Cancer remains a leading cause of morbidity and mortality worldwide, imposing a substantial burden on global health (1, 2). Although cancer predominantly affects individuals aged>50 years, recent years there has been a notable increase in early-onset cancers (diagnosed in individuals aged<50 years) across various regions (3). This increase in early-onset cancers has far-reaching consequences on individuals and society, adding the overall disease burden (4, 5). Moreover, the adverse effects of cancer treatments at a younger age may result in long-term health complications, further worsening the burden associated with early-onset cancers (6).
Similarly, the incidence of early-onset head and neck squamous cell carcinoma (HNSCC) is increasing. HNSCC accounts for approximately 6% of all cancers globally and is ranked the sixth most common cancer (7, 8). Each year, approximately 900,000 new cases of head and neck cancers are diagnosed worldwide, with over 400,000 deaths annually (9).
While most HNSCC cases are diagnosed in older individuals (median age of 65 years) (10), the incidence among younger patients has increased, particularly in Asia, with marked increases in oral and oropharyngeal squamous cell carcinomas, particularly tongue and tonsil cancers (7, 11, 12). Traditional risk factors for HNSCC include tobacco and alcohol use; however, additional etiologies include Epstein-Barr virus (EBV) infection in the nasopharynx and human papillomavirus (HPV) infection in the oropharynx (13, 14). In Southeast Asia, the increasing use of betel nuts and the rising prevalence of HPV infections among younger populations have contributed to the growing incidence of HNSCC in this demographic (11, 15). Studies indicate that young patients with HNSCC (typically defined as individuals aged ≤30 to ≤45, accounting for 1%–8% of all HNSCC cases) exhibit distinct disease characteristics and progression patterns compared to older patients (16, 17); however, the mechanisms underlying these differences and the associated potential for targeted treatment remain unclear.
Emerging evidence suggests that younger cancer patients with cancer, including those with HNSCC, may present with unique biological and tumor microenvironmental (TME) characteristics. The TME comprises diverse cellular components and molecular signals, including immune cells, fibroblasts, endothelial cells, and various cytokines (18, 19), exhibiting distinct features in terms of inflammatory responses, immune evasion, and microenvironmental regulation. Studies have shown that the TME in younger patients may contain elevated levels and activity of immunosuppressive cells, such as regulatory T cells and myeloid-derived suppressor cells (20, 21), or a higher proportion of programmed death ligand 1 (PD-L1) expressing antigen presenting cells (APCs) (22), which could influence their response to immunotherapy. Therefore, exploring the unique TME in young patients with HNSCC is essential for understanding its underlying pathogenesis and to laying the groundwork for future, targeted therapeutic approaches.
In this study, we reviewed the current findings of the TME in early-onset HNSCC patients. The characteristics of the included studies were carefully presented and assessed, and the relevant findings (including TME profile and correlation factors) were summarized. Consequently, the potential immunotherapy for this age group was also discussed.
2 Materials and methodsLiterature containing early-onset or young HNSCC in its the title or abstract from the last 20 years up to the end of October 2024) was retrieved through searching PubMed, Web of Science Core Collection, MEDLINE and Embase.
Inclusion criteria: Original articles investigating the characteristics of early-onset HNSCC.
Exclusion criteria: Review, meeting abstract, original research or bioinformatic analysis without real pathological specimen data, and literature not in English.
The exact search strategy, results and selection flow chart are presented in the Supplementary Materials.
3 Results3.1 Characterization of included studiesAfter the screening, 31 studies were included in the final analysis. Different definitions of ‘younger’ and ‘older’ patients groups were observed (Figure 1A). Twenty-four studies defined younger patients as those aged<40 years (n=12) or <45 years (n=12), five studies defined younger patients as those aged<50 years, and one study defined younger patients as those aged<70 years. Studies defined the older group as follows: patients >45 years old; patients aged 40 (n=5), 50 (n=4), and 60 years (n=4); and patients aged >70 years (n=2).
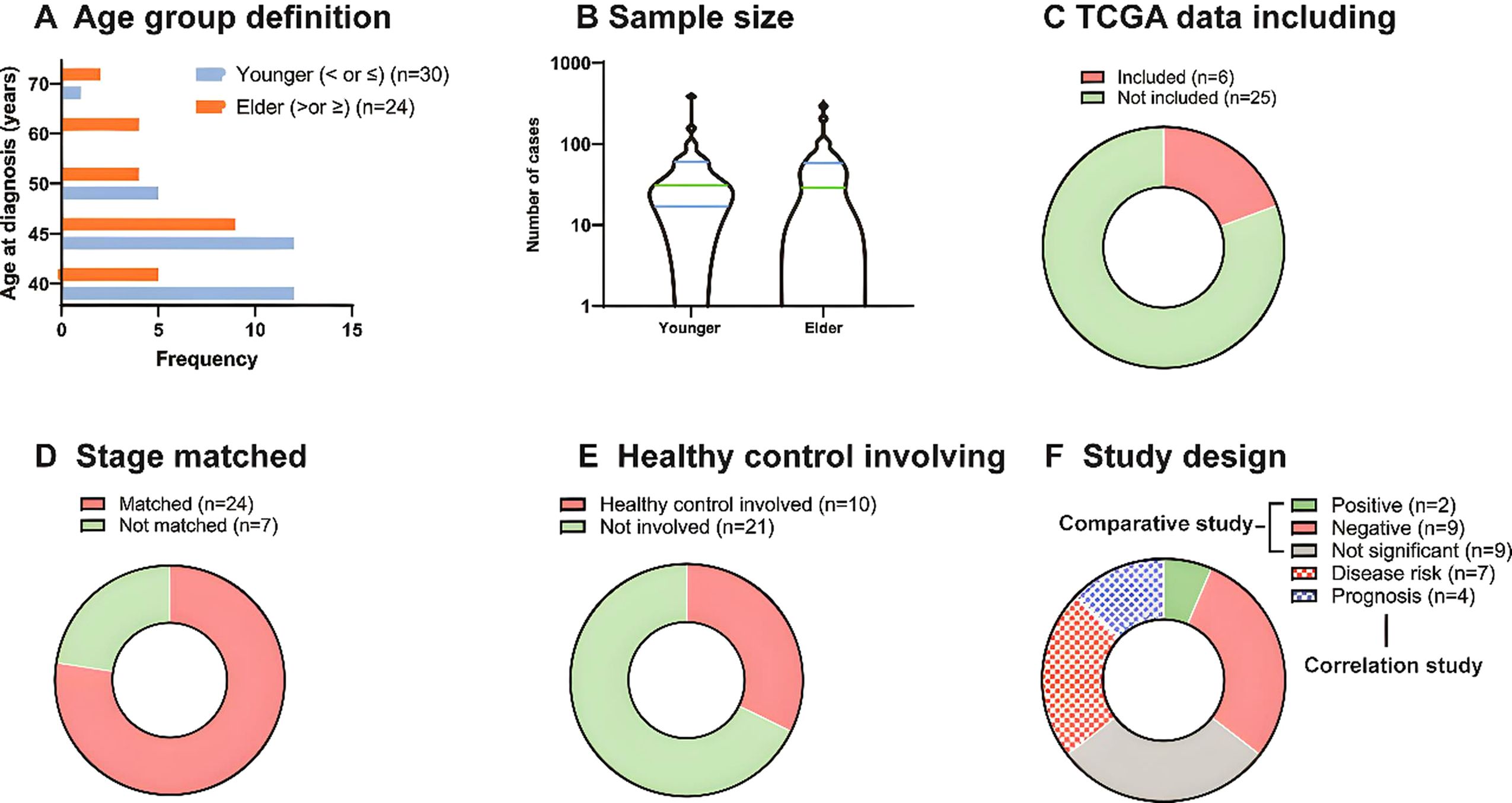
Figure 1. Characterization of included studies. (A) The frequency of age group definition of included studies. (B) The sample size of younger and older patient groups. (C) TCGA data including of included studies. (D) TNM stage match of the included studies. (E) Healthy (blank) control involving of the included studies. (F) Study design of included studies. There were 20 comparative studies and 11 correlation studies in total, and 2, 9, 9 studies reported positive, negative and not significant of younger patients compared to older ones. And 7 studies presented disease correlation and 4 presented prognostic correlation.
The sample sizes also varied (Figure 1B). Nine studies did not include an older group, and the number of younger and older patients ranged from 4 to 386 and 4 to 294, respectively, with a relatively similar median. Among the 31 studies, 6 included The Cancer Genome Atlas (TCGA) data (Figure 1C), 24 matched TNM stages between patient groups (Figure 1D), and 10 used healthy or blank controls for comparison with the study groups (Figure 1E). Finally, among 20 comparative studies, 2 studies showed that younger groups were positive for tumor aggressiveness compared to older groups, 9 showed negative results, and 9 showed non-significant results. In addition, among 11 correlation studies, 7 presented disease risk and 4 prognosis correlations (Figure 1F).
3.2 Possible TME of early-onset HNSCCAmong the 31 studies included in the final analysis, 20 reported comparisons between patients with early- and late- onset HNSCC, and 11 reported potential correlations between specific factors and early onset. The possible TME based on these studies are shown in Figure 2. The two positive results showed that young patients presented a less harmful TME than older patients. These include lower expression of enhancer of zeste homolog 2 (EZH2) (23) and lower neutrophil-to-lymphocyte ratio (NLR) (24) in younger patients, reflecting lower invasion and metastasis of the tumor behavior in this age group. However, the remaining nine studies showed opposing results, indicating that younger tumors have a more aggressive TME. Increased extracellular matrix (ECM) remodeling, epithelial-to-mesenchymal transition (EMT), and a decreased quantity of CD8+T cells lead to a more immunosuppressive TME (20). The enrichment of inflammatory microbes, increased levels of reactive oxygen species (25) and MMP-9 expression (26) result into inflammatory TME. In addition, the increased WGD (27), EGFR level (26, 28, 29), nuclear polymorphism and mitotic index (30), and P16 methylation leads to abnormal cell proliferation and invasion (31).
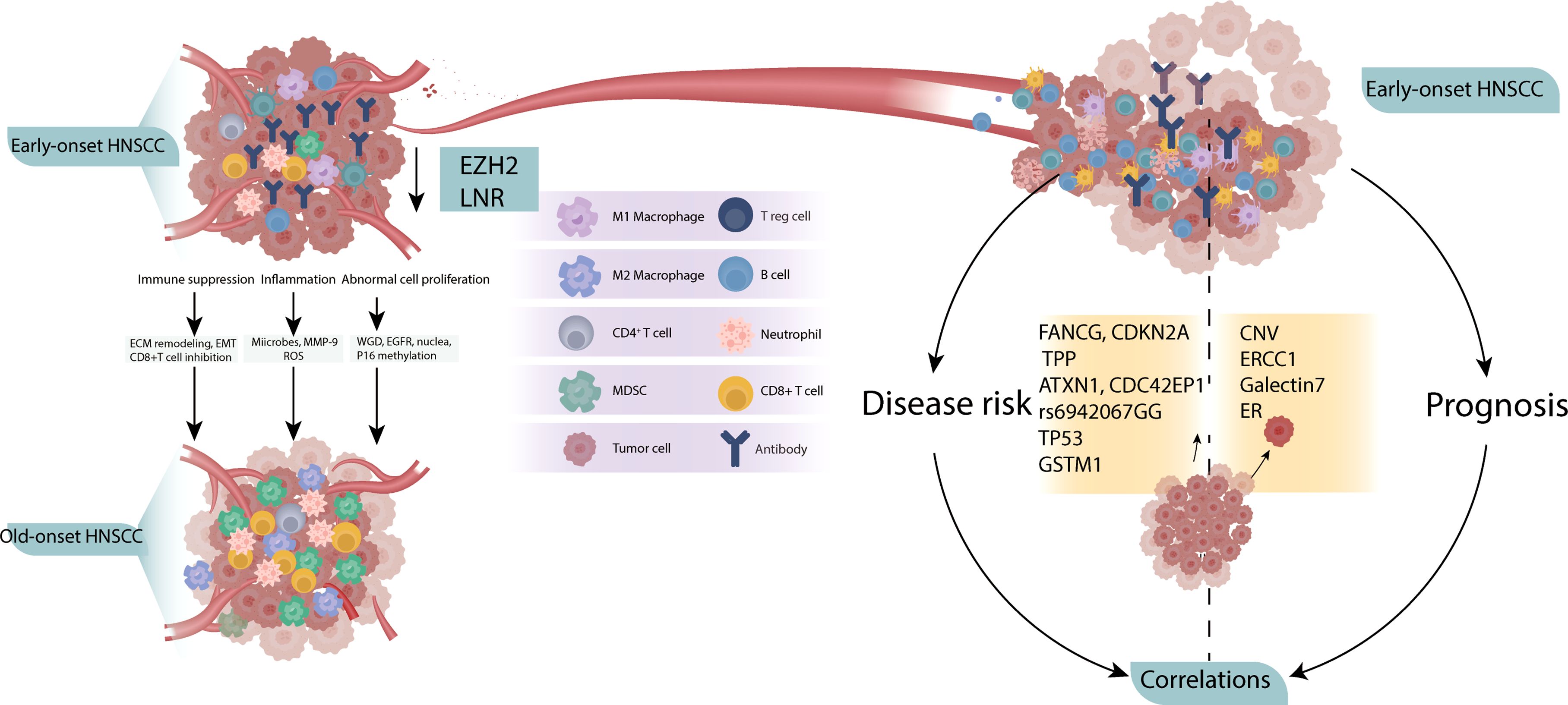
Figure 2. Possible TME of early-onset HNSCC. Compared to old-onset, the early-onset group presented a more aggressive TME (left), with independent correlation studies showing potential disease risk and prognosis (right).
Correlation studies have identified early-onset factors for both disease and prognosis. The following factors have been associated with HNSCC risk in young patients: major histocompatibility complex class I-related chain A (MICA) A5.1 homozygous genotype (32), germline variants in FANCG, CDKN2A and TPP (33), drive genes ATXN1 and CDC42EP1 (34), rs6942067 GG genotype (35) in non-HPV and non-smokers, TP53 variation (36), HPV16 positive (37) and GSTM1 null genotype (38) were reported to be associated with HNSCC risk in young patients. In addition, copy number variation (39), ERCC1 (40), galectins-7 (41) and estrogen hormone receptor expression (42) are associated with the prognosis to young HNSCC patients.
4 Discussion4.1 Altered TME in young groupThe evolving landscape of early-onset HNSCC demands a better understanding of host-tumor interactions in the TME to improve the effectiveness of immunotherapy. However, the impact of complex tumor-infiltrating immune cell profiles on responses to immune checkpoint inhibitors is not fully understood; limited evidence has yielded contradictory findings. Although only direct analysis of the TME was reported, the studies showed that early-onset HNSCC is distinct from average-onset terms of tumor behavior and prognosis, indicating different therapeutic demands.
According to Révész et al. (23) and Zhang et al. ‘s (24) studies, TME of younger patients presented more gently compared to older ones. EZH2 expression and NLR were lower in the young groups than in the older group. Having a well-defined oncogenic role in cancer initiation, progression, metastasis, metabolism, and drug resistance, and in the modulation of antitumor immunity in various cancers, EZH2 has been defined as an effective marker of the tumor aggressiveness and tumorigenic potential and plays essential roles in driving cancer cell immunoediting and as an immune escape regulator (43). Inhibition of EZH2 could suppress oral squamous cell carcinoma (OSCC) progression via modulate EZH2/Wnt/beta-catenin pathway, both in vitro and in vivo (44). Clinically, the use of EZH2 inhibitors in combination with IO represents a compelling strategy to remodel the TME, potentially overcoming immune evasion and enhancing therapeutic outcomes in breast cancer (16), mesothelioma (45), non-Hodgkin lymphoma (46) and other cancers.
Similarly, a low NLR is associated with reduced cancer invasion and metastasis of the cancer. When this is observed in young patients, a better prognosis is usually expected. In patients with muscle-invasive bladder cancer (MIBC), this is associated with increased CD3+ T cells and B cell infiltration, lead to improved response and long-term outcomes (47).
Genetic factors were reported to be associated with the occurrence of HNSCC or prognosis. The membrane protein MICA generate response to various cellular stresses such as infection and oncogenic transformation, its mechanism of A5.1 allele association with disease risk of young OSCC remained unclear. Current evidence shows it’s essential in etiology and immune response of cancer, both positive or negative. Li et al. reported MICA expression positively related to the CD8+ T cell infiltration in hepatocellular carcinoma (48), however Wu et al. found the releasing of MICA progressed tumor immune escape (49).
Cury et al. reported germline variants CDKN2A and RECQL4 are associated with young HNSCC (33). CDKN2A variant closely associated with weak expression of immune-inflammatory pathways in the TME, potentially leading to reduced immune cell activity and weakened immune responses. RECQL4 variant may play a critical role in tumorigenesis and progression by regulating immune responses. Also, these two variants played roles in immune infiltration and the interactions of chemokines and their receptors under immune cells in melanoma (50), and existed in pancreatic ductal adenocarcinoma but are not currently actionable targeted (51). Similarly, ATXN1 and CDC42EP1 have also been reported as driver genes in HNSCC; however, their relevance to young patients cannot be conclusively determined (34). On SNP level, rs6942067 GG genotype is significantly higher in young and in HPV negative non-smoking HNSCC than in other HNSCC, which associated with DCBLD1 expression (35, 52). In addition, TP53 variation, HPV16 positive and GSTM1 null genotype were also mentioned. Different studies also showed CNV, ERCC1 expression, galectin-7 and Estrogen related to prognosis in young HNSCC. Overall, distinct genotypes may either promote or inhibit tumor progression by influencing various components within the TME. In young HNSCC patients, the high expression of specific genotypes has been associated with immune microenvironment activation. In addition, CD8+ T cells are crucial effector cells in anti-tumor immune responses the decreased amount in TME suggested a heightened state of immune suppression. The diminished immune surveillance allows tumor cells to evade detection and destruction by the immune system, thereby promoting tumor growth and metastasis.
Furthermore, the marked acceleration in cell proliferation of younger patient indicated that tumor cells may exhibit dysregulation in the signaling pathways controlling proliferation. Such abnormal proliferation is often associated with imbalances in cell cycle regulation and disruptions in apoptotic mechanisms, further driving rapid tumor growth and dissemination (53, 54).
The crosstalk among cells in the TME plays essential roles in the development and progression of HNSCC. Although there is no direct evidence showing exact cellular crosstalk, it can be inferred that the early-onset group exhibits altered communication between immune cells (lymphocytes and CD8+ T cells) and tumor cells compared to the older group. Additionally, the different crosstalk induced by changes in cellular molecules (e.g., EZH2 protein) also plays an indirect role.
4.2 Heterogeneity of the studiesThe included studies were highly heterogenous. The definition of younger groups had varying age ranges depending on different guidelines. The samples sizes of the young and older groups were relatively similar; however, large cohort data is lacking. Some studies involved supplementary data from TCGA, which increased the number of patients available for comparison; however, considering the characteristics of different ethnicities and nationalities, bias may exist. Most studies matched TNM stages between groups, while less involved healthy patients as blank controls. These variations contribute to the unreliability of the studies, complicating efforts to combine and compare results. To reduce heterogeneity and achieve more definitive conclusions, subgroup analyses and meta-analyses are recommended when sufficient data points are available.
4.3 Personalized immunotherapy tailoring based on current TME findingsPersonalized immunotherapy emphasizes treatment to each patient’s unique tumor profile and immune response, significantly enhancing effectiveness and reducing side effects compared to standard approaches (55–57). To develop personalized immunotherapy for specific cohorts, understanding the TME is essential (58, 59). Although current evidence is limited in scale and fragmented, it can be concluded that immunotherapy for early-onset HNSCC patients should focus on targeting EGFR, inhibiting ECM remodeling and EMT, and paying attention to high P16 methylation and specific coexisting microbial infections.
Additionally, defined genetic risk factors, including variations in MICA A5.1, FANCG, CDKN2A, and TPP, as well as alterations in ATXN1, CDC42EP1, and TP53, offer potential therapeutic and preventive pharmacological targets. Furthermore, CNV, ERCC1, galectins-7, and ER expression are promising candidate predictive biomarkers. It can be learned that multi-dimensional approaches including blood test, immunohistochemistry, PCR, RNA-sequencing, whole exosome sequencing, microbiota and whole genome have been used from comparative and correlation studies.
For young patients, the focus should be on strategies that restore CD8+ T cell function, regulate associated genetic factors, and target immune escape mechanisms (such as MICA shedding). EZH2 inhibitors have shown potential in remodeling the TME and enhancing immune responses. Therefore, in young patients, if the immune suppression in the TME is low, the combination of EZH2 inhibitors with immunotherapy therapies may be particularly effective. For older patients, this combination therapy may also be effective, but the treatment regimen should be adjusted based on the specific TME characteristics.
4.4 Future worksOverall, based on the current findings, larger-scale clinical studies are necessary in the future to verify these results. Further investigations into TME cellular characteristics, such as EZH2, MICA expression, and NLR, would also provide valuable evidence. Additionally, developing new therapeutic targets and predictive biomarkers based on these findings and translating them into real clinical practice is anticipated.
5 ConclusionIn conclusion, early-onset HNSCC demonstrates unique TME characteristics, often marked by heightened aggressiveness and immune suppression compared to HNSCC in older patients. These findings highlight the need for further investigation into the specific mechanisms driving these age-related differences. Personalized immunotherapy provide potential as an effective therapeutic strategy for early-onset HNSCC, underscoring the importance of tailored approaches in addressing the distinct clinical and biological features of this patient cohort.
Author contributionsYS: Writing – original draft, Writing – review & editing. DH: Writing – original draft. FY: Writing – review & editing. WH: Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1522820/full#supplementary-material
References1. Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. (2023) 9:465–72. doi: 10.1001/jamaoncol.2022.7826
PubMed Abstract | Crossref Full Text | Google Scholar
2. Ma X, Yu H. Global burden of cancer. Yale J Biol Med. (2006) 79:85–94.
3. Ugai T, Sasamoto N, Lee H-Y, Ando M, Song M, Tamimi RM, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. (2022) 19:656–73. doi: 10.1038/s41571-022-00672-8
PubMed Abstract | Crossref Full Text | Google Scholar
4. He T-C, Li J-A, Xu Z-H, Chen Q-D, Yin H-L, Pu N, et al. Biological and clinical implications of early-onset cancers: A unique subtype. Crit Rev Oncology/Hematology. (2023) 190:104120. doi: 10.1016/j.critrevonc.2023.104120
PubMed Abstract | Crossref Full Text | Google Scholar
5. Hamilton AC, Donnelly DW, Fitzpatrick D, Coleman HG. Early-onset cancers in adults: A review of epidemiology, supportive care needs and future research priorities. Cancers (Basel). (2022) 14(16):4021. doi: 10.3390/cancers14164021
PubMed Abstract | Crossref Full Text | Google Scholar
6. Lustberg MB, Kuderer NM, Desai A, Bergerot C, Lyman GH. Mitigating long-term and delayed adverse events associated with cancer treatment: implications for survivorship. Nat Rev Clin Oncol. (2023) 20:527–42. doi: 10.1038/s41571-023-00776-9
PubMed Abstract | Crossref Full Text | Google Scholar
8. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
PubMed Abstract | Crossref Full Text | Google Scholar
10. Hsieh Ronan W, Gooding William E, Nilsen M, Kubik M, Kelly Z, Sridharan S, et al. Association of patient and tumor characteristics with outcomes in young head and neck squamous cell carcinoma patients. Clin Otolaryngol. (2025) 50(1):15–21. doi: 10.1111/coa.14215
PubMed Abstract | Crossref Full Text | Google Scholar
11. Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J. (2022) 233:780–6. doi: 10.1038/s41415-022-5166-x
PubMed Abstract | Crossref Full Text | Google Scholar
12. Chen Z, Chan ABW, Kam LS, Chan MH, Chan JYK, Lee WT, et al. Changes in the incidence and human papillomavirus-positive portion of oropharyngeal squamous cell carcinoma in Hong Kong. Cancers (Basel). (2024) 16(1):226. doi: 10.3390/cancers16010226
PubMed Abstract | Crossref Full Text | Google Scholar
13. Broccolo F, Ciccarese G, Rossi A, Anselmi L, Drago F, Toniolo A. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non- keratinizing squamous cell carcinoma of the oropharynx. Infect Agent Cancer. (2018) 13:32. doi: 10.1186/s13027-018-0205-6
PubMed Abstract | Crossref Full Text | Google Scholar
14. Makvandi M, Jalilian S, Faghihloo E, Khanizadeh S, Ramezani A, Bagheri S, et al. Prevalence of human papillomavirus and co-infection with epstein-barr virus in oral and oropharyngeal squamous cell carcinomas. Asian Pac J Cancer Prev. (2022) 23:3931–7. doi: 10.31557/APJCP.2022.23.11.3931
PubMed Abstract | Crossref Full Text | Google Scholar
15. Joshi P, Dutta S, Chaturvedi P, Nair S. Head and neck cancers in developing countries. Rambam Maimonides Med J. (2014) 5:e0009. doi: 10.5041/RMMJ.20769172
Crossref Full Text | Google Scholar
16. Zhou LL, Yu CW. Epigenetic modulations in triple-negative breast cancer: Therapeutic implications for tumor microenvironment. Pharmacol Res. (2024) 204:107205. doi: 10.1016/j.phrs.2024.107205
PubMed Abstract | Crossref Full Text | Google Scholar
18. Wei R, Liu S, Zhang S, Min L, Zhu S. Cellular and extracellular components in tumor microenvironment and their application in early diagnosis of cancers. Anal Cell Pathol (Amst). (2020) 2020:6283796. doi: 10.1155/2020/6283796
PubMed Abstract | Crossref Full Text | Google Scholar
20. Estephan LE, Kumar G, Stewart M, Banoub R, Linnenbach A, Harshyne LA, et al. Altered extracellular matrix correlates with an immunosuppressive tumor microenvironment and disease progression in younger adults with oral cavity squamous cell carcinoma. Front Oncol. (2024) 14:1412212. doi: 10.3389/fonc.2024.1412212
PubMed Abstract | Crossref Full Text | Google Scholar
21. Bai X, Attrill GH, Gide TN, Ferguson PM, Nahar KJ, Shang P, et al. Stroma-infiltrating T cell spatiotypes define immunotherapy outcomes in adolescent and young adult patients with melanoma. Nat Commun. (2024) 15:3014. doi: 10.1038/s41467-024-47301-9
PubMed Abstract | Crossref Full Text | Google Scholar
22. Griffith BD, Lazarus J, McGue J, Krishnan S, D’Angelica MI, Shia J, et al. Unique characteristics of the tumor immune microenvironment in young patients with metastatic colorectal cancer. Front Immunol. (2023) 14:1289402. doi: 10.3389/fimmu.2023.1289402
PubMed Abstract | Crossref Full Text | Google Scholar
23. Révész M, Oberna F, Slezák A, Tóth E, Ferenczi Ö, Kenessey I, et al. EZH2 expression in head-and-neck squamous cell cancer in young patients. Int J Mol Sci. (2024) 25(10):5250. doi: 10.3390/ijms25105250
PubMed Abstract | Crossref Full Text | Google Scholar
24. Zhang B, Du W, Gan K, Fang Q, Zhang X. Significance of the neutrophil-to-lymphocyte ratio in young patients with oral squamous cell carcinoma. Cancer Manag Res. (2019) 11:7597–603. doi: 10.2147/CMAR.S211847
PubMed Abstract | Crossref Full Text | Google Scholar
25. Zhang Z, Feng Q, Li M, Li Z, Xu Q, Pan X, et al. Age-related cancer-associated microbiota potentially promotes oral squamous cell cancer tumorigenesis by distinct mechanisms. Front Microbiol. (2022) 13:852566. doi: 10.3389/fmicb.2022.852566
PubMed Abstract | Crossref Full Text | Google Scholar
26. Miranda Galvis M, Santos-Silva AR, Freitas Jardim J, Paiva Fonseca F, Lopes MA, de Almeida OP, et al. Different patterns of expression of cell cycle control and local invasion-related proteins in oral squamous cell carcinoma affecting young patients. J Oral Pathol Med. (2018) 47:32–9. doi: 10.1111/jop.2018.47.issue-1
PubMed Abstract | Crossref Full Text | Google Scholar
27. Satgunaseelan L, Strbenac D, Willet C, Chew T, Sadsad R, Wykes J, et al. Whole genome duplication in oral squamous cell carcinoma in patients younger than 50 years: implications for prognosis and adverse clinicopathological factors. Genes Chromosomes Cancer. (2022) 61:561–71. doi: 10.1002/gcc.23076
PubMed Abstract | Crossref Full Text | Google Scholar
28. Satgunaseelan L, Porazinski S, Strbenac D, Istadi A, Willet C, Chew T, et al. Oral squamous cell carcinoma in young patients show higher rates of EGFR amplification: implications for novel personalized therapy. Front Oncol. (2021) 11:750852. doi: 10.3389/fonc.2021.750852
PubMed Abstract | Crossref Full Text | Google Scholar
29. Costa V, Kowalski LP, Coutinho-Camillo CM, Begnami MD, Calsavara VF, Neves JI, et al. EGFR amplification and expression in oral squamous cell carcinoma in young adults. Int J Oral Maxillofac Surg. (2018) 47:817–23. doi: 10.1016/j.ijom.2018.01.002
PubMed Abstract | Crossref Full Text | Google Scholar
30. Ur Rahaman SM, Ahmed Mujib B. Histopathological correlation of oral squamous cell carcinoma among younger and older patients. J Oral Maxillofac Pathol. (2014) 18:183–8. doi: 10.4103/0973-029X.140734
PubMed Abstract | Crossref Full Text | Google Scholar
31. Su PF, Huang WL, Wu HT, Wu CH, Liu TY, Kao SY. p16(INK4A) promoter hypermethylation is associated with invasiveness and prognosis of oral squamous cell carcinoma in an age-dependent manner. Oral Oncol. (2010) 46:734–9. doi: 10.1016/j.oraloncology.2010.07.002
PubMed Abstract | Crossref Full Text | Google Scholar
32. Tani R, Ito N, Matsui K, Yamasaki S, Hamada A, Tokumaru K, et al. MICA A5.1 homozygous genotype is associated with a risk for early-onset oral cancer. Oral Oncol. (2021) 116:105256. doi: 10.1016/j.oraloncology.2021.105256
PubMed Abstract | Crossref Full Text | Google Scholar
33. Cury SS, Miranda PM, Marchi FA, Canto LMD, Chulam TC, Petersen AH, et al. Germline variants in DNA repair genes are associated with young-onset head and neck cancer. Oral Oncol. (2021) 122:105545. doi: 10.1016/j.oraloncology.2021.105545
PubMed Abstract | Crossref Full Text | Google Scholar
34. Campbell BR, Chen Z, Faden DL, Agrawal N, Li RJ, Hanna GJ, et al. The mutational landscape of early- and typical-onset oral tongue squamous cell carcinoma. Cancer. (2021) 127:544–53. doi: 10.1002/cncr.v127.4
PubMed Abstract | Crossref Full Text | Google Scholar
35. Cardin GB, Bernard M, Bahig H, Nguyen-Tan PF, Ballivy O, Filion E, et al. Single nucleotide polymorphism rs6942067 is a risk factor in young and in non-smoking patients with HPV negative head and neck squamous cell carcinoma. Cancers (Basel). (2019) 12(1):55. doi: 10.3390/cancers12010055
PubMed Abstract | Crossref Full Text | Google Scholar
36. Braakhuis BJ, Rietbergen MM, Buijze M, Snijders PJ, Bloemena E, Brakenhoff RH, et al. TP53 mutation and human papilloma virus status of oral squamous cell carcinomas in young adult patients. Oral Dis. (2014) 20:602–8. doi: 10.1111/odi.2014.20.issue-6
PubMed Abstract | Crossref Full Text | Google Scholar
37. Chen X, Sturgis EM, Lei D, Dahlstrom K, Wei Q, Li G. Human papillomavirus seropositivity synergizes with MDM2 variants to increase the risk of oral squamous cell carcinoma. Cancer Res. (2010) 70:7199–208. doi: 10.1158/0008-5472.CAN-09-4733
PubMed Abstract | Crossref Full Text | Google Scholar
38. Gawecki W, Kostrzewska-Poczekaj M, Gajecka M, Milecki P, Szyfter K, Szyfter W. The role of genetic factor in etiopathogenesis of squamous cell carcinoma of the head and neck in young adults. Eur Arch Otorhinolaryngol. (2007) 264:1459–65. doi: 10.1007/s00405-007-0386-x
PubMed Abstract | Crossref Full Text | Google Scholar
39. Gu X, Coates PJ, Boldrup L, Wang L, Krejci A, Hupp T, et al. Copy number variation: A prognostic marker for young patients with squamous cell carcinoma of the oral tongue. J Oral Pathol Med. (2019) 48:24–30. doi: 10.1111/jop.2019.48.issue-1
PubMed Abstract | Crossref Full Text | Google Scholar
40. Gong Y, Ju H, Ren G, Wu Y. Cisplatin based induction chemotherapy modified by ERCC1 improved the outcome of young adults with locally advanced oral squamous cell carcinoma. J Cancer. (2019) 10:2083–90. doi: 10.7150/jca.28959
PubMed Abstract | Crossref Full Text | Google Scholar
41. Mesquita JA, Queiroz LM, Silveira ÉJ, Gordon-Nunez MA, Godoy GP, Nonaka CF, et al. Association of immunoexpression of the galectins-3 and -7 with histopathological and clinical parameters in oral squamous cell carcinoma in young patients. Eur Arch Otorhinolaryngol. (2016) 273:237–43. doi: 10.1007/s00405-014-3439-y
PubMed Abstract | Crossref Full Text | Google Scholar
42. Grimm M, Biegner T, Teriete P, Hoefert S, Krimmel M, Munz A, et al. Estrogen and Progesterone hormone receptor expression in oral cavity cancer. Med Oral Patol Oral Cir Bucal. (2016) 21:e554–8. doi: 10.4317/medoral.21182
PubMed Abstract | Crossref Full Text | Google Scholar
43. Singh V, Nandi S, Ghosh A, Adhikary S, Mukherjee S, Roy S, et al. Epigenetic reprogramming of T cells: unlocking new avenues for cancer immunotherapy. Cancer Metastasis Rev. (2024) 43:175–95. doi: 10.1007/s10555-024-10167-w
PubMed Abstract | Crossref Full Text | Google Scholar
44. Campolo M, Scuderi SA, Filippone A, Bova V, Lombardo SP, Colarossi L, et al. EZH2 inhibition to counteract oral cancer progression through wnt/β-catenin pathway modulation. Pharmaceuticals. (2024) 17(8):1102. doi: 10.3390/ph17081102
PubMed Abstract | Crossref Full Text | Google Scholar
45. Zauderer MG, Szlosarek PW, Le Moulec S, Popat S, Taylor P, Planchard D, et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated Malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol. (2022) 23:758–67. doi: 10.1016/S1470-2045(22)00277-7
留言 (0)