 35 mm, right atrium 38 mm, left ventricle 44 mm, right ventricle 16 mm), a giant CAA (RCA 6.0 mm [z score, 8.55]; LCA 9.5 mm [z score, 11.64]) and thrombosis, a giant Valsalva sinus aneurysm (inner diameter, 60 mm), and ascending aorta dilation (inner diameter, 35 mm) (Figure 1). A cardiac computed tomography angiography (CCTA) further showed an enlarged left ventricle, a dilated ascending aorta (36 mm), a right coronary sinus aneurysm (23 mm × 17 mm), a non-coronary sinus aneurysm (35 mm × 39 mm) compressing the left atrium, a left coronary sinus aneurysm (41 mm × 29 mm); an RCA aneurysm [7.9 mm (z score, 11.86)], thrombosis and calcifications in the distal right coronary artery; a left main coronary artery aneurysm [LMCA, 7.5 mm (z score, 9.19)], a LAD [3 mm (z score, 2.51)], and an LCX [5 mm (z score, 6.13)] (Figure 2); and absence of coronary-pulmonary artery fistulas. We attributed these cardiac complications to the vasculitis produced by the CAEBV after the detailed discussion among the members of the Multiple Disciplinary Team (MDT) comprised of experts in infectious, blood, autoimmune, and cardiac diseases of our hospital. Accordingly, we prescribed a subcutaneous injection of low molecular weight heparin calcium (LMWHC) and sequential therapy with oral warfarin, combined with aspirin and clopidogrel. Three weeks later, the patient was finally discharged home. Unfortunately, one month later, the fever attacked this patient. Owning to economic difficulties, the patient's guardian had refused further treatment and took the patient home. During our subsequent follow-up visit, the girl subsequently passed away (Figure 3).
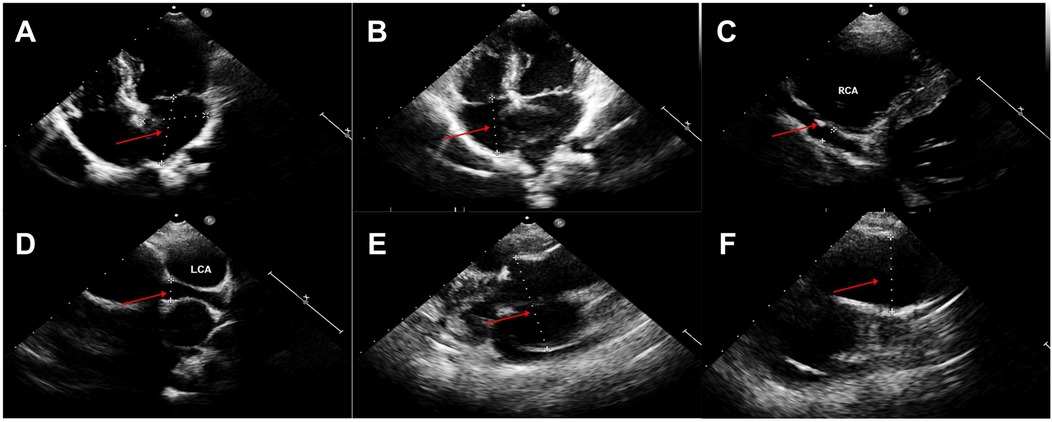
Figure 1. Echocardiography findings (A,B). Enlarged atrium and ventricle (left atrium 44 × 35 mm, right atrium 38 mm, left ventricle 44 mm, right ventricle 16 mm), (C) Giant right coronary artery aneurysm (6.0 mm) and thrombosis (red arrow), (D) Left coronary artery aneurysm (9.5 mm), (E) Giant Valsalva sinus aneurysm (60 mm), (F) Ascending aorta dilation (35 mm).
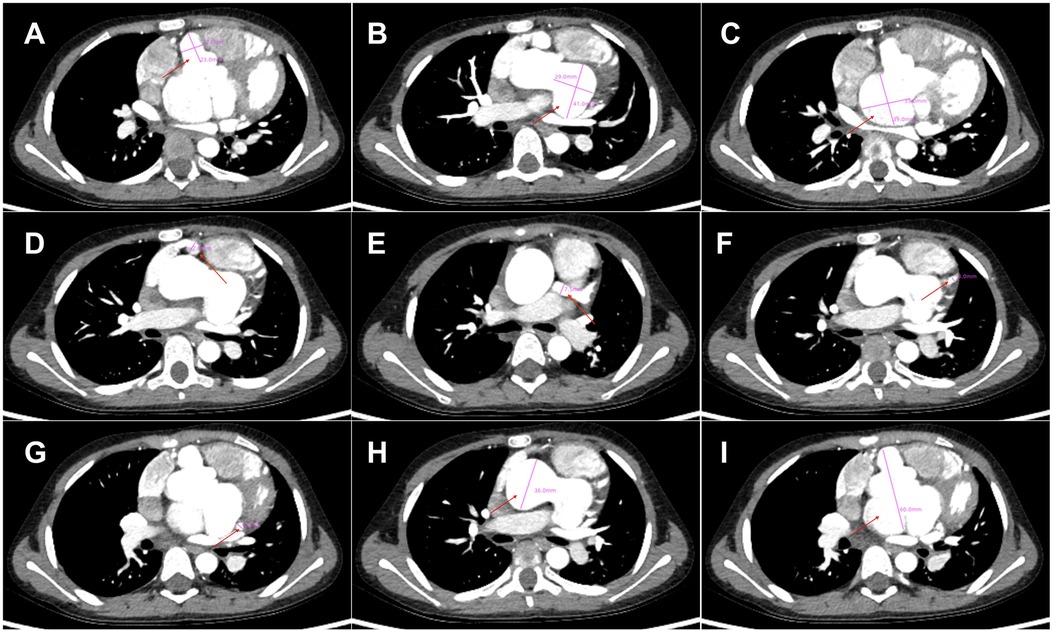
Figure 2. Cardiac computed tomography angiography findings. (A) Right coronary sinus aneurysm (23 mm × 17 mm, red arrow), (B) Left coronary sinus aneurysm (41 mm × 29 mm, red arrow), (C) Non-coronary sinus aneurysm (35 mm × 39 mm, red arrow) compressing the left atrium; (D) Right coronary artery aneurysm (7.9 mm, red arrow), thrombosis and calcifications in the distal right coronary artery; (E–G). Left main coronary artery aneurysm (LMCA, 7.5 mm), LAD (3 mm) and LCX (5 mm); (H) Dilated ascending aorta (36 mm, red arrow); (I). Giant Valsalva sinus aneurysm (60 mm).
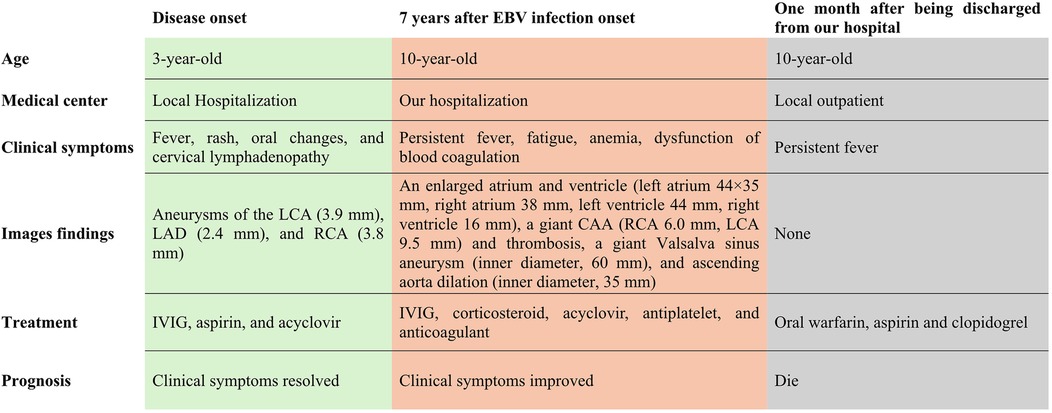
Figure 3. Clinical course of this patient.
DiscussionAlthough primary EBV infection in children is usually asymptomatic and self-limiting with a favorable prognosis (2), some patients with an immune deficiency or impairment develop CAEBV, HLH, and tumors. EBV-HLH is a high-mortality, high-morbidity disease with life-threatening complications. Approximately 8.5% of patients with CAEBV present with CALs (4); whereas the incidence of CALs in KD is approximately 4% after IVIG treatment (5). In addition to CALs, severe EBV infections may cause myocarditis, valvular heart disease, heart failure, and PAH (6). However, we found only two reports of patients with CAEBV presenting with Valsalva sinus involvement at the onset of their disease (7, 8). To the best of our knowledge, this is the first report of a pediatric case of EBV infection that progressed to CAEBV and EBV-HLH and developed fatal cardiovascular complications seven years after the disease onset, presenting with giant CAA and thrombosis, giant Valsalva sinus aneurysm, and ascending aorta dilation. Clinicians need to be aware of the potential cardiac complications in patients with EBV infection, especially in those with CAEBV and/or EBV-HLH.
On the pathogenesis mechanism of cardiovascular complications involving EBV infection, there is ample evidence showing how EBV directly damages the heart, and viral genome analyses by quantitative PCR have shown EBV-DNA copies in endomyocardial biopsies (EMB) from patients with cardiomyopathy (14, 15). The EMB from a patient with ongoing perimyocarditis was shown to contain high numbers of EBV-encoded RNA copies in CD8+ T-lymphocytes, demonstrating that the EBV infection contributed to a severe, chronic active infection in the myocardium (16). Animal myocarditis models have shown that EBV-associated cardiomyocytes negative for EBV-DNA copies still presented a severe inflammatory infiltration (17). Moreover, myocardial necrosis was not observed in B- and T-lymphocyte-deficient mice with high EBV-DNA copy numbers, suggesting that the myocardial injury might not have completely been attributed to viral replication within the cardiac tissues (17). EBV-related coronary artery lesions associated with lymphoid vasculitis are considered a kind of immune injury (18) due to chemotaxis, recruitment, adhesion, infiltration, cytotoxic injury, and cytokine secretion by the local inflammatory cells (15). EBV can induce local inflammatory infiltration by infecting T cells, natural killer (NK) cells, or B cells (19). Cytotoxic T cells (CTLs) infected with EBV produce excessive amounts of vascular endothelial growth factor (VEGF), which enhances the post-capillary permeability of veins and venules, further promoting vascular wall degradation (20). In addition, vascular lesions may be attributed to adhesion molecules and cytokines secreted by EBV-positive NK/T cells (20). EBV also causes vascular endothelial damage via the production of deoxyuridine triphosphatase (dUTPase) during the viral replication, subsequently increasing interleukin-6 (IL-6) levels (21, 22). Therefore, it was postulated that the infiltration of EBV-infected T- and NK-cells and the related inflammatory reactions mainly accounted for cardiac complications during the progressive disease course. Cardiac complications in patients with CAEBV tend to be associated with high EBV load in peripheral blood samples, rather than with the type of CAEBV or the time interval from disease onset to the development of cardiac disease (23). Muneuchi et al. have found the CAEBV patients with cardiac complications presented fever more frequently than the patients without complications. It lends itself to reason, therefore, a high EBV load, fever, and cytopenias might be risk factors for the development of coronary artery lesion (23). Furthermore, more studies should be carried out to explore the risk factors of cardiac complications in patients with EBV infections. In the case of our patient, she developed CAEBV and EBV-HLH indicating a severe hyper-inflammatory reaction, which was probably responsible for her serious cardiovascular complications. As mentioned, we speculate that our patient's cardiovascular complications might be attributed to direct virus infiltration and immune injury. However, the pathogenesis of cardiovascular complications involving EBV infection remain incompletely understood and advanced animal and cell model studies still need to clarify the relevant mechanisms.
Coronary artery lesions are the most common cardiovascular complications in patients with CAEBV (4). In a nationwide survey, the incidences of coronary artery aneurysms and myocarditis in patients with CAEBV were respectively 8.5% and 6% (4). In children with CAEBV, cardiovascular complications are a significant mortality risk factor (23). As shown in the Table 1, patients with EBV infection shared the similar clinical characteristics, namely younger age of disease onset, prolonged fever (7, 8, 18, 24–26), rash (7, 18, 27), and high EBV–DNA load (7, 8, 26). In addition, most patients with severe cardiac complications were found on follow–up, which indicated that the younger age of disease onset, prolonged fever, and high EBV–DNA load were risk factors for cardiac complications in those patients (7, 8, 24, 26–28). Reports of aortic and aortic branch involvements have been uncommon: abdominal aorta (18, 24, 25, 27, 28), carotid artery (25), subclavian artery (25), common iliac artery (24–26), and thoracic aorta (8, 18). Notably, ours seems to be the third case of CAEBV involving the Valsalva sinus (7, 8). Y. Sato et al. reported the case of an 11-year-old girl diagnosed as having CAEBV complicated with Valsalva sinus dilatation, her pathological findings showed fibroid necrotic changes with infiltration of a few abnormal lymphoid cells in the wall of the Valsalva sinus (7). Qirui Li et al. presented the case of a 5-year-old girl with CAEBV complicated with multiple arterial aneurysms mainly involving the right coronary artery sinus, left main coronary artery, aorta, and its major branches (8). Notably, the cardiovascular complications developed during the initial stages of the disease in the reports by Y. Sato and Li; whereas our patient presented a seven-year gap between the initial. Infection and the development of the fatal cardiovascular complications. Moreover, her giant Valsalva sinus aneurysm involved simultaneously the right and left coronary sinuses, and the non-coronary aneurysm. In addition, her giant non-coronary sinus aneurysm compressed the left atrium. At the same time, our patient had a giant CAA and thrombosis, and an ascending aorta dilation. Congenital Valsalva sinus aneurysms are caused by connective tissue diseases, such as those in Apert's, Marfan's, and Ehlers-Danlos' syndromes (29–31). By contrast, acquired Valsalva sinus aneurysms can be found in connective tissue disease, infectious etiologies (syphilis, bacterial endocarditis, and tuberculosis), vasculitis diseases (Takayasu's Arteritis), chronic atherosclerotic changes, medial cystic necrosis, chest trauma, and iatrogenic injury during aortic valve surgery (32–37). Thus, clinicians should know that severe EBV infection, especially for CAEBV and/or EBV-HLH, may also cause Valsalva sinus lesions besides the coronary artery and aorta lesions. Moreover, clinicians need to be aware of these complications during the long-term follow-up of patients with EBV infection, especially for patients with CAEBV and/or EBV-HLH.
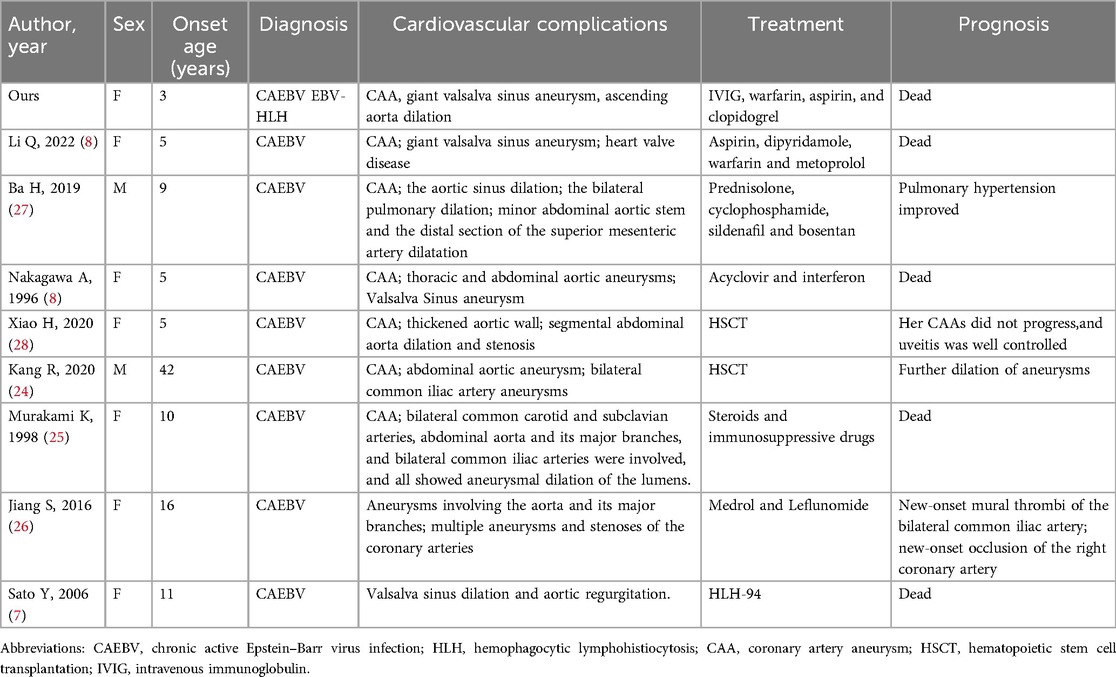
Table 1. Summary of patients with CAEBV involving coronary artery, valsalva sinus, and aorta.
Regarding the management of cardiovascular complications associated with EBV infection, medical therapy should be provided according to the types of cardiovascular complications (38, 39). Moreover, surgical repair should be considered for patients with ruptured or symptomatic non-ruptured Valsalva sinus aneurysm since this condition can be fatal (40, 41). In any case, cardiovascular complications are mostly observed in patients with CAEBV, and the cornerstone of therapy should be to address the CAEBV. Unfortunately, the effectiveness of antiviral therapy is limited. Allogeneic hematopoietic stem cell transplantation (HSCT) is often curative for CAEBV disease, and the treatment should be considered early in the course of the disease as patients are more likely to tolerate the procedure then (42). The conventional treatment (including rituximab, immunosuppressive therapy, cytotoxic chemotherapy, and autologous CTLs) may yield a temporary remission, but the effect of these agents is not curative (43). Chemotherapy is used to reduce viral load and control disease activity in CAEBV before performing HSCT. In addition, chemotherapy could reduce the risk of complications related to HSCT, and may control the disease activity of CAEBV, which contributes to improving the outcome of HSCT (3). In this case, due to financial constraints, the patient's guardian declined the optimal treatment plan. As an alternative, we administered high-dose IVIG and glucocorticoids to suppress the inflammatory cytokine storm, while also providing antiviral and supportive care, in order to save the patient's life to the greatest extent possible.
ConclusionWe reported the rare case of a pediatric patient with EBV infection progressing to CAEBV/EBV-HLH. The patient developed uncommon and fatal cardiovascular complications seven years after the onset of the initial infection. Clinicians need to be aware of the possibility of these long-term EBV infection complications during the follow-up of patients, especially in those with CAEBV and/or EBV-HLH.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statementWritten informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributionsZF: Writing – original draft. HD: Writing – original draft. LW: Data curation, Visualization, Writing – review & editing. HY: Data curation, Visualization, Writing – review & editing. KZ: Writing – review & editing. YH: Writing – review & editing. CW: Writing – review & editing. XL: Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science-Technology Support Plan Projects in Sichuan Province (2023ZYD0119, 2023YFH0036) and National Natural Science Foundation of China (No. 82370236, No. 82070324).
AcknowledgmentsWe are grateful to the patients and families for their contributions to this work.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. (2005) 80(1):64–9. doi: 10.1002/ajh.20398
PubMed Abstract | Crossref Full Text | Google Scholar
3. Kawada JI, Ito Y, Ohshima K, Yamada M, Kataoka S, Muramatsu H, et al. Updated guidelines for chronic active Epstein-Barr virus disease. Int J Hematol. (2023) 118(5):568–76. doi: 10.1007/s12185-023-03660-5
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. (2003) 187(4):527–33. doi: 10.1086/367988
PubMed Abstract | Crossref Full Text | Google Scholar
6. Chen X, Li Y, Deng L, Wang L, Zhong W, Hong J, et al. Cardiovascular involvement in Epstein-Barr virus infection. Front Immunol. (2023) 14:1188330. doi: 10.3389/fimmu.2023.1188330
PubMed Abstract | Crossref Full Text | Google Scholar
7. Sato Y, Tsuboi T, Mikami T, Kurosawa H, Kanou K, Sugita K, et al. Chronic active Epstein-Barr virus infection with dilatation of the valsalva sinus. Pediatr Int. (2006) 48(6):643–5. doi: 10.1111/j.1442-200X.2006.02283.x
PubMed Abstract | Crossref Full Text | Google Scholar
8. Li Q, Li G, Shao D, Yarrabolu T, Yue Y. Case report: pediatric chronic active Epstein-Barr virus infection with giant Sinus of valsalva aneurysms and aorta and its branch dilations. Front Pediatr. (2021) 9:779806. doi: 10.3389/fped.2021.779806
PubMed Abstract | Crossref Full Text | Google Scholar
9. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135(17):e927–99. doi: 10.1161/CIR.0000000000000484
PubMed Abstract | Crossref Full Text | Google Scholar
10. Wei A, Ma H, Zhang L, Li Z, Guan Y, Zhang Q, et al. Clinical analysis of chronic active EBV infection with coronary artery dilatation and a matched case-control study. Orphanet J Rare Dis. (2021) 16(1):50. doi: 10.1186/s13023-021-01689-5
PubMed Abstract | Crossref Full Text | Google Scholar
11. Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007) 48(2):124–31. doi: 10.1002/pbc.21039
PubMed Abstract | Crossref Full Text | Google Scholar
12. China NHCotPsRo. Guideline for diagnosis and treatment of chronic active epstein-barr virus infection in children (2021 edition). Clin Educ Gen Pract. (2021) 19(11):964–584.
13. Subspecialty Group of Infectious Diseases tSoP, Chinese Medical Association; National Children’s Epstein-Barr Virus Infection Cooperative Group. Experts consensus on diagnosis and treatment of Epstein-Barr virus infection-related diseases in children. Zhonghua Er Ke Za Zhi. (2021) 59(11):905–11. doi: 10.3760/cma.j.cn112140-20210618-00513
PubMed Abstract | Crossref Full Text | Google Scholar
14. Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. (2005) 111(7):887–93. doi: 10.1161/01.CIR.0000155616.07901.35
Crossref Full Text | Google Scholar
15. Tschope C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. (2021) 18(3):169–93. doi: 10.1038/s41569-020-00435-x
PubMed Abstract | Crossref Full Text | Google Scholar
16. Richter J, Quintanilla-Martinez L, Bienemann K, Zeus T, Germing U, Sander O, et al. An unusual presentation of a common infection. Infection. (2013) 41(2):565–9. doi: 10.1007/s15010-012-0321-y
PubMed Abstract | Crossref Full Text | Google Scholar
17. Häusler M, Sellhaus B, Scheithauer S, Gaida B, Kuropka S, Siepmann K, et al. Myocarditis in newborn wild-type BALB/c mice infected with the murine gamma herpesvirus MHV-68. Cardiovasc Res. (2007) 76(2):323–30. doi: 10.1016/j.cardiores.2007.06.025
PubMed Abstract | Crossref Full Text | Google Scholar
18. Nakagawa A, Ito M, Iwaki T, Yatabe Y, Asai J, Hayashi K. Chronic active Epstein-Barr virus infection with giant coronary aneurysms. Am J Clin Pathol. (1996) 105(6):733–6. doi: 10.1093/ajcp/105.6.733
PubMed Abstract | Crossref Full Text | Google Scholar
19. Savoldo B, Huls MH, Liu Z, Okamura T, Volk HD, Reinke P, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. (2002) 100(12):4059–66. doi: 10.1182/blood-2002-01-0039
PubMed Abstract | Crossref Full Text | Google Scholar
20. Kanno H, Watabe D, Shimizu N, Sawai T. Adhesion of Epstein-Barr virus-positive natural killer cell lines to cultured endothelial cells stimulated with inflammatory cytokines. Clin Exp Immunol. (2008) 151(3):519–27. doi: 10.1111/j.1365-2249.2007.03584.x
PubMed Abstract | Crossref Full Text | Google Scholar
21. Dogan A, Tuzun N, Turker Y, Akcay S, Kaya S, Ozaydin M. Matrix metalloproteinases and inflammatory markers in coronary artery ectasia: their relationship to severity of coronary artery ectasia. Coron Artery Dis. (2008) 19(8):559–63. doi: 10.1097/MCA.0b013e3283109079
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ariza ME, Glaser R, Kaumaya PT, Jones C, Williams MV. The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J Immunol. (2009) 182(2):851–9. doi: 10.4049/jimmunol.182.2.851
PubMed Abstract | Crossref Full Text | Google Scholar
23. Muneuchi J, Ohga S, Ishimura M, Ikeda K, Yamaguchi K, Nomura A, et al. Cardiovascular complications associated with chronic active Epstein-Barr virus infection. Pediatr Cardiol. (2009) 30(3):274–81. doi: 10.1007/s00246-008-9343-8
PubMed Abstract | Crossref Full Text | Google Scholar
24. Kang R, Tanaka TD, Ogasawara Y, Yoshimura M. A rare complication of chronic active Epstein-Barr virus infection. JACC Case Rep. (2020) 2(5):756–9. doi: 10.1016/j.jaccas.2020.03.022
PubMed Abstract | Crossref Full Text | Google Scholar
25. Murakami K, Ohsawa M, Hu SX, Kanno H, Aozasa K, Nose M. Large-vessel arteritis associated with chronic active Epstein-Barr virus infection. Arthritis Rheum. (1998) 41(2):369–73. doi: 10.1002/1529-0131(199802)41:2%3C369::AID-ART22%3E3.0.CO;2-S
PubMed Abstract | Crossref Full Text | Google Scholar
26. Jiang S, Li X, Cao J, Wu D, Kong L, Lin L, et al. Early diagnosis and follow-up of chronic active Epstein-Barr-virus-associated cardiovascular complications with cardiovascular magnetic resonance imaging: a case report. Medicine (Baltimore). (2016) 95(31):e4384. doi: 10.1097/MD.0000000000004384
PubMed Abstract | Crossref Full Text | Google Scholar
27. Ba H, Xu L, Peng H, Lin Y, Li X, Wang H, et al. Chronic active Epstein-Barr virus infection with systemic vasculitis and pulmonary arterial hypertension in a child. Front Pediatr. (2019) 7:219. doi: 10.3389/fped.2019.00219
PubMed Abstract | Crossref Full Text | Google Scholar
28. Xiao H, Hu B, Luo R, Hu H, Zhang J, Kuang W, et al. Chronic active Epstein-Barr virus infection manifesting as coronary artery aneurysm and uveitis. Virol J. (2020) 17(1):166. doi: 10.1186/s12985-020-01409-8
PubMed Abstract | Crossref Full Text | Google Scholar
29. Aldabain L, Haddaden M, Bandaru S, Camire L, Weisman DS. Ruptured Sinus of valsalva aneurysm in apert syndrome: case report. J Community Hosp Intern Med Perspect. (2022) 12(1):68–72. doi: 10.55729/2000-9666.1013
PubMed Abstract | Crossref Full Text | Google Scholar
30. Takach TJ, Reul GJ, Duncan JM, Cooley DA, Livesay JJ, Ott DA, et al. Sinus of valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. (1999) 68(5):1573–7. doi: 10.1016/S0003-4975(99)01045-0
PubMed Abstract | Crossref Full Text | Google Scholar
31. Leier CV, Call TD, Fulkerson PK, Wooley CF. The spectrum of cardiac defects in the Ehlers-Danlos syndrome, types I and III. Ann Intern Med. (1980) 92(2 Pt 1):171–8. doi: 10.7326/0003-4819-92-2-171
PubMed Abstract | Crossref Full Text | Google Scholar
32. Deng Z, Liu Q, Liu H, Yuan J, Li J. Right sinus of valsalva aneurysm rupture into the right ventricle in a rare cardiovascular case of syphilis. Arch Med Sci. (2023) 19(6):1920–2. doi: 10.5114/aoms/172098
PubMed Abstract | Crossref Full Text | Google Scholar
33. Batiste C, Bansal RC, Razzouk AJ. Echocardiographic features of an unruptured mycotic aneurysm of the right aortic sinus of valsalva. J Am Soc Echocardiogr. (2004) 17(5):474–7. doi: 10.1016/j.echo.2004.01.002
PubMed Abstract | Crossref Full Text | Google Scholar
34. Koh KK, Lee KH, Kim SS, Lee SC, Jin SH, Cho SW. Ruptured aneurysm of the sinus of valsalva in a patient with Behcet’s disease. Int J Cardiol. (1994) 47(2):177–9. doi: 10.1016/0167-5273(94)90186-4
PubMed Abstract | Crossref Full Text | Google Scholar
35. Nakano T, Okano H, Konishi T, Takezawa H. Aneurysm of the left aortic sinus caused by Takayasu’s arteritis: compression of the left coronary artery producing coronary insufficiency. J Am Coll Cardiol. (1986) 7(3):696–700. doi: 10.1016/S0735-1097(86)80483-1
PubMed Abstract | Crossref Full Text | Google Scholar
36. Guler N, Eryonucu B, Tuncer M, Asker M. Aneurysm of sinus of valsalva dissecting into interventricular septum: a late complication of aortic valve replacement. Echocardiography. (2004) 21(7):645–8. doi: 10.1111/j.0742-2822.2004.03128.x
留言 (0)