Mycoplasmas are the smallest and simplest self-replicating prokaryotic organisms that often parasitize mucus surfaces in the respiratory and genitourinary tracts (Benedetti et al., 2020). Mycoplasma pneumoniae (M. pneumoniae) is one of the most common pathogens responsible for community-acquired pneumonia, especially in children and adolescents (Su et al., 2021). In the USA, an estimated 2 million cases of M. pneumoniae occur annually, resulting in approximately 100,000 hospitalizations, with school-age children and adolescents being the most commonly affected (Waites et al., 2019). M. pneumoniae produces a variety of virulence factors, such as membrane lipoproteins, polysaccharides, and invasive nucleases, which induce proinflammatory cytokines, lymphocyte activation, airway pathology, and dysfunction, and can lead to a variety of pulmonary disorders (Ramasamy et al., 2018; Luo et al., 2021).
The epidemiologic cycle of M. pneumoniae infection is 3 to 7 years. In late 2019 and early 2020, M. pneumoniae simultaneously became endemic in several European and Asian countries. The epidemiologic surveillance of acute respiratory infections in China showed that the M. pneumoniae infection rate ranked third among childhood pneumonia cases and first among adult pneumonia cases (Li et al., 2021). Interestingly, the positive detection rate of M. pneumoniae RNA decreased significantly from 40% to 10% during the pandemic period of COVID-19 (Li et al., 2023). However, in 2023, M. pneumoniae infections rapidly increased to 4.12% in European countries, and the positive detection rate in China rose to 61.1% (Meyer Sauteur and Beeton, 2023; Gong et al., 2024; Yan et al., 2024).
Because Mycoplasma has no cell wall, the first-choice treatment for M. pneumoniae infection is macrolide antibiotics which act on bacterial ribosomes and inhibit protein synthesis, along with tetracyclines and fluoroquinolones (Waites and Talkington, 2004). However, since the beginning of this century, many studies have reported macrolide-resistant M. pneumoniae (MRMP) worldwide (Waites et al., 2017). In China, MRMP is very common, with a prevalence of 83% to 95%. Patients infected with MRMP usually respond poorly to macrolides, and exhibit more extensive lung solidification or necrosis, pleural effusion, and longer periods of fever and hospitalization (Yen et al., 2023).
Therefore, it is necessary to monitor the prevalence of MRMP and raise a warning of increased MRMP prevalence. Due to the difficulty in isolating and cultivating of M. pneumoniae, only a few reports on using minimum inhibitory concentrations (MICs) to evaluate the antibiotic sensitivity of M. pneumoniae isolates can be found (Wu et al., 2020; Xiao et al., 2020; Burgos et al., 2023). In this study, we collected and cultured isolates from children hospitalized with M. pneumoniae pneumonia (MPP) at the Children’s Hospital affiliated to Capital Institute of Pediatrics, Beijing, China, from 2021 to 2023. We selected six antibiotics to determine the MICs of M. pneumoniae isolates, analyzed molecular characteristics including P1 typing and multi-locus variable-number tandem-repeat analysis (MLVA), and macrolide resistance associated mutations. We also collected patient information, and analyzed the clinical features. We intended to identify the reasons for the resurgence of M. pneumoniae outbreaks and high severity rate of pneumonia in China in 2023.
2 Materials and methods2.1 Ethics statementThis study was performed in compliance with the Ethical Principles for Medical Research Involving Human Subjects of the Declaration of Helsinki, and was approved by the research board of the Ethics Committee of the Capital Institute of Pediatrics in Beijing, China (SHERLL2023092). Informed consent was obtained during admission for patient clinical records and specimens to be collected. Data was accessed anonymously and used in statistical analysis.
2.2 Clinical dataIn this study, we retrospectively analyzed 62 pediatric cases diagnosed with MPP and hospitalized in the Children’s Hospital affiliated to Capital Institute of Pediatrics in Beijing, China, between January 1, 2021 and December 31, 2023. For each case, the clinical presentation and laboratory detection data were recorded. Severe M. pneumoniae pneumonia (SMPP) was diagnosed on the basis of fever (>39°C) ≥5 days or fever ≥7 days, radiological deterioration or consolidation present in >2/3 of the lung lobes, and intra‐ and extrapulmonary complications, according to the National Health Commission’s ‘Guidelines for the Diagnosis and Treatment of M. pneumoniae in Children (2023 edition)’ (Yan et al., 2019; Commission, 2023).
2.3 M. pneumoniae culture and quantificationAll isolates were obtained from sputum or bronchoalveolar lavage fluid collected from MPP patients. The specimens were obtained and promptly transported to the bacterial laboratory and M. pneumoniae was detected using the nucleic acid and resistance mutation site detection kit (Jiangsu Mole BioScience Co., Ltd), with positive ones were selected for culturing (Dorigo-Zetsma et al., 1999). After centrifugation (12000rpm, 5min), the DNA of M. pneumoniae was extracted using the Bacteria Genomic DNA Kit (Tiangen, YDP302). The Real-time PCR mixture was prepared in a total volume of 25 μl, containing 5 μl of sample DNA. Real-time PCR was performed under the following conditions: initial activation at 95°C for 30s, followed by 40 cycles at 95°C for 5 s and 63°C for 30s. According to the quantitative result, the concentration of M. pneumoniae was adjusted to 104–105 colony-forming units for experimental use.
2.4 Antimicrobial susceptibility testingFollowing the guidelines of the Clinical and Laboratory Standards Institute (CLSI) M43-A (2011 edition) (Waites et al., 2011), six antibiotics (erythromycin, azithromycin, levofloxacin, chloramphenicol, acetylspiramycin, and tetracycline) were dissolved and diluted, and two-fold serial dilutions ranging from 1024 µg/mL to a minimum of 0.125 µg/mL were conducted. The MIC of each antibiotic was determined using the broth microdilution method. In each well of a 96-well plate, 100 µL of the M. pneumoniae dilution and 100 µL of the antibiotic was added. The positive control strain was M. pneumoniae M129 (ATCC29342), along with drug dilution and medium as the negative control. The plates were placed in a CO2 incubator at 37°C for 4-6 days until the positive control well first showed color change. Each strain was tested in triplicates.
2.5 Molecular characteristics analysisFor analysis of molecular characteristics of M. pneumoniae, the genomic DNA of each isolate was extracted. Subsequently, polymerase chain reaction restriction fragment length polymorphism analysis (PCR-RFLP) was used to analyze the P1 genotypes. Products of type 2 specimens were sequenced to identify type 2 variants (Sun et al., 2013). Variable‐number tandem-repeat loci (Mpn13, Mpn14, Mpn15, and Mpn16) were amplified in a single reaction using a multiplex PCR‐capillary electrophoresis assay (Dumke and Jacobs, 2011; Yan et al., 2019). This approach facilitated the differentiation of various repetitive sequences derived from the M. pneumoniae P1 gene. Furthermore, the sequence of the V domain of the 23S rRNA gene associated with macrolide resistance was amplified and sequenced. All primers synthesis and products sequencing were completed by Biotech (Shanghai) Co., Ltd.
2.6 Detection of co-infectionRespiratory Pathogens Nucleic Acid Detection Kit (CapitalBio Technology Co., Ltd.) was used to detect Streptococcus pneumoniae, Staphylococcus aureus, Methicillin-resistant Staphylococcus, Klebsiella pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Acinetobacter baumannii and Serratia marcescens. Resp®13 Respiratory Pathogen Multiplex Kit (Ningbo Health Gene Technology Co., LTD.) was used to detect Chlamydia pneumoniae and respiratory viruses. The specimens were inoculated on Sabouraud’s AGAR medium and cultured at 37°C and 5% CO2 for 48h for fungal identification.
2.7 Statistical analysisThe collected data were analyzed using SPSS Version 27.0, employing the chi-square test for categorical variables and the rank sum test for rank variables. A p value < 0.05 was considered to indicate statistical significance.
3 Results3.1 Antimicrobial susceptibility analysis of M. pneumoniae isolatesSixty-two M. pneumoniae isolates collected from the years 2021 (13/62), 2022 (18/62), and 2023 (31/62) were tested. Notably, all isolates exhibited resistance to erythromycin (14-membered macrolides) and azithromycin (15-membered macrolides). The MICs of erythromycin ranged from 64 μg/mL to 1024 μg/mL, while that for azithromycin ranged from 1 μg/mL to 256 μg/mL. A comparative analysis of the MICs of azithromycin across the three years revealed a significant increase over time (p < 0.001). Specifically, the MICs of azithromycin in 2023 was notably higher than those in 2021 (p = 0.002) and 2022 (p = 0.025). Furthermore, the MIC50 of azithromycin rose from 16 μg/mL in 2021 to 64 μg/mL in 2023, while the MIC90 increased from 64 μg/mL to 128 μg/mL. Similarly, the MIC90 of erythromycin exhibited an upward trend from 512 μg/mL in 2021 to 1024 μg/mL in 2023. Acetylspiramycin (16-membered macrolides) displayed a lower MIC range of <4 μg/mL, with MIC50 and MIC90 values of 0.25 μg/mL and 1 μg/mL, respectively. Over the years, the MIC50 of acetylspiramycin increased from <0.125 μg/mL in 2021 to 0.5 μg/mL in 2023, while the MIC90 rose from 1 μg/mL to 2 μg/mL, consistent with the increasing trend of erythromycin and azithromycin (Figure 1).
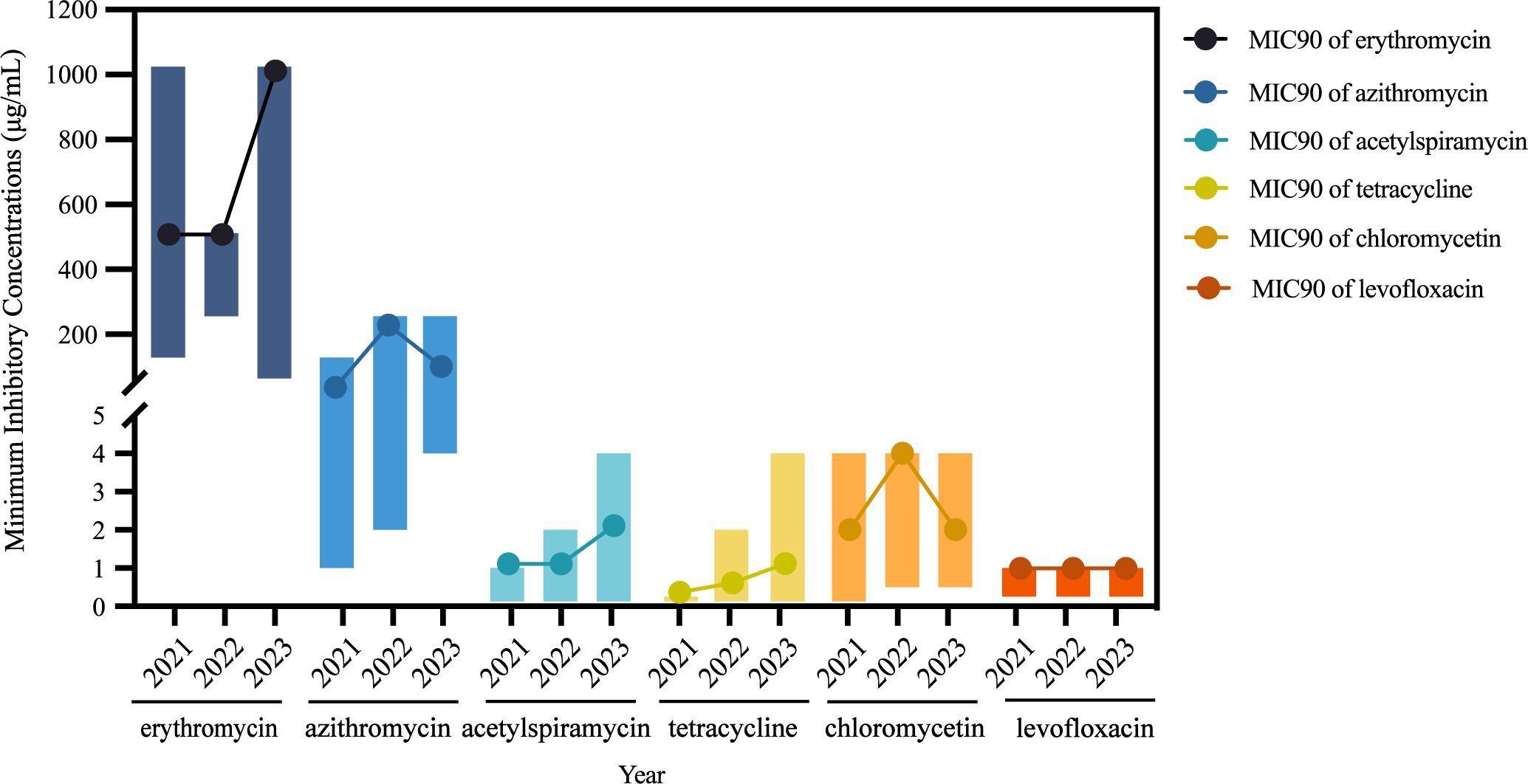
Figure 1. Variation of minimum inhibitory concentrations of Mycoplasma pneumoniae isolates.
All isolates, including the reference strain M129, demonstrated susceptibility to tetracycline, with MIC ranging from <0.125 μg/mL to 1 μg/mL. Moreover, all isolates are sensitive to levofloxacin, with MIC ranging from <0.25 μg/mL to 1 μg/mL. Chloramphenicol displayed a MIC range of <0.125–4 μg/mL, with MIC50 and MIC90 values of 2 μg/mL and 4 μg/mL, respectively (Table 1).
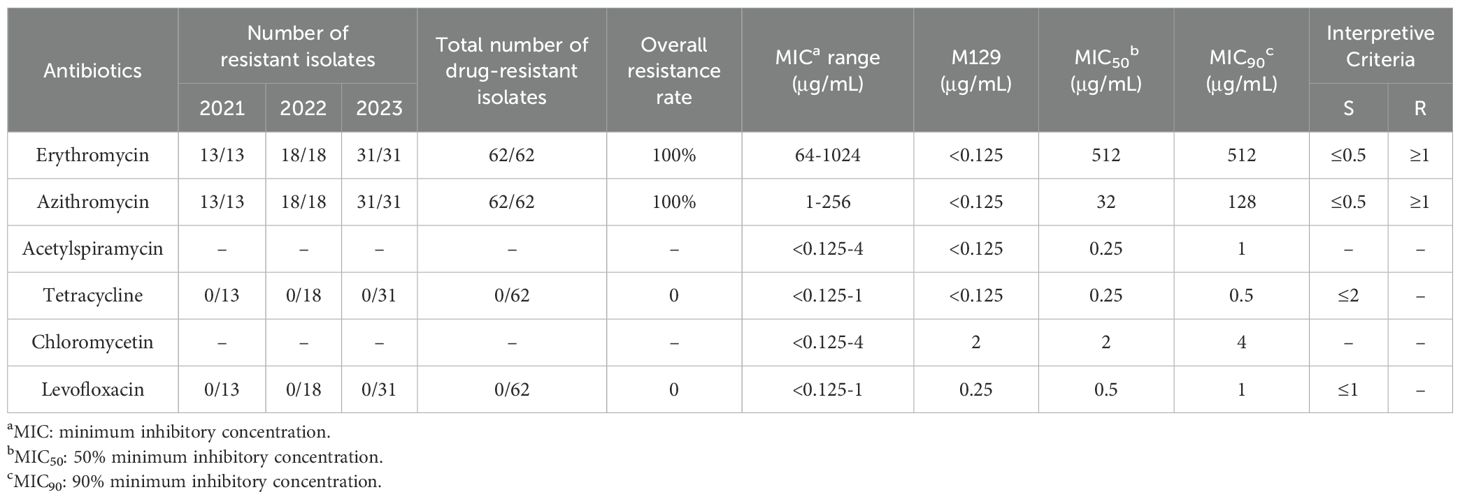
Table 1. Minimum inhibitory concentrations of six antimicrobial drugs against 62 Mycoplasma pneumoniae Isolates.
3.2 Molecular characteristics of M. pneumoniae isolatesAmong the 62 isolates, 74.2% (46/62) were classified as P1 type 1 and 25.8% (16/62) were P1 type 2. When comparing the prevalence of P1 type 1 isolates across different years, we observed a significant increase in 2023 compared with that in 2021 and 2022 (p < 0.001) (Figure 2A). The MIC of erythromycin ranged from 64 μg/mL to 1024 μg/mL in type 1 isolates and ranged from 256 μg/mL to 1024 μg/mL in type 2 isolates. The MIC for azithromycin ranged from 1 μg/mL to 256 μg/mL in type 1 isolates and ranged from 2 μg/mL to 256 μg/mL in type 2 isolates. Eight distinct MLVA types were identified: M4-5-7-2 (61.3%, 38/62), M3-5-6-2 (22.6%, 14/62), M4-4-7-2 (3.2%, 2/62), M3-5-7-2 (3.2%, 2/62), M5-5-7-2 (3.2%, 2/62), M4-5-5-2 (3.2%, 2/62), M4-5-6-2 (1.6%, 1/62), and M4-5-5-1 (1.6%, 1/62) (Figure 2B). M4-5-7-2 and M3-5-6-2 were the main types. Additionally, 80.4% (37/46) of the P1 type 1 isolates exhibited MLVA type M4-5-7-2, whereas 75% (12/16) of the type 2 isolates displayed MLVA type M3-5-6-2. The MIC of erythromycin ranged from 64 μg/mL to 1024 μg/mL in M4-5-7-2 isolates and ranged from 256 μg/mL to 1024 μg/mL in M3-5-6-2 isolates. The MIC of azithromycin ranged from 1 μg/mL to 256 μg/mL in M4-5-7-2 isolates and ranged from 4 μg/mL to 256 μg/mL in M3-5-6-2 isolates (Table 2). All isolates possessed the A2063G mutation within the V domain of the 23S rRNA gene, mutations at site 2064, 2611 and 2617 were not detected. All clinical isolates collected in this study were MRMP.
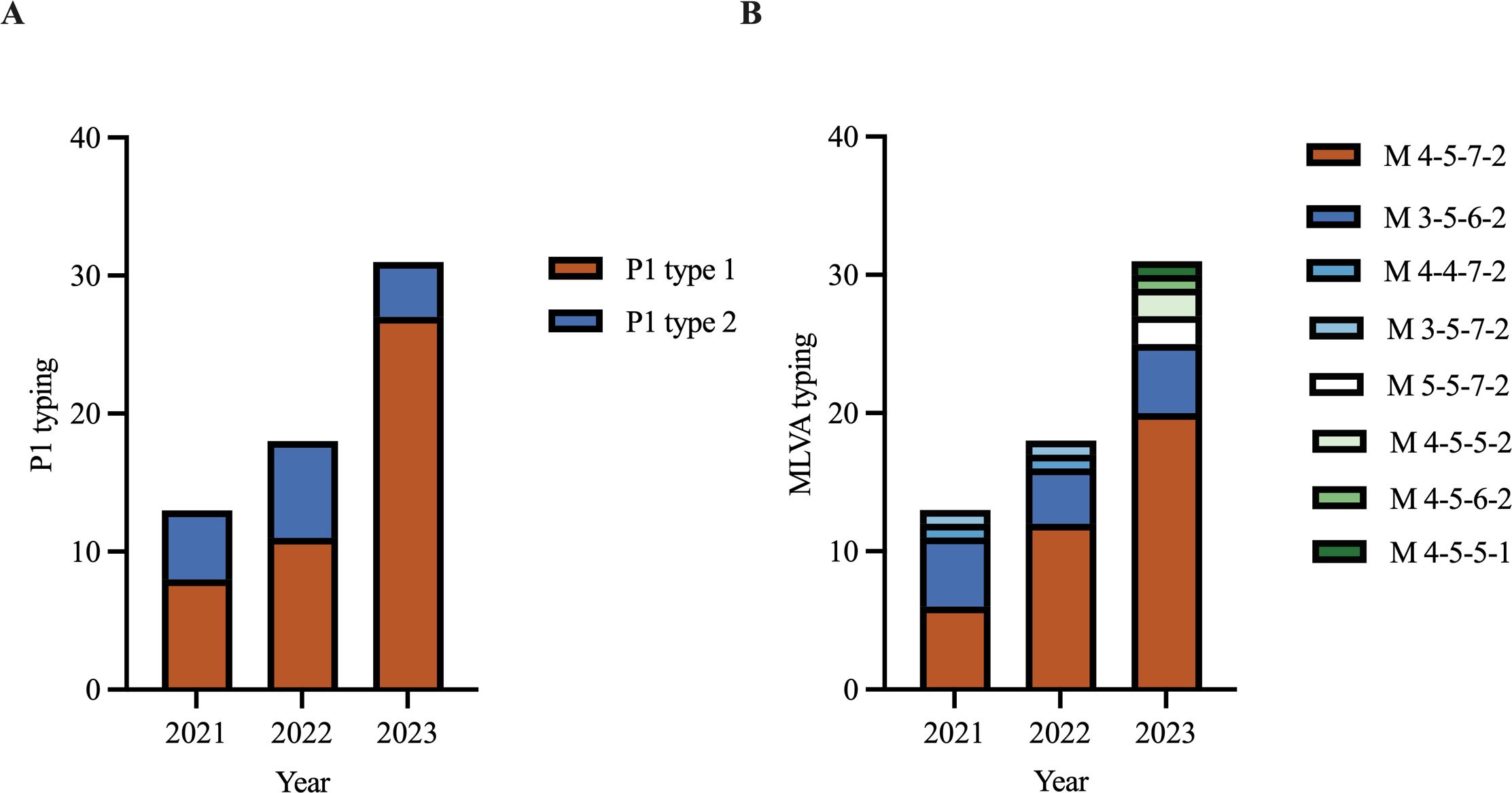
Figure 2. P1 typing and MLVA typing of Mycoplasma pneumoniae in 2021‐2023. (A) P1 typing of isolates in 2021‐2023. (B) MLVA typing of isolates in 2021‐2023.
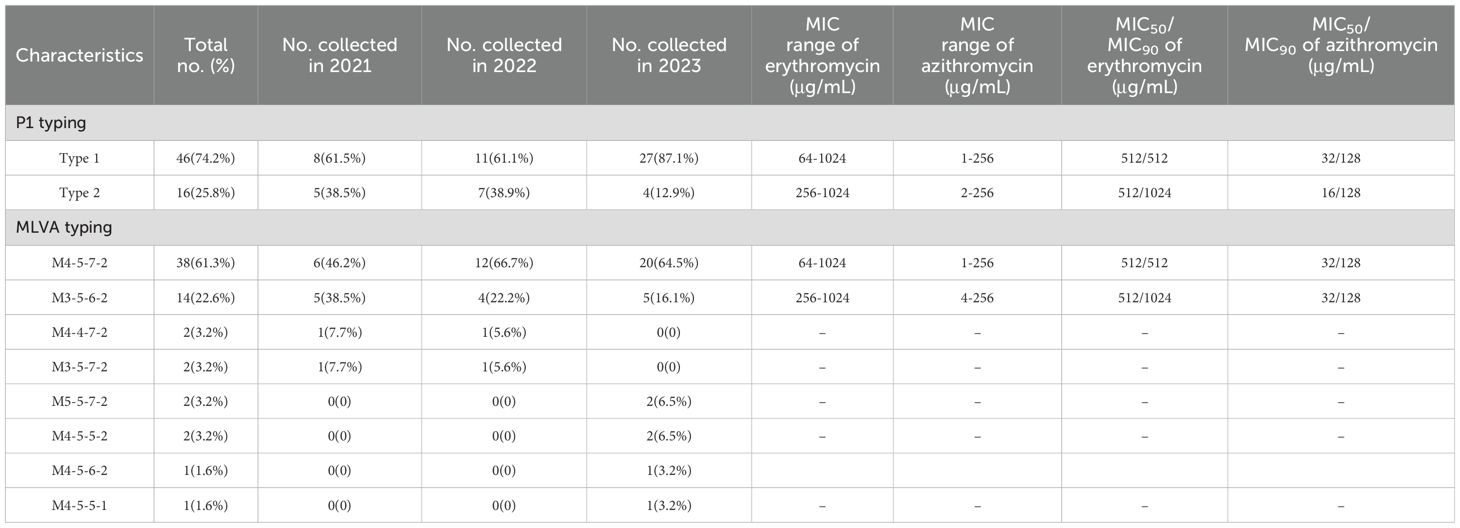
Table 2. Correlation between genotypes and MICs distribution of Mycoplasma pneumoniae Isolates.
3.3 Clinical characteristics of M. pneumoniae pneumonia patientsAmong the 62 M. pneumoniae pneumonia patients, complete clinical information was available for 59 cases. Of them, 76.3% (45/59) cases exhibited mild symptoms, while 23.7% (14/59) cases were SMPP. Among the 26 cases with M. pneumoniae mono-infection, 19.2% (5/26) cases were SMPP and 80.8% (21/26) were general M. pneumoniae pneumonia (GMPP) (80.8%, 21/26). The MIC range of erythromycin against isolates from SMPP patients was 256-1024 μg/mL, and that of azithromycin was 16-256 μg/mL. For M. pneumoniae isolates from GMPP patients, the MIC range of erythromycin was 64-1024 μg/mL and that of azithromycin was 4-256 μg/mL. The MIC of M. pneumoniae isolates obtained from SMPP patients was higher than that from GMPP patients, but statistical analysis revealed no significant difference.
Thirty-three patients (55.9%) were co-infected with bacteria or viruses. Among them, Streptococcus pneumoniae (13.5%) and Haemophilus influenzae (13.5%) are the most prevalent co-pathogens, followed by Epstein–Barr virus (10.2%). Parainfluenza virus, adenovirus, rhinovirus, and coxsackievirus accounted for 5.1%, while cytomegalovirus accounted for 3.4%, and methicillin-resistant S. aureus, Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Serratia marcescens, influenza virus, and oral Candida accounted for only 1.7%. However, there was no statistically significant disparity between the MICs of erythromycin and azithromycin and co-infection.
In MPP patients, fever lasted on an average of 12 days (1-30 days), the duration of fever after initiation treatment of macrolide antibiotics was 8 days (1-22 days), and the hospitalization time was 7 days (2-20 days). Seven patients did not feel better after the macrolide antibiotic was used and they were switched to the usage of levofloxacin, doxycycline, or cephalosporin.
4 DiscussionThis study reported the in vitro antimicrobial susceptibility of M. pneumoniae isolates to six antibiotics and explored the molecular and clinical characteristics of these isolates obtained from the Children’s Hospital affiliated to Capital Institute of Pediatrics in Beijing, China, from 2021 to 2023. These antibiotics can be categorized into as four classes. Among them, erythromycin, azithromycin, and acetylspiramycin belong to 14-membered, 15-membered, and 16-membered macrolides, respectively, which are the first-line drugs for treating M. pneumoniae infection (Lee et al., 2018c; Metlay et al., 2019; Olson and Davis, 2020). Levofloxacin belongs to quinolone antibiotics, chloramphenicol belongs to chloramphenicol antibiotics, and tetracycline belongs to tetracycline antibiotics. The reference strain of M. pneumoniae M129, was selected as the control strain during the antimicrobial susceptibility testing. The results revealed that M129 displayed sensitivity to macrolides and tetracyclines, exhibiting MIC values of less than 0.125 μg/mL. The MICs of levofloxacin and chloramphenicol were recorded as 0.25 μg/mL and 2 μg/mL, respectively. Our study marks the report of erythromycin and azithromycin resistance reaching 100% (62/62), accompanied by a 100% (62/62) A2063G mutation.
At present, there are limited reports on the in vitro antimicrobial susceptibility of M. pneumoniae. In previous study, 65.4% of M. pneumoniae isolates in Beijing exhibited resistance to erythromycin and azithromycin, while maintaining susceptibility to levofloxacin and tetracycline in 2014–2016 in China (Zhao et al., 2019b). In Weihai, Shandong Province, China, the macrolide resistance rate reached 98.8% in 2019 (Guo et al., 2022). Similarly, in Japan, a significant increase in the MICs of macrolides has been reported (Ishiguro et al., 2016; Oishi et al., 2022).
Since M. pneumoniae was recognized and named in 1962, the antibiotic resistance rate of M. pneumoniae is increasing (Chanock, 1963). Mutations occurring on the peptidyl transferase loop of the 23S rRNA gene, particularly at loci 2063 or 2064, have rendered M. pneumoniae less susceptible to macrolide antibiotics than earlier strains (Lucier et al., 1995). In 2022, a systematic review and meta-analysis revealed the global prevalence of MRMP infections, with the highest rates observed in East Asia, reaching 53.4% (Kim et al., 2022). Over the past two decades, Korea has encountered five outbreaks of M. pneumoniae with a surge in macrolide resistance rate from 0% to 84.4% (Lee et al., 2018b). Similarly, in Japan in recent years, a rise in macrolide resistance rates from 53.7% to 62.3% was observed (Ishiguro et al., 2016; Oishi et al., 2022). Moreover, the macrolides resistance rate among M. pneumoniae isolates in various regions of China was around 79.9% during 2017–2018, with 66.7% in Beijing (Zhao et al., 2019a). In late 2023, the mutation rate of macrolide-resistant genes in 23S rRNA was up to 97.1% in Beijing (Yan et al., 2024). In this study, all of the M. pneumoniae isolates presented to be A2063G mutation, this rate was higher than previous reports. Although our results showed a 100% in vitro resistance rate to azithromycin and a 100% mutation rate at A2063G site, only seven patients switched to the usage of other antibiotics instead of macrolide antibiotics, which shows that the majority of children were effectively treated with azithromycin. It may be related to the metabolic time of azithromycin. In children, the metabolism of azithromycin is slow and the clearance rate is low (Neu, 1991; Southern and Barker, 2004; Zhang et al., 2024). After one or two courses of treatment, the concentration of azithromycin can be maintained at a relatively high level which can inhibit M. pneumoniae (Matzneller et al., 2013; Dekyi et al., 2024). In addition, efficacy of macrolide drugs is related to each individual’s immune level (Zheng et al., 2018).
The genotype transition from type 1 to type 2 initiated early. Between 2003 and 2016, the proportion of M. pneumoniae P1 type 2 escalated from 17.4% to 39.4%, and reached 54.5% in 2019 in Beijing (Zhao et al., 2015; Sun et al., 2017; Zhao et al., 2019a, 2019b). The macrolide resistance rate among P1 type 1 M. pneumoniae remains considerable, but this rate for type P1 type 2 has been observed to be increasing (Zhao et al., 2015, 2019a). In the current study, wherein the proportion of type P1 type 1 isolates collected in 2023 markedly exceeded that of the previous two years (p < 0.001). M. pneumoniae strains with MLVA type M3-5-6-2 and P1 type 2 were not correlated with drug resistance (Qu et al., 2013; Kenri et al., 2020). However, recent findings reveal a significant rise in macrolide resistance among strains typed M3-5-6-2 (Wang et al., 2021). In particular, we indicated a 100% resistance rate for erythromycin and azithromycin and 100% A2063G mutation rate among strains type M3-5-6-2 and P1 type 2, potentially contributing to the resurgence of M. pneumoniae in China in 2023, which is specifically evident in the substantial rise in pediatric cases (Conroy, 2023; Gong et al., 2024). After evaluating the antibiotics sensitivity between M. pneumoniae type P1 type 1 and type P1 type 2, we found that the MIC range of erythromycin and azithromycin of P1 type 1 isolates (64-1024 μg/mL;1-256 μg/mL) was slightly wider than the range of P1 type 2 isolates (256-1024 μg/mL;2-256 μg/mL), the MIC90 of erythromycin of P1 type 1 isolates (512 μg/mL) was lower than P1 type 2 isolates (1024 μg/mL), while the MIC50 of azithromycin of P1 type 1 isolates (32 μg/mL) was higher than P1 type 2 isolates (16 μg/mL). Furthermore, while specific investigations have suggested that P1 type 2 M. pneumoniae exhibits elevated levels of CARDS toxins and greater virulence (Lluch-Senar et al., 2015), there are currently no reports on the correlation between typing and MIC.
The duration of fever in non-hospitalized M. pneumoniae patients was 10 days (7-13 days) in Beijing in 2010 (Cao et al., 2010), it was 7 days (2-9 days) in macrolide-resistant M. pneumoniae infected children in Ningbo, Zhejiang province, China, in 2019-2022 (Chen et al., 2024). Both of the fever days were shorter than the duration in this study (12 days). The duration of fever after initiation of macrolide antibiotics was 4 days (0-8 days), which was also shorter than that of 8 days (1-22 days) in the present study, suggesting that MRMP infection increases the duration of fever and the duration of macrolide antibiotic use. Once resistance to erythromycin and azithromycin emerged, the doctors are urged to choose 16-membered cyclic macrolide antibiotics and tetracyclines when warranted, particularly if patients remain febrile or chest radiographs exhibit deterioration 48–72 hours post treatment (Lee et al., 2018a). Clinically, when azithromycin treatment is ineffective and fever persists in children with M. pneumoniae, tetracyclines are preferred as substitutes, among which doxycycline has fewer side effects and a higher fever reduction rate than minocycline, so doxycycline is preferred. Quinolones may also be considered in children over 8 years of age. Methylprednisolone can be used when necessary in severely ill children (Song et al., 2024).
There are some limitations in our study. Firstly, we only analyzed 62 isolates, which is a limited number that may have led to data bias and may not comprehensively and objectively represent the actual situation in Beijing. In future studies, a larger sample size covering a greater number of surveillance sites is needed. Secondly, we only detected the mutation sites of M. pneumoniae relevant to macrolide antibiotics in 23S rRNA gene, mutations in other sites were not included. Thirdly, the period from 2021 to 2023 is the pandemic period of COVID-19, and the M. pneumoniae isolates resistance data of several years before and after the COVID-19 epidemic will be added for comparison in next study.
In conclusion, this study analyzed MICs distribution of 62 M. pneumoniae isolates collected in Beijing from 2021 to 2023. The MIC of azithromycin in 2023 was notably higher than those in 2021 and 2022. All M. pneumoniae isolates possessed the A2063G mutation, conferring 100% resistance to erythromycin and azithromycin. Our findings confirm that MRMP may be the causative factor of the M. pneumoniae epidemic in late 2023 in Beijing, China. It is important for the doctors to pay more attention to detection of MRMP and the antibiotics choose. Acetylspiramycin, which is a 16-membered macrolide antibiotic, appears to be a promising alternative treatment, especially for resistant isolates.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributionsXJ: Data curation, Formal analysis, Writing – original draft. YC: Data curation, Formal analysis, Writing – original draft. YG: Data curation, Formal Analysis, Writing – original draft. XR: Resources, Supervision, Writing – original draft. BD: Resources, Supervision, Writing – original draft. HZ: Resources, Supervision, Writing – original draft. YF: Resources, Supervision, Writing – original draft. GX: Resources, Supervision, Writing – original draft. JC: Resources, Supervision, Writing – original draft. LG: Resources, Supervision, Writing – original draft. JF: Resources, Supervision, Writing – original draft. ZF: Resources, Supervision, Writing – original draft. TF: Resources, Supervision, Writing – original draft. ZX: Resources, Supervision, Writing – original draft. ZY: Resources, Supervision, Writing – original draft. YY: Resources, Supervision, Writing – original draft. SZ: Resources, Supervision, Writing – original draft. LH: Resources, Supervision, Writing – original draft. YK: Resources, Supervision, Writing – original draft. CL: Resources, Supervision, Writing – original draft, Conceptualization. CY: Writing – review & editing, Funding acquisition, Methodology, Resources, Conceptualization. JY: Writing – review & editing, Funding acquisition, Resources, Conceptualization.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by Beijing Natural Science Foundation (7222014, 7232007, L232071, 7242015), Beijing High-Level Public Health Technical Talent Project (2023–02-08), Beijing Hospitals Authority’s Ascent Plan (DFL20241301), Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes (JYY2023-10), National Natural Science Foundation of China (32170201), and Research Foundation of Capital Institute of Pediatrics (JHYJ-2023–05).
AcknowledgmentsWe thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBenedetti, F., Curreli, S., Zella, D. (2020). Mycoplasmas-host interaction: mechanisms of inflammation and association with cellular transformation. Microorganisms 8, 1351. doi: 10.3390/microorganisms8091351
PubMed Abstract | Crossref Full Text | Google Scholar
Burgos, R., Garcia-Ramallo, E., Shaw, D., Lluch-Senar, M., Serrano, L. (2023). Development of a serum-free medium to aid large-scale production of mycoplasma-based therapies. Microbiol. Spectr. 11, e0485922. doi: 10.1128/spectrum.04859-22
PubMed Abstract | Crossref Full Text | Google Scholar
Cao, B., Zhao, C. J., Yin, Y. D., Zhao, F., Song, S. F., Bai, L., et al. (2010). High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin. Infect. Dis. 51, 189–194. doi: 10.1086/653535
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, Y., Zhang, Y., Tang, Q. N., Shi, H. B. (2024). Efficacy of doxycycline therapy for macrolide-resistant Mycoplasma pneumoniae pneumonia in children at different periods. Ital J. Pediatr. 50, 38. doi: 10.1186/s13052-024-01615-y
PubMed Abstract | Crossref Full Text | Google Scholar
Commission, N. H. (2023). Guidelines for the Diagnosis and Treatment of Mycoplasma pneumoniae in Children(2023 edition). Infect. Dis. Info 36, 291.
Dekyi, Xiao, Y., Wang, X., Feng, S., Wang, Y., Liao, L., et al. (2024). Predominance of A2063G mutant strains in the Mycoplasma pneumoniae epidemic in children: A clinical and epidemiological study in 2023 in Wuhan, China. Int. J. Infect. Dis. 145, 107074. doi: 10.1016/j.ijid.2024.107074
PubMed Abstract | Crossref Full Text | Google Scholar
Dorigo-Zetsma, J. W., Zaat, S., Wertheim-van Dillen, P. M., van Waveren, G., et al. (1999). Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J. Clin. Microbiol. 37, 14–17. doi: 10.1128/JCM.37.1.14-17.1999
PubMed Abstract | Crossref Full Text | Google Scholar
Dumke, R., Jacobs, E. (2011). Culture-independent multi-locus variable-number tandem-repeat analysis (MLVA) of Mycoplasma pneumoniae. J. Microbiol. Methods 86, 393–396. doi: 10.1016/j.mimet.2011.06.008
PubMed Abstract | Crossref Full Text | Google Scholar
Gong, C., Huang, F., Suo, L., Guan, X., Kang, L., Xie, H., et al. (2024). Increase of respiratory illnesses among children in Beijing, China, during the autumn and winter of 2023. Euro Surveill 29, 2300704. doi: 10.2807/1560-7917.Es.2024.29.2.2300704
PubMed Abstract | Crossref Full Text | Google Scholar
Guo, Z., Liu, L., Gong, J., Han, N., He, L., Wang, W., et al. (2022). Molecular features and antimicrobial susceptibility of Mycoplasma pneumoniae isolates from pediatric inpatients in Weihai, China: Characteristics of M. pneumoniae In Weihai. J. Glob Antimicrob. Resist. 28, 180–184. doi: 10.1016/j.jgar.2022.01.002
PubMed Abstract | Crossref Full Text | Google Scholar
Ishiguro, N., Koseki, N., Kaiho, M., Kikuta, H., Togashi, T., Oba, K., et al. (2016). Regional Differences in Prevalence of Macrolide Resistance among Pediatric Mycoplasma pneumoniae Infections in Hokkaido, Japan. Jpn J. Infect. Dis. 69, 186–190. doi: 10.7883/yoken.JJID.2015.054
PubMed Abstract | Crossref Full Text | Google Scholar
Kenri, T., Suzuki, M., Sekizuka, T., Ohya, H., Oda, Y., Yamazaki, T., et al. (2020). Periodic genotype shifts in clinically prevalent mycoplasma pneumoniae strains in Japan. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00385
PubMed Abstract | Crossref Full Text | Google Scholar
Kim, K., Jung, S., Kim, M., Park, S., Yang, H. J., Lee, E. (2022). Global trends in the proportion of macrolide-resistant mycoplasma pneumoniae infections: A systematic review and meta-analysis. JAMA Netw. Open 5, e2220949. doi: 10.1001/jamanetworkopen.2022.20949
PubMed Abstract | Crossref Full Text | Google Scholar
Lee, H., Yun, K. W., Lee, H. J., Choi, E. H. (2018a). Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev. Anti Infect. Ther. 16, 23–34. doi: 10.1080/14787210.2018.1414599
PubMed Abstract | Crossref Full Text | Google Scholar
Lee, J. K., Lee, J. H., Lee, H., Ahn, Y. M., Eun, B. W., Cho, E. Y., et al. (2018b). Clonal expansion of macrolide-resistant sequence type 3 mycoplasma pneumoniae, South Korea. Emerg. Infect. Dis. 24, 1465–1471. doi: 10.3201/eid2408.180081
PubMed Abstract | Crossref Full Text | Google Scholar
Lee, M. S., Oh, J. Y., Kang, C. I., Kim, E. S., Park, S., Rhee, C. K., et al. (2018c). Guideline for antibiotic use in adults with community-acquired pneumonia. Infect. Chemother. 50, 160–198. doi: 10.3947/ic.2018.50.2.160
PubMed Abstract | Crossref Full Text | Google Scholar
Li, X., Li, T., Chen, N., Kang, P., Yang, J. (2023). Changes of Mycoplasma pneumoniae prevalence in children before and after COVID-19 pandemic in Henan, China. J. Infect. 86, 256–308. doi: 10.1016/j.jinf.2022.12.030
PubMed Abstract | Crossref Full Text | Google Scholar
Li, Z. J., Zhang, H. Y., Ren, L. L., Lu, Q. B., Ren, X., Zhang, C. H., et al. (2021). Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12, 5026. doi: 10.1038/s41467-021-25120-6
PubMed Abstract | Crossref Full Text | Google Scholar
Lluch-Senar, M., Cozzuto, L., Cano, J., Delgado, J., Llórens-Rico, V., Pereyre, S., et al. (2015). Comparative “-omics” in mycoplasma pneumoniae clinical isolates reveals key virulence factors. PloS One 10, e0137354. doi: 10.1371/journal.pone.0137354
PubMed Abstract | Crossref Full Text | Google Scholar
Lucier, T. S., Heitzman, K., Liu, S. K., Hu, P. C. (1995). Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 39, 2770–2773. doi: 10.1128/aac.39.12.2770
PubMed Abstract | Crossref Full Text | Google Scholar
Luo, H., He, J., Qin, L., Chen, Y., Li, R.. (2021). Mycoplasma pneumoniae lipids license TLR-4 for activation of NLRP3 inflammasome and autophagy to evoke a proinflammatory response. Clin. Exp. Immunol. 203, 66–79.
PubMed Abstract | Google Scholar
Matzneller, P., Krasniqi, S., Kinzig, M., Sörgel, F., Hüttner, S., Lackner, E., et al. (2013). Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 57, 1736–1742. doi: 10.1128/aac.02011-12
PubMed Abstract | Crossref Full Text | Google Scholar
Metlay, J. P., Waterer, G. W., Long, A. C., Anzueto, A., Brozek, J., Crothers, K., et al. (2019). Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of america. Am. J. Respir. Crit. Care Med. 200, e45–e67. doi: 10.1164/rccm.201908-1581ST
PubMed Abstract | Crossref Full Text | Google Scholar
Meyer Sauteur, P. M., Beeton, M. L. (2023). Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. 5, e100-e101 doi: 10.1016/s2666-5247(23)00344-0
PubMed Abstract | Crossref Full Text | Google Scholar
Oishi, T., Yoshioka, D., Nakano, T., Ouchi, K. (2022). Recent Trend of Antimicrobial Susceptibility among Mycoplasma pneumoniae Isolated from Japanese Children. Microorganisms 10, 2428. doi: 10.3390/microorganisms10122428
PubMed Abstract | Crossref Full Text | Google Scholar
Qu, J., Yu, X., Liu, Y., Yin, Y., Gu, L., Cao, B., et al. (2013). Specific multilocus variable-number tandem-repeat analysis genotypes of Mycoplasma pneumoniae are associated with diseases severity and macrolide susceptibility. PloS One 8, e82174. doi: 10.1371/journal.pone.0082174
PubMed Abstract | Crossref Full Text | Google Scholar
Ramasamy, K., Balasubramanian, S., Manickam, K., Pandranki, L., Taylor, A. B., Hart, P. J., et al. (2018). Mycoplasma pneumoniae community-acquired respiratory distress syndrome toxin uses a novel KELED sequence for retrograde transport and subsequent cytotoxicity. mBio 9, e01663-17. doi: 10.1128/mBio.01663-17
PubMed Abstract | Crossref Full Text | Google Scholar
Song, X., Zhou, N., Lu, S., Gu, C., Qiao, X. (2024). New-generation tetracyclines for severe macrolide-resistant Mycoplasma pneumoniae pneumonia in children: a retrospective analysis. BMC Infect. Dis. 24, 1166. doi: 10.1186/s12879-024-10070-3
PubMed Abstract | Crossref Full Text | Google Scholar
Su, X., You, X., Luo, H., Liang, K., Chen, L., Tian, W., et al. (2021). Community-acquired respiratory distress syndrome toxin: unique exotoxin for M. pneumoniae. Front. Microbiol. 12, 766591. doi: 10.3389/fmicb.2021.766591
PubMed Abstract | Crossref Full Text | Google Scholar
Sun, H., Xue, G., Yan, C., Li, S., Cao, L., Yuan, Y., et al. (2013). Multiple-locus variable-number tandem-repeat analysis of mycoplasma pneumoniae clinical specimens and proposal for amendment of MLVA nomenclature. PloS One 8, e64607. doi: 10.1371/journal.pone.0064607
PubMed Abstract | Crossref Full Text | Google Scholar
Sun, H., Xue, G., Yan, C., Li, S., Zhao, H., Feng, Y., et al. (2017). Changes in molecular characteristics of mycoplasma pneumoniae in clinical specimens from children in beijing between 2003 and 2015. PloS One 12, e0170253. doi: 10.1371/journal.pone.0170253
留言 (0)