Pancreatic cancer is projected to become the second leading cause of cancer-related deaths by 2030, with only 15% of patients presenting with resectable disease at diagnosis (1). Early-stage pancreatic cancer often presents with mild gastrointestinal symptoms such as nausea, vomiting, abdominal distension, and low back pain. Consequently, many patients are misdiagnosed and treated for other conditions, leading to delays in the appropriate management of pancreatic cancer (2–4). Surgical resection remains the gold standard for achieving a radical cure; however, for borderline resectable pancreatic cancer, R0 resection following chemoradiotherapy is considered superior to the treatment for locally advanced pancreatic cancer (LAPC). According to the DPCG criteria, LAPC is defined as involving >90° arterial encasement (such as the superior mesenteric artery, celiac trunk, or any hepatic artery) and/or >270° involvement or occlusion of the portal vein and/or superior mesenteric vein (5). Palliative chemotherapy (with or without radiotherapy) and supportive care are typically the primary treatment options for patients with LAPC (6).
The standard treatment for LAPC remains a subject of debate internationally. The most recent clinical guidelines, including the 2024 REDISCOVER guidelines, “Conversion Surgery” position paper from the joint meeting of the International Association of Pancreatology, and Japan Pancreas Society in 2022, suggest that in cases of favorable response to neoadjuvant chemotherapy, surgical resection can be considered following discussion with the patient and their family. However, both guidelines emphasize the lack of sufficient evidence to recommend a specific timeline for surgical resection after neoadjuvant chemotherapy (7, 8).
Current treatment options for LAPC are evolving, with recent studies exploring the combination of stereotactic body radiotherapy (SBRT), chemotherapy, and immune checkpoint inhibitors (ICIs). Notably, the combination of FOLFIRINOX (FFX) chemotherapy followed by SBRT has shown the potential for improving survival outcomes in patients with LAPC, especially in selected individuals. This approach may also increase the likelihood of radical resection in patients whose tumors were initially deemed unresectable (9). Additionally, ICIs targeting CTLA-4, PD-1, and PD-L1 have demonstrated superior efficacy compared to cytotoxic chemotherapy alone (10, 11). However, both strategies are still in the clinical research phase, and further studies are required to validate these findings.
McCarthy et al. reported a case involving a patient with locally advanced pancreatic ductal adenocarcinoma who underwent treatment with PD-1 inhibitors and radiotherapy, followed by surgical resection, achieving nearly complete pathological remission after surgery (10). In contrast, we present a case of chemotherapy combined with targeted immunotherapy, without radiotherapy or surgical resection, which resulted in a favorable prognosis. We also reviewed the current literature for reports on the use of targeted immunotherapy for the treatment of pancreatic cancer, highlighting its potential role in improving outcomes in patients with LAPC.
2 Case presentationA 60-year-old female presented with a history of intermittent upper abdominal pain and discomfort lasting >10 d. Contrast-enhanced CT of the upper abdomen revealed a mass in the pancreatic neck with dilatation of the pancreatic duct, suggestive of a tumor (Figure 1).

Figure 1. Contrast-enhanced plain CT showed the mass in close contact with the surrounding blood vessels (A, B). After 2 months of targeted immunotherapy, the tumor was slightly reduced, and the relationship with the surrounding blood vessels was clearer (C, D). After conservative treatment for more than 1 year, the mass was significantly reduced and a ring of high-density shadow appeared around it, which was considered to form a calcification package (E–H). The size of the mass remained the same, whereas the density of the annular calcification layer increased (I, J).
2.1 Auxiliary examinationsRoutine blood tests, liver function tests, electrolyte levels, coagulation profiles, and preoperative evaluation revealed no significant abnormalities. However, serum tumor marker levels were elevated: carcinoembryonic antigen (CEA), 36.94 ng/mL (reference range: 0–10 ng/mL) and cancer antigen 125 (CA125), 50.46 U/mL (reference range: 0–35 U/mL). The patient’s lymphocyte percentage (LY%) was 15.9% (reference range: 20–50%), and the peripheral monocyte percentage (MO%) was 6.1% (reference range: 3–10%) (Figure 2).
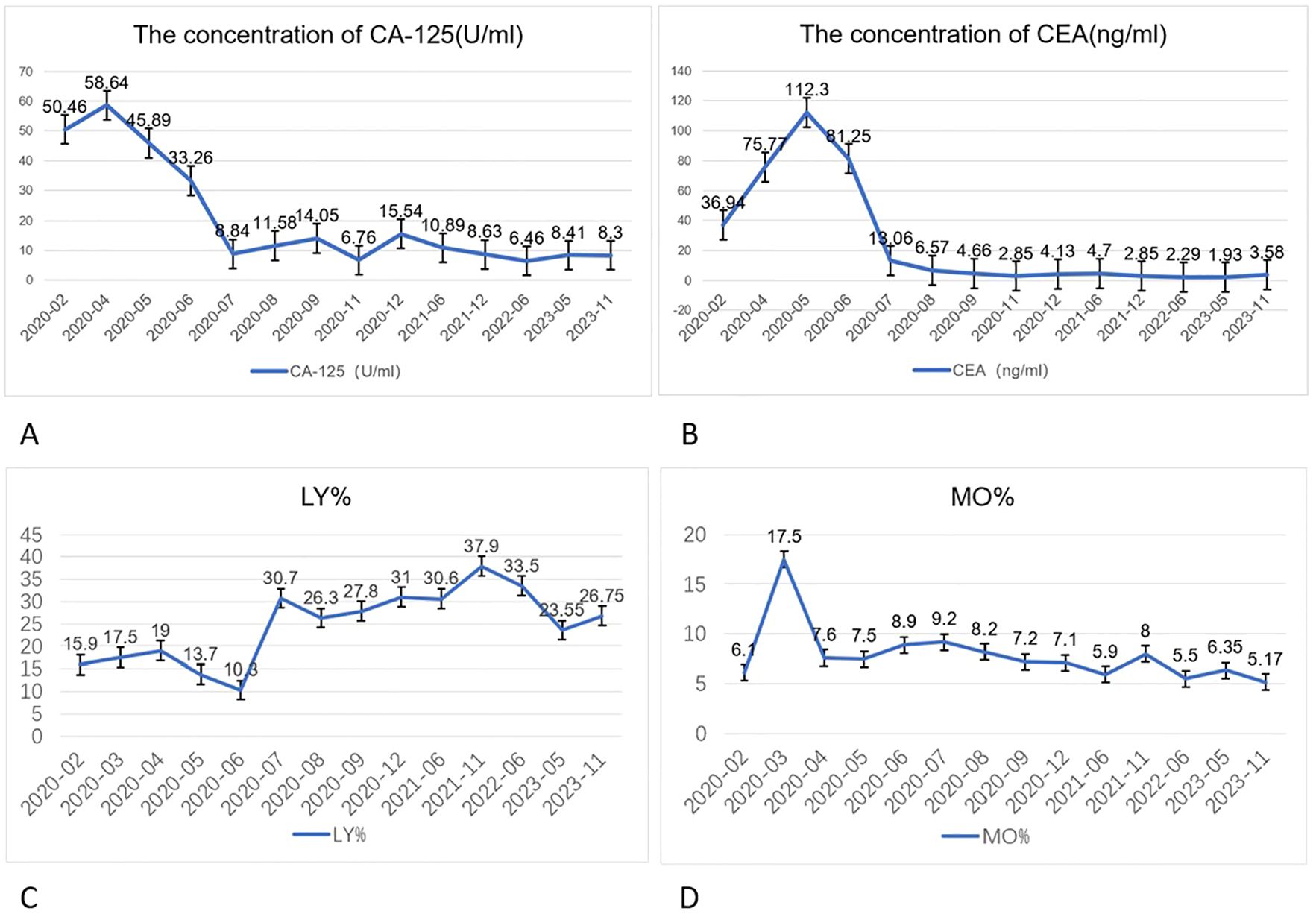
Figure 2. Changes in CA125 (A) (reference range: 0–35) and CEA (B) (reference range: 0–10) levels in the patient before and after treatment. These tumor markers showed a brief elevation in the initial treatment stage, followed by a decrease below the baseline level. Lymphocyte percentage (LY%) (C) returned to normal after three cycles of targeted immunotherapy (reference range: 20.00–50.00 %), and peripheral monocyte percentage (MO%) (D) returned to normal after the first cycle of targeted immunotherapy (reference range: 3.00–10.00 %).
Endoscopic ultrasound-guided fine-needle aspiration confirmed LAPC. Genetic testing revealed and mutation rates of 8.82% and 6.17% for the KRAS and TP53, respectively. Additionally, PD-L1 mRNA expression was moderately positive (28%).
2.2 Initial treatmentThe patient initially received a chemotherapy regimen consisting of gemcitabine, oxaliplatin, and S-1 (G&S regimen) for three cycles, along with oral pain management using acetaminophen and oxycodone tablets. Follow-up CT of the upper abdomen revealed a slightly enlarged pancreatic neck mass with persistent pancreatic duct dilatation. Serum CEA levels continued to rise, indicating a limited response to the initial chemotherapy.
2.3 Treatment modificationThe treatment regimen was modified based on the results of genetic testing. The treatment was switched to a combination of albumin-bound paclitaxel and capecitabine, along with the PD-1 inhibitor camrelizumab (200 mg, administered for six cycles). After three cycles of this new regimen, the patient developed multiple skin hemangiomas, necessitating the addition of apatinib. However, apatinib induced hypertension, which was treated with lenvatinib.
2.4 Further response and follow-upThe patient refused surgery throughout treatment. Follow-up imaging via enhanced CT showed that the pancreatic head mass had developed a high-density shadow at its periphery, suggesting the formation of a calcified layer surrounding the tumor. There was no evidence of metastasis to surrounding tissues or organs. Tumor marker levels gradually returned to normal, and the patient’s condition stabilized.
3 OutcomeForty-five months after the diagnosis, the patient’s serum CEA level decreased to 3.58 ng/mL, and the CA125 level was reduced to 8.30 U/mL. Treatment was discontinued, and there has been no evidence of recurrence.
This case highlights the potential of targeted immunotherapy combined with chemotherapy in the treatment of LAPC, particularly in patients who are not candidates for surgical resection.
4 DiscussionHerein, we presented the case of a 60-year-old woman with LAPC who initially received ineffective chemotherapy (G&S regimen). Genetic testing revealed mutations in KRAS (mutation rate: 8.82%) and TP53 (mutation rate: 6.17%). Subsequently, the patient was treated with the PD-1 inhibitor camrelizumab, which led to a remarkable outcome, with complete remission after 45 months of treatment, despite the patient’s consistent refusal to undergo surgery (Figure 3).
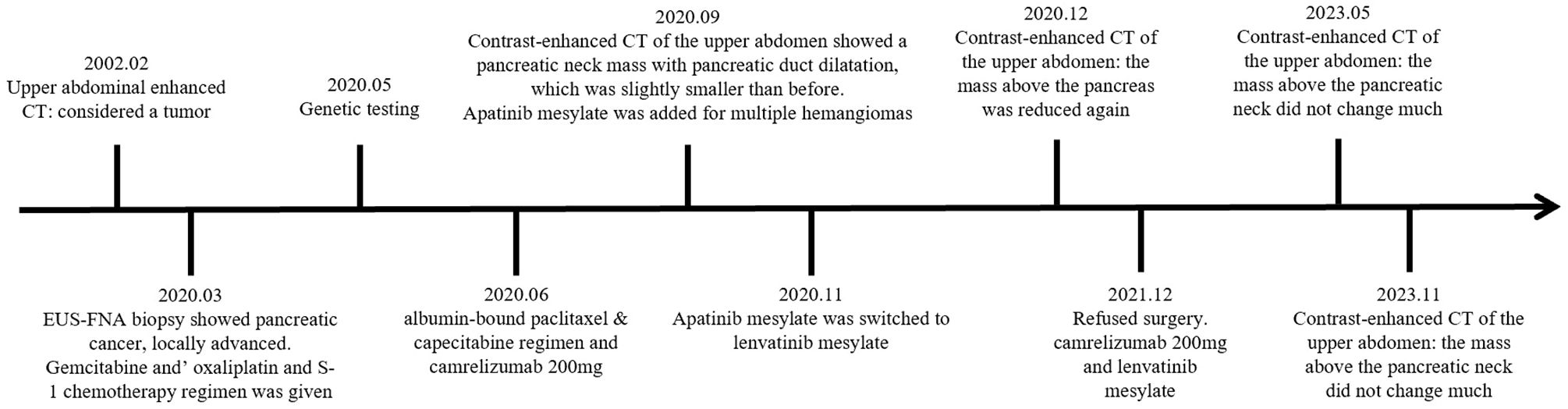
Figure 3. Timeline of the patient’s treatment.
The management approach for this case aligns with the American Society of Clinical Oncology Clinical Practice Guidelines, which suggest that targeted therapies such as PD-1 inhibitors could be considered in cases of LAPC with specific genetic alterations (12). After six cycles of targeted immunotherapy, an in-hospital discussion concluded that the patient had reached resectable conditions, leading to a recommendation for surgical resection. This is consistent with the latest clinical guidelines, which emphasize the potential utility of baseline CEA and CA125 in monitoring patients with LAPC. In this case, both tumor markers normalized after just three cycles of targeted immunotherapy.
Interestingly, the MO% decreased to normal levels after the first cycle of targeted immunotherapy, while the LY% returned to normal after three cycles. Monocytes have long been considered cytotoxic to various tumor cells and are often used as a prognostic marker in cancer, reflecting the tumor microenvironment (TME). The peripheral monocyte count correlates with the density of tumor-associated macrophages (TAMs), which play a critical role in immune evasion. In colorectal and pancreatic cancer, TAMs are known to suppress immune responses (13, 14). TAMs can be regulated by Treg cells, which prevent T-cell activation and inhibit the immune response via the fatty acid synthesis pathway mediated by sterol regulatory element-binding protein 1 (15, 16). The reduction in monocyte levels in this case may indicate a favorable modulation of the TME following immunotherapy.
Recent studies have suggested that patients with KRAS mutations tend to respond poorly to chemotherapy combined with immunotherapy. The KRAS G12D mutation induces immunosuppressive tumor environments in the early stages of malignancy, partly by expanding and reprogramming cancer-associated fibroblasts (CAFs) (17, 18). The deletion of CAFs has been shown to restore T-cell effector function and reduce tumor proliferation (19). However, in this case, the combination of albumin-bound paclitaxel, capecitabine chemotherapy, and camrelizumab demonstrated an unexpectedly favorable effect on the patient’s prognosis despite the presence of KRAS mutations.
Camrelizumab is a humanized PD-1 inhibitor that blocks the interaction between PD-L1 and its receptor PD-1, preventing immune evasion by tumors. In pancreatic cancer, PD-L1 expression is closely associated with CD163+ TAMs, and the cytokine TNF-α significantly increases PD-L1 mRNA expression by activating NF-κB signaling (20–22). Camrelizumab has demonstrated positive results in liver and advanced gastric cancer (23, 24). Furthermore, studies have shown that combining PD-1 inhibitors with neoadjuvant chemoradiotherapy improves the LAPC resection rate without significantly increasing postoperative complications (25). Animal experiments have shown that combined immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling can regulate PD-L1 expression at the transcriptional level through the p65 NF-κB pathway, induce the activation of CD8+ T cells in the pancreatic ductal adenocarcinoma (PDAC) microenvironment, and induce strong anti-tumor immune memory and secondary prevention to achieve better therapeutic effects (26). Other studies have shown that third-generation PD-L1-targeted CAR T cells enhance T cell persistence (27). In this case, after treatment with camrelizumab, follow-up CT revealed a significant reduction in the pancreatic mass, making surgical resection a feasible option.
Despite the development of multiple hemangiomas that were manageable with medication, the patient’s overall response to the targeted immunotherapy regimen was promising. This case highlights the potential of targeted immunotherapy, particularly in patients with LAPC who are not candidates for surgery or show inadequate responses to conventional chemotherapy. These results suggest that further research on targeted immunotherapy for LAPC is warranted, especially for patients with high PD-L1 expression, or those who do not tolerate or respond to cytotoxic chemotherapy.
The traditional treatment for pancreatic cancer mainly relies on surgery, chemotherapy, and radiation therapy. Only 15% of pancreatic cancer cases are considered resectable at the time of diagnosis. A retrospective study showed that only 4.3% of the 413 patients who received FFX or gemcitabine plus albumin-bound paclitaxel treatment successfully underwent surgery, with a median overall survival (OS) of 32.9 months (2, 28). In a study of induction chemotherapy combined with stereotactic body radiotherapy, the local recurrence rate of patients with LAPC who underwent conversion therapy for surgical resection was as high as 33% (29). One study showed that the median OS of 54 patients treated with proton beam therapy was 18.2 months, with only one patient surviving for >5 years (30). Mustafa Suker et al. showed that the median OS for patients undergoing FFX therapy was 24.2 months (95% CI 21.7–26.8) (31). A recent single-center retrospective cohort study showed that the median OS of patients with advanced pancreatic cancer treated with a sequence of FFX and gemcitabine/albumin-bound paclitaxel was 10.3 months (32). Passardi et al. followed up on 73 patients who received gemcitabine and oxaliplatin and large fractionated stereotactic radiotherapy for LAPC and showed that the median progression-free survival and OS were 10.2 (95% CI 7.8–13.2) and 14.3 (95% CI 12.0–18.1) months, respectively (33). A study that included 1047 patients who underwent LAPC radiofrequency ablation (RFA) showed that the median OS ranged from 6 to 33 months (34). Compared to traditional treatments, targeted immunotherapy may bring about a new era in the treatment of pancreatic cancer, especially unresectable pancreatic cancer. There are few large-sample prognostic studies on targeted immunotherapy for LAPC, and successful cases without surgery are even rarer. The significant effects observed in this case may promote the development of targeted immunotherapy and individualized treatments for pancreatic cancer.
Additionally, this case did not consider the costs or accessibility of the treatments, nor did it explore alternative therapies. Furthermore, the relationship between ICIs and TAMs warrants further investigation, as these interactions may provide new therapeutic opportunities.
5 Literature reviewA literature search was conducted using PubMed, Scopus, Web of Science, JAMA Network, and NEJM to identify cases in which targeted immunotherapy was successfully used to treat pancreatic cancer. Among the cases reviewed, one patient with PDAC underwent surgical resection after a combination of radiotherapy and targeted immunotherapy. Another case involved a patient with pancreatic head cancer and liver metastasis, who received targeted immunotherapy combined with RFA. Another case involved a patient with pancreatic acinar cell carcinoma, who had enlarged retroperitoneal lymph nodes and multiple pulmonary nodules. The patient responded well to targeted immunotherapy after chemotherapy failure. In all three cases, the patients tolerated the treatment well and showed no evidence of disease recurrence (Table 1).
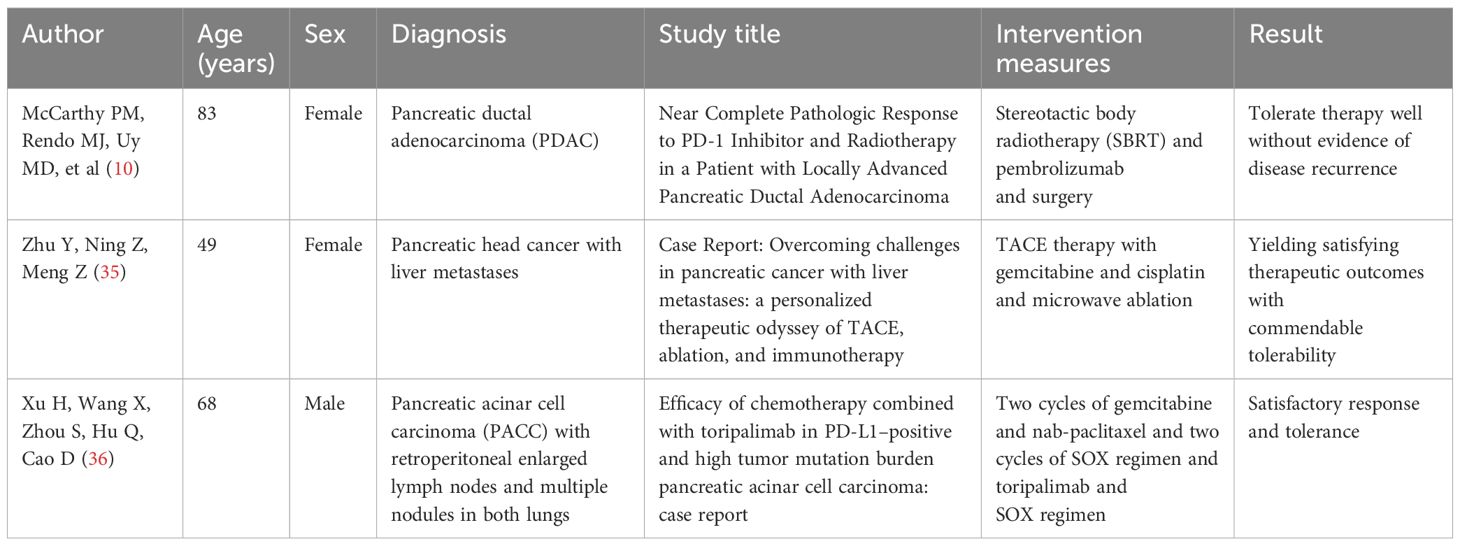
Table 1. Literature review.
In summary, the treatment method in this case has met the requirements of the latest international guidelines. However, due to the small sample size and high heterogeneity, our treatment method is not universally applicable to patients. However, this case report contributes to the growing body of evidence supporting the use of targeted immunotherapy in LAPC, particularly when combined with chemotherapy, without the need for radiotherapy or surgical resection. The favorable prognosis in this case provides valuable insights into potential treatment strategies for patients with LAPC.
6 ConclusionIn the present case, targeted immunotherapy led to a rare phenomenon in which the pancreatic head tumor was encased in a calcified layer encapsulating the cancerous tissue. Despite recent studies indicating that patients with KRAS mutations often respond poorly to chemotherapy combined with immunotherapy, this case highlights an unexpected and favorable outcome. The combination of albumin-bound paclitaxel, capecitabine chemotherapy, and camrelizumab significantly improved patient prognosis, demonstrating the potential of targeted immunotherapy in treating LAPC.
The traditional treatment for pancreatic cancer relies primarily on surgery, chemotherapy, and radiation therapy. This case highlights the promising efficacy of ICIs in LAPC, offering hope to patients with advanced unresectable disease. However, further research is needed to define the specific criteria for patient selection, as the clinical application of ICIs in LAPC remains complex and individualized. The remarkable results observed in this case may help advance targeted immunotherapy and personalized treatment for pancreatic cancer. Targeted immunotherapy should be considered a viable treatment option for frail or unresectable LAPCs.
Data availability statementThe original contributions presented in this study are included in the article/supplementary materials. For further inquiries, please contact the corresponding author.
Ethics statementThis study was approved by the Ethics Committee of the Affiliated Hospital of the Second Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsYB: Funding acquisition, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. HY: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Writing – review & editing. XM: Methodology, Resources, Supervision, Validation, Writing – review & editing. SC: Resources, Supervision, Validation, Writing – original draft.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
PubMed Abstract | Crossref Full Text | Google Scholar
3. Walter FM, Mills K, Mendonça SC, Abel GA, Basu B, Carroll N, et al. Symptoms and patient factors associated with diagnostic intervals for pancreatic cancer (SYMPTOM pancreatic study): a prospective cohort study. Lancet Gastroenterol Hepatol. (2016) 1:298–306. doi: 10.1016/S2468-1253(16)30079-6
PubMed Abstract | Crossref Full Text | Google Scholar
4. Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. (2015) 22:1153–9. doi: 10.1245/s10434-014-4225-1
PubMed Abstract | Crossref Full Text | Google Scholar
5. Stoop TF, Seelen L, Van’t Land FR, Lutchman KRD, van Dieren S, Lips DJ, et al. ASO visual abstract: nationwide use and outcome of surgery for locally advanced pancreatic cancer following induction chemotherapy. Ann Surg Oncol. (2024) 31:2640–53. doi: 10.1245/s10434-023-14650-6
PubMed Abstract | Crossref Full Text | Google Scholar
6. Stoop TF, Seelen L, van ‘t Land FR, Lutchman KRD, van Dieren S, Lips DJ, et al. Nationwide use and outcome of surgery for locally advanced pancreatic cancer following induction chemotherapy. Ann Surg Oncol. (2023) 25(S2):S192–287. doi: 10.1016/j.hpb.2023.07.176
PubMed Abstract | Crossref Full Text | Google Scholar
7. Boggi U, Kauffmann EF, Napoli N, Barreto SG, Besselink MG, Fusai GK, et al. REDISCOVER guidelines for borderline-resectable and locally advanced pancreatic cancer: management algorithm, unanswered questions, and future perspectives. Updates Surg. (2024) 76:1573–91. doi: 10.1007/s13304-024-01860-0
PubMed Abstract | Crossref Full Text | Google Scholar
8. Oba A, Del Chiaro M, Fujii T, Okano K, Stoop TF, Wu YHA, et al. Conversion surgery” for locally advanced pancreatic cancer: A position paper by the study group at the joint meeting of the International Association of Pancreatology (IAP) & Japan Pancreas Society (JPS) 2022. Pancreatology. (2023) 23:712–20. doi: 10.1016/j.pan.2023.06.005
PubMed Abstract | Crossref Full Text | Google Scholar
9. Teriaca MA, Loi M, Suker M, Eskens F, van Eijck C, Nuyttens JJ. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): Long-term outcome. Radiother Oncol. (2021) 155:232–6. doi: 10.1016/j.radonc.2020.11.006
PubMed Abstract | Crossref Full Text | Google Scholar
10. McCarthy PM, Rendo MJ, Uy MD, Adams AM, O'Shea AE, Nelson DW, et al. Near complete pathologic response to PD-1 inhibitor and radiotherapy in a patient with locally advanced pancreatic ductal adenocarcinoma. Onco Targets Ther. (2021) 14:3537–44. doi: 10.2147/OTT.S311661
PubMed Abstract | Crossref Full Text | Google Scholar
12. Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, et al. Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2016) 34:2654–68. doi: 10.1200/JCO.2016.67.5561
PubMed Abstract | Crossref Full Text | Google Scholar
13. Yang S, Liu Q, Liao Q. Tumor-associated macrophages in pancreatic ductal adenocarcinoma: origin, polarization, function, and reprogramming. Front Cell Dev Biol. (2020) 8:607209. doi: 10.3389/fcell.2020.607209
PubMed Abstract | Crossref Full Text | Google Scholar
14. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Matsutani S, et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC Cancer. (2017) 17:404. doi: 10.1186/s12885-017-3395-1
PubMed Abstract | Crossref Full Text | Google Scholar
15. Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8+ T cell-derived interferon-γ. Immunity. (2019) 51:381–397.e6. doi: 10.1016/j.immuni.2019.06.017
PubMed Abstract | Crossref Full Text | Google Scholar
18. Velez-Delgado A, Donahue KL, Brown KL, Du W, Irizarry-Negron V, Menjivar RE, et al. Extrinsic KRAS signaling shapes the pancreatic microenvironment through fibroblast reprogramming. Cell Mol Gastroenterol Hepatol. (2022) 13:1673–99. doi: 10.1016/j.jcmgh.2022.02.016
PubMed Abstract | Crossref Full Text | Google Scholar
19. Krishnamurty AT, Shyer JA, Thai M, Gandham V, Buechler MB, Yang YA, et al. LRRC15(+) myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature. (2022) 611:148–54. doi: 10.1038/s41586-022-05272-1
PubMed Abstract | Crossref Full Text | Google Scholar
20. Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J Exp Clin Cancer Res. (2019) 38:87. doi: 10.1186/s13046-019-1085-3
PubMed Abstract | Crossref Full Text | Google Scholar
21. Khasraw M, Reardon DA, Weller M, Sampson JH. PD-1 Inhibitors: Do they have a Future in the Treatment of Glioblastoma. Clin Cancer Res. (2020) 26:5287–96. doi: 10.1158/1078-0432.CCR-20-1135
PubMed Abstract | Crossref Full Text | Google Scholar
22. Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W, et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. (2023) 22:58. doi: 10.1186/s12943-023-01725-x
PubMed Abstract | Crossref Full Text | Google Scholar
23. Cai J, Wang D, Zhang G, Guo X. The role of PD-1/PD-L1 axis in treg development and function: implications for cancer immunotherapy. Onco Targets Ther. (2019) 12:8437–45. doi: 10.2147/OTT.S221340
PubMed Abstract | Crossref Full Text | Google Scholar
24. Liu H, Li Y, Yao Y, Chen K, Gan J. Meta-analysis of efficacy and safety of karelizumab combined with apatinib in the treatment of advanced gastric cancer. Dis Markers. (2022) 2022:6971717. doi: 10.1155/2022/6971717
PubMed Abstract | Crossref Full Text | Google Scholar
25. Du J, Lu C, Mao L, Zhu Y, Kong W, Shen S, et al. PD-1 blockade plus chemoradiotherapy as preoperative therapy for patients with BRPC/LAPC: A biomolecular exploratory, phase II trial. Cell Rep Med. (2023) 4:100972. doi: 10.1016/j.xcrm.2023.100972
PubMed Abstract | Crossref Full Text | Google Scholar
26. Zhang X, Lao M, Xu J, Duan Y, Yang H, Li M, et al. Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors. J Immunother Cancer. (2022) 10:e003982. doi: 10.1136/jitc-2021-003982
PubMed Abstract | Crossref Full Text | Google Scholar
27. Yang CY, Fan MH, Miao CH, Liao YJ, Yuan RH, Liu CL. Engineering chimeric antigen receptor T cells against immune checkpoint inhibitors PD-1/PD-L1 for treating pancreatic cancer. Mol Ther Oncolytics. (2020) 17:571–85. doi: 10.1016/j.omto.2020.05.009
PubMed Abstract | Crossref Full Text | Google Scholar
28. Ide Y, Otsuka T, Shimokawa M, Koga F, Ueda Y, Nakazawa J, et al. Conversion surgery for unresectable pancreatic cancer treated with FOLFIRINOX or gemcitabine plus nab-paclitaxel. Anticancer Res. (2023) 43:1817–26. doi: 10.21873/anticanres.16335
PubMed Abstract | Crossref Full Text | Google Scholar
29. Hill C, Sehgal S, Fu W, Hu C, Reddy A, Thompson E, et al. High local failure rates despite high margin-negative resection rates in a cohort of borderline resectable and locally advanced pancreatic cancer patients treated with stereotactic body radiation therapy following multi-agent chemotherapy. Cancer Med. (2022) 11:1659–68. doi: 10.1002/cam4.v11.7
PubMed Abstract | Crossref Full Text | Google Scholar
30. Seto I, Yamaguchi H, Takagawa Y, Azami Y, Takayama K, Suzuki M, et al. Clinical outcomes of proton beam therapy for unresectable locally advanced pancreatic cancer: A single-center retrospective study. Adv Radiat Oncol. (2024) 9:101577. doi: 10.1016/j.adro.2024.101577
PubMed Abstract | Crossref Full Text | Google Scholar
31. Queck A, Elango S, Koch C, Walter D, Schmidt J, Trebicka J, et al. Sequence therapy with FOLFIRINOX and gemcitabine/nab-paclitaxel for patients with advanced pancreatic cancer: A monocentre retrospective cohort study. Oncol Res Treat. (2022) 45:79–87. doi: 10.1159/000521258
PubMed Abstract | Crossref Full Text | Google Scholar
32. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. (2016) 17:801–10. doi: 10.1016/S1470-2045(16)00172-8
PubMed Abstract | Crossref Full Text | Google Scholar
33. Granata V, Grassi R, Fusco R, Belli A, Palaia R, Carrafiello G, et al. Local ablation of pancreatic tumors: State of the art and future perspectives. World J Gastroenterol. (2021) 27:3413–28. doi: 10.3748/wjg.v27.i23.3413
PubMed Abstract | Crossref Full Text | Google Scholar
34. Passardi A, Rapposelli IG, Scarpi E, Sullo FG, Bartolini G, Neri E, et al. Multimodal treatment with GEMOX plus helical tomotherapy in unresectable locally advanced pancreatic cancer: A pooled analysis of two phase 2 studies. Biomolecules. (2021) 11:1200. doi: 10.3390/biom11081200
PubMed Abstract | Crossref Full Text | Google Scholar
35. Zhu Y, Ning Z, Meng Z. Case Report: Overcoming challenges in pancreatic cancer with liver metastases: a personalized therapeutic odyssey of TACE, ablation, and immunotherapy. Front Immunol. (2023) 14:1275782. doi: 10.3389/fimmu.2023.1275782
PubMed Abstract | Crossref Full Text | Google Scholar
36. Xu H, Wang X, Zhou S, Hu Q, Cao D. Efficacy of chemotherapy combined with toripalimab in PD-L1-positive and high tumor mutation burden pancreatic acinar cell carcinoma: case report. Tumori. (2021) 107:NP24–7. doi: 10.1177/0300891620980792
留言 (0)