Functional gastrointestinal disorders (FGIDs) encompass a cluster of chronic conditions affecting the alimentary system, characterized by recurrent symptoms without identifiable structural or biochemical abnormalities. Common FGIDs include irritable bowel syndrome (IBS), functional dyspepsia (FD), and functional constipation (FC) (Black et al., 2020). According to global epidemiological studies, FGIDs affect approximately 10–20% of the world’s population (Suares and Ford, 2011; Lovell and Ford, 2012; Aro et al., 2015; Kano et al., 2018; Sperber et al., 2021), leading to substantial healthcare utilization, impaired quality of life, and significant economic burden. IBS presents with abdominal pain and altered bowel habits, FC is characterized by infrequent bowel movements and difficulty in defecation, while FD involves persistent upper abdominal pain or discomfort without an identifiable organic cause. These disorders manifest with symptoms such as abdominal pain, bloating, altered bowel habits, and indigestion, often resulting in reduced productivity and psychological distress among patients (Black et al., 2020; Sperber et al., 2021). Understanding the prevalence and impact of FGIDs is crucial for effective management and public health interventions to alleviate the burden on individuals and healthcare systems.
The FGIDs are closely associated with psychological and emotional states, often overlapping with conditions such as depression and anxiety. Psychological factors such as stress and negative life events can influence gut motility, visceral sensitivity, and immune function, contributing to the onset and exacerbation of FGIDs (Black et al., 2020). Research has shown that individuals with FGIDs frequently experience comorbid psychiatric conditions. For example, a longitudinal study by Aro et al. (2015) found that anxiety was related to new-found indigestion in the population of Sweden, suggesting a potential causal relationship between psychological distress and gastrointestinal symptoms. Similarly, a meta-analysis by Lovell and Ford (2012) revealed a higher prevalence of anxiety and depression in patients with IBS compared to the general population. The gut-brain axis is recognized as pivotal in the development of FGIDs, facilitating bidirectional communication between the central nervous system and the enteric nervous system (Mayer et al., 2022). This axis plays a critical role in modulating both gastrointestinal function and emotional responses (Mayer et al., 2022; Gong et al., 2023). Dysregulation of the gut-brain axis, characterized by altered neurotransmitter signaling, immune activation, and changes in gut microbiota composition, has been implicated in the pathophysiology of FGIDs (Mayer et al., 2015a; Mayer et al., 2015b). For example, stress-induced alterations in gut microbiota composition can impact gut permeability and immune function, contributing to intestinal inflammation and visceral hypersensitivity observed in FGIDs (Mayer et al., 2015b), which might underlie the patients’ gastrointestinal symptoms.
Recent advances in neuroimaging technology have facilitated the exploration of brain function through non-invasive methods, with resting-state functional magnetic resonance imaging (rs-fMRI) emerging as a prominent technique. Among this, regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF), and fractional ALFF (fALFF) are commonly utilized approaches for exhibiting local spontaneous activity in rs-fMRI data (Zang et al., 2015). Each of these metrics offers unique insights into regional spontaneous brain activities, collectively enhancing our understanding of brain function in health and disease (Zang et al., 2015; Salvia et al., 2019). Numerous neuroimaging studies have revealed abnormal brain structure and function in individuals with FGIDs, emphasizing the significance of the gut-brain axis in the development of these disorders (Kano et al., 2018). However, current findings are inconsistent, possibly due to the heterogeneity in samples and imaging methodologies.
Meta-analysis serves as a robust method to consolidate neuroimaging observations from diverse researches, offering an all-sided synthesis of regional alterations. This method not only addresses discrepancies among neuroimaging studies but also distinguishes between spurious results and reproducible findings. By aggregating data across studies, meta-analysis provides a unified perspective and facilitates the integration of vast amounts of information (Muller et al., 2018). Among these techniques, the seed-based d mapping with permutation of subject images (SDM-PSI) is a notable progressive statistical method for coordinate-based meta-analysis (CBMA) (Radua et al., 2012). SDM-PSI allows for the objective and quantitative integration of diverse neuroimaging findings. One former meta-analysis investigated irregular local spontaneous functional activity during resting states in IBS patients, identifying transformations in brain regions associated with emotional management and somatic sensation (Su et al., 2022). Similarly, a systematic review by Yu et al. (2022) highlighted altered resting brain functions in IBS patients, implicating disruptions in neural networks involved in pain modulation and emotional regulation. Furthermore, another meta-analysis examined alterations in default mode network functionality and gray matter structure in IBS patients, emphasizing abnormalities in brain regions responsible pain perception, transmission and interpretation (Zhao et al., 2023). Besides, a CBMA found changes in brain regions responsible for visceral sensation, pain modulation, and emotion regulation in FD patients (Mao et al., 2023). One recent review indicated that FC is linked to changes in brain function and structure, especially in regions and networks related to emotion regulation, motor control, somatic sensation, and self-referential processing (Feng et al., 2023). These studies provided valuable insights into the neuropathological characteristics of FGIDs and underscored the importance of understanding the brain-gut axis in these disorders. However, these studies conducted meta-analysis focusing on a specific disorder, which might contain certain limitations.
This present study aims to enhance CBMA across various FGIDs, focusing on consolidating regional aberrations in brain activity utilizing ReHo, ALFF, and fALFF metrics. Additionally, with the approach of meta-regression, we seek to explore the latent influence of demographics and clinical parameters on brain functions, such as age and disease duration. We expect these findings will provide valuable insights to advance the diagnosis and treatment of FGIDs in clinical practice. Based on previous research findings, we hypothesize that FGIDs patients may manifest altered brain activations in regions associated with emotion regulation, pain perception, and sensory processing such as the frontal gyrus and insula.
Methods Literature search strategyThe protocol for this CBMA was duly registered with PROSPERO (registration number: CRD42024536106; accessible at http://www.crd.york.ac.uk/PROSPERO), underscoring our commitment to methodological rigor and transparency. The current meta-analysis adhered strictly to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., 2009; Moher et al., 2009a,b; Page et al., 2021) as well as the rules for neuroimaging meta-analysis (Muller et al., 2018) to ensure transparency and reliability in our methods. Relevant literature was systematically sourced through comprehensive searches of the PubMed, Web of Science, and Cochrane Library databases, encompassing publications up to January 31, 2024. The search strategy involved keywords including (“functional gastrointestinal disorders” or “FGIDs” or “irritable bowel syndrome” or “IBS” or “functional constipation” or “functional dyspepsia”) in conjunction with [(“resting state” or “resting-state” or “at rest” or “resting”) or (“amplitude of low frequency fluctuation” or “fractional amplitude of low frequency fluctuation” or “ALFF” or “fALFF”) or (“regional homogeneity” or “ReHo” or “local connectivity” or “coherence”)]. Moreover, for the purpose of preventing oversight, we conducted a manual examination of the reference lists of approved studies and correlated reviews.
Study selectionThe criteria for the selection of studies were delineated as follows: (1) Studies comparing ReHo, ALFF, or fALFF values between patients with FGIDs and HC through whole-brain analyses were included. (2) Results needed to be provided in either Talairach or Montreal Neurological Institute (MNI) coordinates. (3) Utilization of a significance threshold; (4) publication in peer-reviewed journals and composed in the English language. The criteria for exclusion were as below: (1) meta-analyses, reviews, and case reports were excluded. (2) Studies that did not include direct between-group comparisons were excluded. (3) Studies were excluded if the peak coordinates or parametric maps were inaccessible.
Quality assessment and data extractionThe process of quality assessment and data extraction was executed by two authors (S.Y. and W.B.) respectively. They performed literature searches, evaluated the quality of retrieved articles, and extracted and cross-validated data from eligible articles. Additionally, both authors independently assessed the quality of the final studies in accordance with guidelines for neuroimaging meta-analyses (Muller et al., 2018). We documented the following parameters: lead author, sample size, participant characteristics (such as age and gender), criteria for diagnosis, illness duration, imaging protocols, methods of data processing, and the statistical thresholds applied in individual studies.
Meta-analysisWe performed meta-analyses using SDM software (Radua and Mataix-Cols, 2009; Albajes-Eizagirre et al., 2019) to investigate discrepancies in local brain activation between FGIDs patients and HC. The SDM-PSI technique, known for its robust statistical approach, utilizes peak coordinates to evaluate disparities in cerebral activity (Radua et al., 2012). The detailed procedures of SDM-PSI, outlined extensively elsewhere (Duan et al., 2022; Liu et al., 2022; Wang et al., 2022), and are succinctly summarized as follows: (1) the software generated effect-size maps illustrating differences in regional activities between patients and HC for each study, based on peak coordinates of effects and associated statistics, such as t-statistics. Significant cluster Z- or p-values were transformed to t-statistics using the SDM online converter. (2) Peak coordinates for each study were reconstructed utilizing a standard MNI map of effect size for group differences in neuroimaging, employing an anisotropic Gaussian kernel (Radua et al., 2014). (3) A comprehensive meta-analysis was performed to generate a mean map through voxel-wise computation of the random-effects mean of the study maps.
Following the methodology established by Radua et al. (2012), we adopted p = 0.005 in SDM-PSI analyses. Furthermore, we employed a peak height threshold of Z = 1.00 and a cluster size threshold of 10 voxels to ensure robustness in our findings.
Sensitivity analysesWe then conducted sensitivity analyses to assess the repeatability of our findings. If a particular brain region consistently demonstrated significance across the majority or all combinations of studies during the jackknife sensitivity analysis, it was considered highly replicable (Radua and Mataix-Cols, 2009).
Subgroup meta-analysesWe conducted subgroup meta-analyses focusing exclusively on each subtype of the FGIDs (IBS, FD, and FC).
Meta-regression analysesMeta-regression analyses were performed in each patient group to explore potential demographic variables on neuroimaging changes. We used p < 0.0005 to serve as a threshold for significance (Radua and Mataix-Cols, 2009). We only considered brain regions identified in the main effect.
Results Sample characteristics of included studiesFigure 1 depicts a flow diagram outlining the process of including studies related to FGIDs. Table 1 summarizes the demographic characteristics and neuroimaging methodologies utilized in each subgroup corresponding to the studied diseases. We identified 1895 studies under the search strategy, with 12 fitting the predefined inclusion criteria (Zhou et al., 2013; Nan et al., 2014; Ke et al., 2015; Ma et al., 2015; Qi et al., 2016; Lee et al., 2018; Jin et al., 2019; Qi et al., 2020; Ao et al., 2021; Chen et al., 2021; Li et al., 2021; Cai et al., 2024). Notably, the study by Qi et al. (2020) included two subgroups of FD, while the study by Li et al. (2021) encompassed two subgroups of functional constipation FC patients. Additionally, Chen et al. (2021) conducted both ALFF and ReHo analyses. The final sample comprised 463 patients and 469 healthy controls, with 99 coordinates extracted from 15 datasets.
Figure 1. Flow diagram for the identification and exclusion of studies.
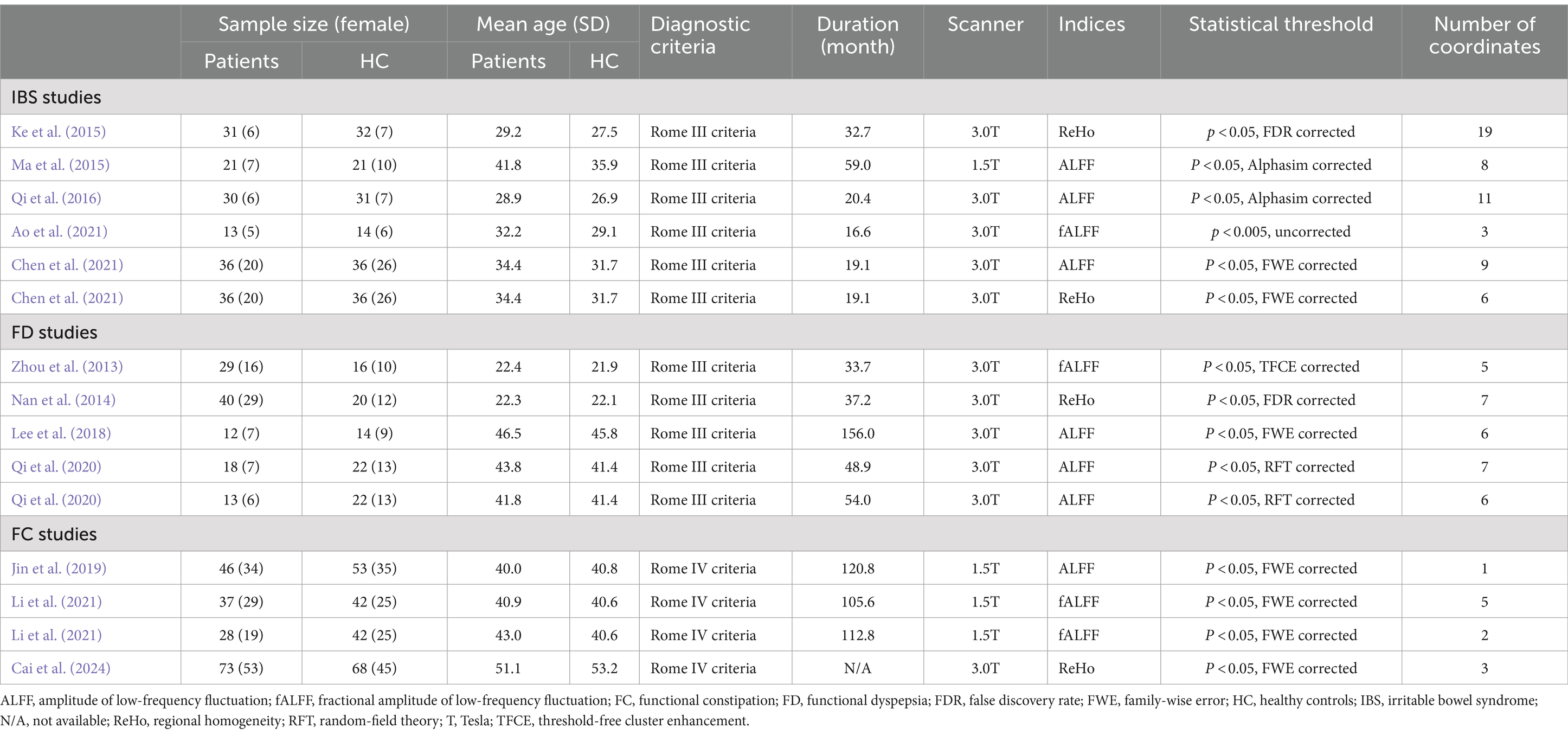
Table 1. Demographic and clinical characteristics and the neuroimaging approaches of the participants in the studies included in the meta-analyses.
Meta-analyses findingsThe primary meta-analysis unveiled that FGIDs patients displayed significantly heightened brain activities across five clusters, notably encompassing the right postcentral gyrus, left calcarine fissure/surrounding cortex, right superior frontal gyrus (medial), right insula, and the left superior frontal gyrus (orbital part). Additionally, three clusters exhibited decreased brain activities, including the left posterior cingulate gyrus, right median cingulate/paracingulate gyri, and the left caudate nucleus. These results are visually shown in Figure 2 and thoroughly summarized in Table 2.
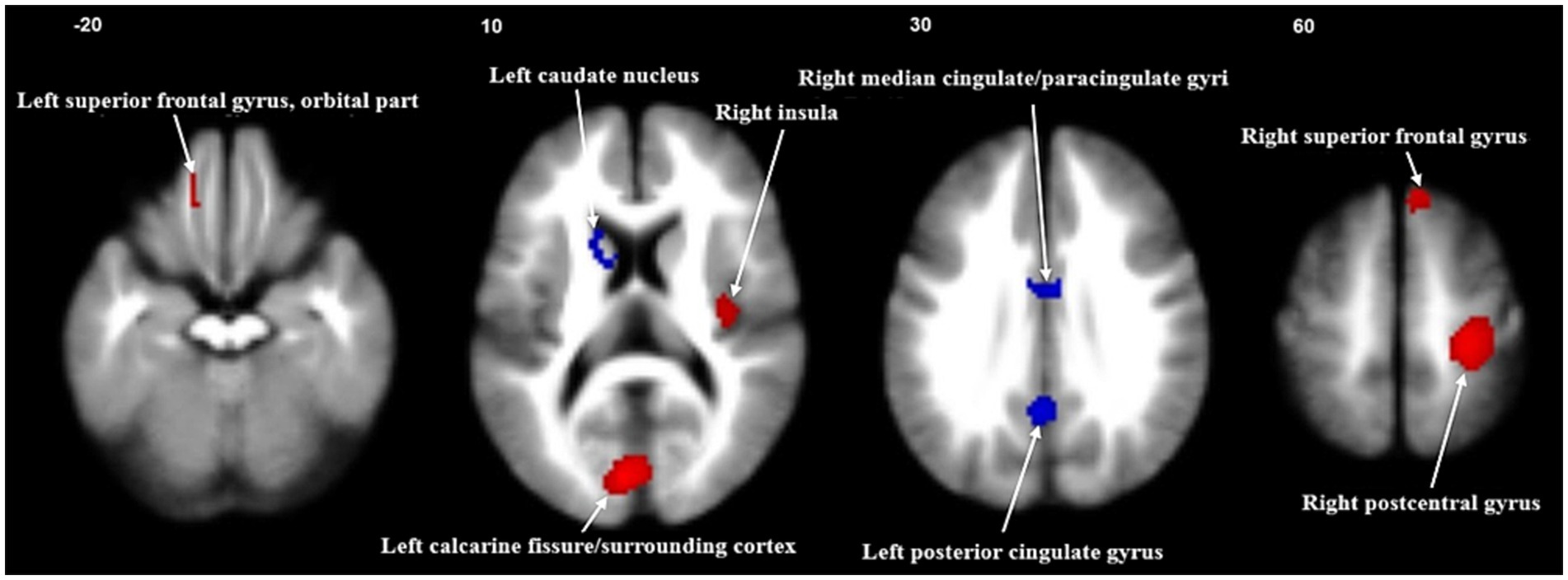
Figure 2. Meta-analysis of regional abnormal resting-state brain activities in functional gastrointestinal disorders. Significant clusters are overlaid on MRIcron template for Windows for display purposes only.
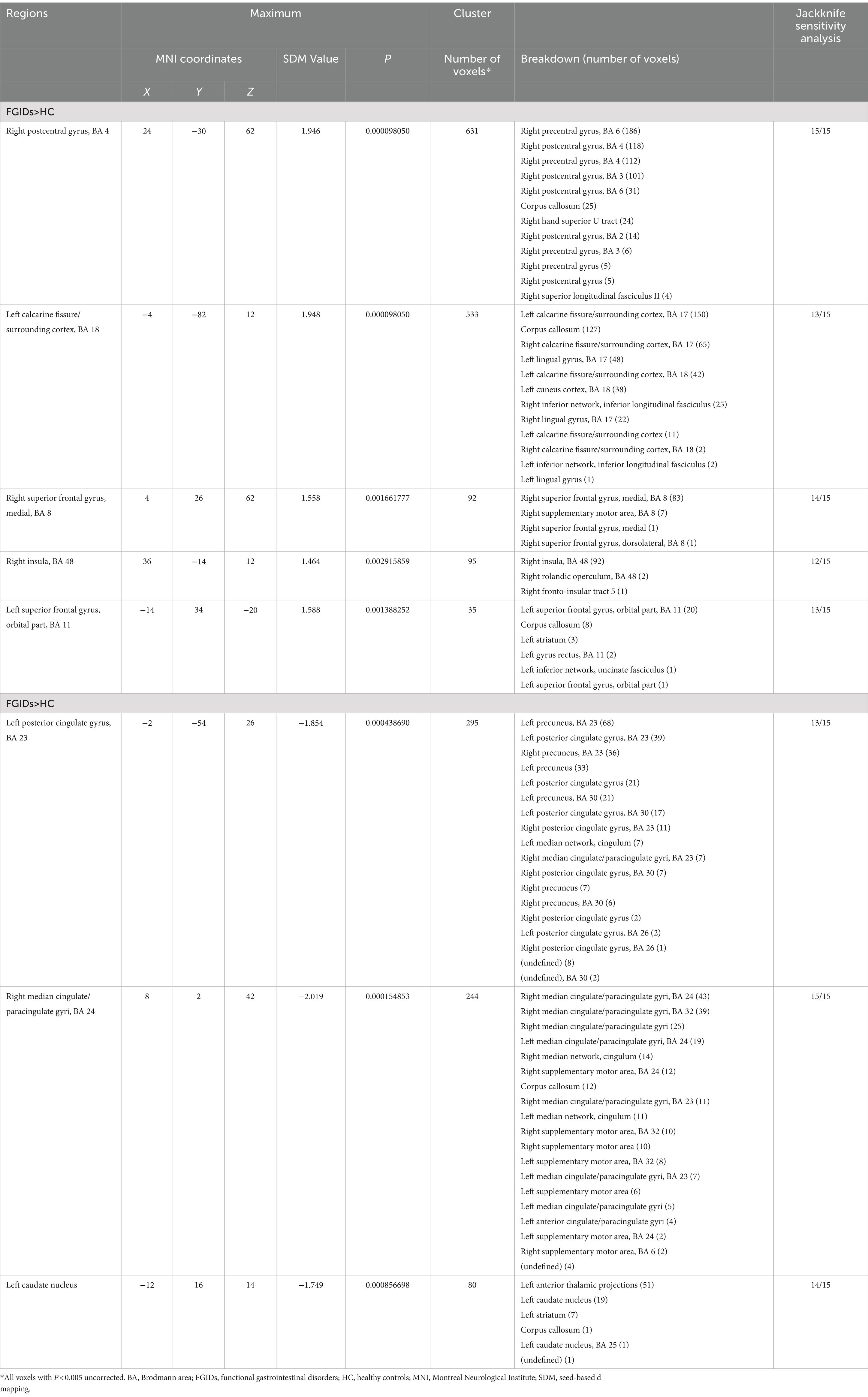
Table 2. Regional brain spontaneous activity changes in functional gastrointestinal disorders.
Sensitivity analysisThe sensitivity analysis, conducted through whole-brain jackknife analyses, demonstrated high reproducibility of the results. Specifically, consistently significant findings across all dataset combinations included increased brain activity in the right postcentral gyrus and reduced activity in the right median cingulate/paracingulate gyri. Likewise, heightened brain activity in the right superior frontal gyrus (medial) and reduced brain activity in the left caudate nucleus remained consistently significant across all but one dataset combination. Additionally, the remaining clusters retained significance in all but three dataset combinations. Detailed information can be found in Table 2.
Subgroup analysisDespite the inclusion of a sample size below the recommended minimum of 10 datasets for SDM meta-analyses, we conducted an exploratory analysis of different subtypes of FGIDs. Detailed results of the subtypes of FGIDs are provided in Supplementary Table S1. The findings from subgroups partially aligned with the pooled meta-analysis.
Meta-regression analysisMeta-regression analysis unveiled a negative correlation between the duration of FGIDs in patients and brain activity changes, particularly within the right median cingulate/paracingulate gyri and the left caudate nucleus (Table 3).
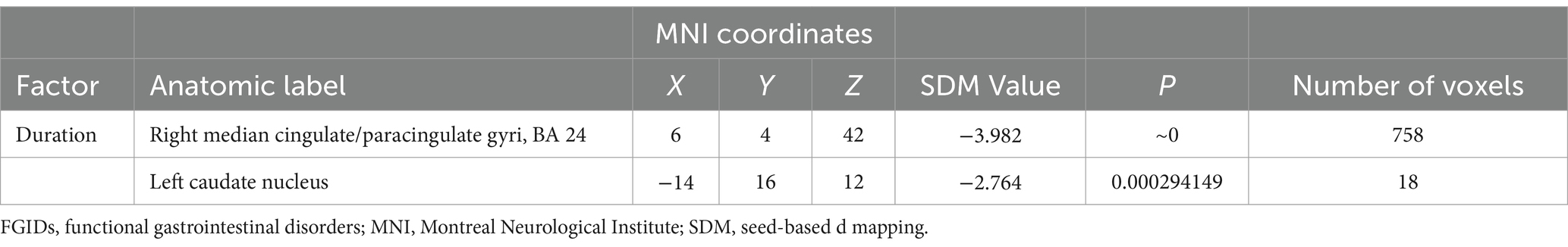
Table 3. Negative correlations between duration and regional brain activity alterations in FGIDs patients revealed by meta‐regression analysis.
DiscussionConsistent with prior studies, our study revealed significantly changed regional brain functions in several regions including increased brain activities in the postcentral gyrus, calcarine fissure/surrounding cortex, superior frontal gyrus, and insula, as well as lower activation in the left posterior cingulate gyrus, right median cingulate/paracingulate gyri, and the left caudate nucleus. These findings reflected the complex interplay between the gut and brain in FGIDs. The observed negative association between the duration of FGIDs and alterations in specific brain regions suggested potential disease progression effects on neural function, highlighting the dynamic nature of gut-brain interactions in FGIDs patients.
The identified alterations in brain function observed in various regions implicated in sensory processing, emotional regulation, and cognitive control provide valuable insights into the pathophysiology of FGIDs (Yu et al., 2022; Mao et al., 2023). The increased brain activities detected in the postcentral gyrus, calcarine fissure/surrounding cortex, superior frontal gyrus, and insula are indicative of heightened sensory processing and visceral sensation perception (Hong et al., 2013). The postcentral gyrus, known for its role in somatosensory processing, may contribute to the heightened perception of visceral pain commonly reported by FGIDs patients (Dong et al., 2022). Similarly, the increased activity in the insula, a key region involved in interoception and emotional processing, suggests dysregulated visceral sensation and emotional responses in FGIDs patients (Zhang et al., 2023). Notably, one previous fMRI study demonstrated that major depressive disorder (MDD) patients with gastrointestinal symptoms showed increased functional asymmetry than those without gastrointestinal symptoms (Fu et al., 2021), this suggests an imbalance in the regulation of brain-gastrointestinal function, emphasizing the involvement of the frontal gyrus in FGIDs.
On the other hand, decreased activation in regions such as the left posterior cingulate gyrus, right median cingulate/paracingulate gyri, and the left caudate nucleus underscored the multifaceted nature of FGIDs (Mayer et al., 2015a). The left posterior cingulate gyrus, known for its involvement in self-awareness, cognition, and emotion regulation, exhibits reduced activity in FGIDs patients compared to healthy controls (Labus et al., 2013). This diminishment in activity might reflect abnormalities in emotional regulation and self-awareness commonly observed in FGIDs patients, which are often linked to anxiety and depression. Additionally, the posterior cingulate gyrus is implicated in pain processing, suggesting its involvement in the altered perception of pain experienced by FGIDs patients (Hong et al., 2014). The cingulate cortex plays crucial roles in emotional regulation and autonomic nervous system function (Berman et al., 2008). The decreased activation observed in right median cingulate/paracingulate gyri might contribute to the emotional dysregulation and autonomic dysfunction often observed in FGIDs patients. Prospective studies have highlighted heightened stress responses and autonomic nervous system dysfunction in FGIDs patients, aligning with the observed reduction in activity in these brain regions (Kano et al., 2018). The caudate nucleus, associated with motor control and reward processing, exhibits decreased activation in IBS patients (Weng et al., 2017). This reduction in activity may be related to the motor dysfunction and altered reward processing observed in FGIDs patients, including changes in gastrointestinal motility and appetite regulation (Kano et al., 2018). It is noteworthy that these brain regions exhibiting aberrant intrinsic brain activities were commonly implicated in emotional disorders such as anxiety and depression (Elsenbruch et al., 2010; Labus et al., 2013), providing microscopic evidence for the frequent comorbidity of FGIDs with mood disturbances. This indicated the intricate interplay between gut-brain interactions and emotional regulation in FGIDs, highlighting the need for comprehensive therapeutic interventions addressing both gastrointestinal symptoms and associated emotional distress. Taken together, the identified alterations in brain activation patterns highlighted the widespread impact of gut dysfunction on brain function in FGIDs. These findings provided insights for further research to elucidate the precise mechanisms underlying these alterations and seek potential interventions targeting these brain regions to relieve symptoms and improve quality of life in FGIDs patients. We propose that future treatments for FGIDs might benefit from targeted interventions aimed at the brain regions implicated in our study, potentially through neuromodulation therapies or cognitive-behavioral strategies.
The gut-brain axis serves as a crucial interface between the gastrointestinal system and the central nervous system, playing a pivotal role in the pathophysiology of FGIDs (Mayer et al., 2022). In line with the predictive coding theory, our findings of altered brain activity in FGIDs may reflect disruptions in the brain’s predictive mechanisms, particularly in processing interoceptive signals from the gut. Dysfunctional gut-brain communication, influenced by factors such as gut microbiota, neuroendocrine signaling, and immune responses, contributes to the onset, progression, and perpetuation of FGIDs symptoms (Mayer et al., 2015a). Reflecting in neuroimaging, our findings demonstrated the significant alterations in brain function observed in FGID patients, highlighted the multifaceted influence of the gut-brain axis on neural processing, and indicated a disrupted homeostatic balance between the gut and brain (Al Omran and Aziz, 2014). In addition, a compelling correlation emerged between the duration of FGIDs and specific alterations in brain regions, suggesting a progressive impact of the disease on neural function. Therefore, it emphasizes the importance of implementing effective treatments and interventions for FGIDs as early as possible. Moreover, in contrast to previous studies, this research conducted an analysis by amalgamating all FGIDs and solely focusing on local brain functional metrics based on ReHo, ALFF, and fALFF, without incorporating other imaging modalities such as positron emission computed tomography (PET) and functional connectivity metrics of fMRI. Subgroup analyses concerning different disorders revealed that FGIDs constitute a complex spectrum of conditions, with IBS, FD, and FC each exhibiting distinct neurobiological mechanisms. However, these findings warrant cautious interpretation given the limited sample size.
The study acknowledges several limitations that should be taken into consideration. Firstly, the heterogeneous nature of data acquisition parameters and clinical variables across the included studies introduced potential biases not fully addressed by statistical methods alone. Secondly, the absence of longitudinal studies in both our meta-analysis and the literature reviewed herein restricted our ability to explore the dynamicity and reversibility of neural activities associated with FGIDs. Longitudinal studies and task-based functional MRI studies are crucial for elucidating the temporal dynamics of brain functions in FGIDs. These studies are especially crucial in examining the temporal interplay between brain activity and the progression of FGID symptoms. Future studies might usefully adopt methodologies that either track these changes longitudinally or involve the presentation of controlled gut-related stimuli to participants. Thirdly, our meta-analysis exclusively focused on changes in resting-state regional spontaneous brain activity in FGIDs, neglecting other valuable aspects such as functional connectivity, graph theory, independent component analysis (ICA), and task-based fMRI studies. Incorporating these methodologies in future investigations could offer a more comprehensive understanding of the functional patterns associated with FGIDs. Lastly, methodological constraints precluded direct comparisons between different subtypes of FGIDs in this study. Future research efforts should strive to overcome these limitations through advancements in analytical techniques.
ConclusionIn conclusion, our study shed light on the intricate relationship between FGIDs and aberrant brain activity, providing valuable insights into the underlying pathophysiology of these conditions. Our findings revealed significant regional abnormalities in brain activities, implicating areas involved in sensory processing, emotional regulation, and cognitive control. Notably, heightened brain activity in sensory processing regions and reduced activation in areas associated with emotional regulation highlighted the complex interplay between gut dysfunction and brain function in FGIDs.
Author contributionsYS: Investigation, Methodology, Software, Writing – original draft, Data curation. BW: Data curation, Formal analysis, Project administration, Writing – review & editing. XZ: Data curation, Methodology, Validation, Writing – review & editing. ZS: Project administration, Resources, Visualization, Writing – review & editing. SH: Formal analysis, Investigation, Project administration, Writing – review & editing. CZ: Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing, Conceptualization.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Medical and Health Science and Technology Development Plan of Shandong Province (202304011343), the Ministry of Education Industry-University Cooperative Education Project (220900242232529), the Key Research and Development Plan of Jining City (2023JNZC141), and the Cultivation Plan of High-level Scientific Research Projects of Jining Medical University (JYGC2021KJ006).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1452216/full#supplementary-material
ReferencesAlbajes-Eizagirre, A., Solanes, A., Vieta, E., and Radua, J. (2019). Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. NeuroImage 186, 174–184. doi: 10.1016/j.neuroimage.2018.10.077
PubMed Abstract | Crossref Full Text | Google Scholar
Ao, W., Cheng, Y., Chen, M., Wei, F., Yang, G., An, Y., et al. (2021). Intrinsic brain abnormalities of irritable bowel syndrome with diarrhea: a preliminary resting-state functional magnetic resonance imaging study. BMC Med. Imaging 21:4. doi: 10.1186/s12880-020-00541-9
PubMed Abstract | Crossref Full Text | Google Scholar
Aro, P., Talley, N. J., Johansson, S. E., Agreus, L., and Ronkainen, J. (2015). Anxiety is linked to new-onset dyspepsia in the Swedish population: a 10-year follow-up study. Gastroenterology 148, 928–937. doi: 10.1053/j.gastro.2015.01.039
PubMed Abstract | Crossref Full Text | Google Scholar
Berman, S. M., Naliboff, B. D., Suyenobu, B., Labus, J. S., Stains, J., Ohning, G., et al. (2008). Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 28, 349–359. doi: 10.1523/JNEUROSCI.2500-07.2008
PubMed Abstract | Crossref Full Text | Google Scholar
Black, C. J., Drossman, D. A., Talley, N. J., Ruddy, J., and Ford, A. C. (2020). Functional gastrointestinal disorders: advances in understanding and management. Lancet 396, 1664–1674. doi: 10.1016/s0140-6736(20)32115-2
Crossref Full Text | Google Scholar
Cai, W., Tian, H., Sun, P., Hua, T., Gong, J., Zhang, R., et al. (2024). Regional homogeneity alterations in patients with functional constipation and their associations with gene expression profiles. Cereb. Cortex 34:403. doi: 10.1093/cercor/bhad403
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, X. F., Guo, Y., Lu, X. Q., Qi, L., Xu, K. H., Chen, Y., et al. (2021). Aberrant intraregional brain activity and functional connectivity in patients with diarrhea-predominant irritable bowel syndrome. Front. Neurosci. 15:721822. doi: 10.3389/fnins.2021.721822
PubMed Abstract | Crossref Full Text | Google Scholar
Dong, X., Yin, T., Yu, S., He, Z., Chen, Y., Ma, P., et al. (2022). Neural responses of acupuncture for treating functional dyspepsia: An fMRI study. Front. Neurosci. 16:819310. doi: 10.3389/fnins.2022.819310
PubMed Abstract | Crossref Full Text | Google Scholar
Duan, W., Liu, X., Ping, L., Jin, S., Yu, H., Dong, M., et al. (2022). Distinct functional brain abnormalities in insomnia disorder and obstructive sleep apnea. Eur. Arch. Psychiatry Clin. Neurosci. 273, 493–509. doi: 10.1007/s00406-022-01485-7
PubMed Abstract | Crossref Full Text | Google Scholar
Elsenbruch, S., Rosenberger, C., Bingel, U., Forsting, M., Schedlowski, M., and Gizewski, E. R. (2010). Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology 139, 1310–1319.e4. doi: 10.1053/j.gastro.2010.06.054
PubMed Abstract | Crossref Full Text | Google Scholar
Feng, Y., Li, G., Hu, Y., Zhang, W., Ji, W., Zhou, H., et al. (2023). Magnetic resonance imaging and functional constipation. J. Clin. Basic Psychosomat. 2:1463. doi: 10.36922/jcbp.1463
Crossref Full Text | Google Scholar
Fu, X., Ding, Y., Chen, J., Liu, F., Li, H., Zhao, J., et al. (2021). Altered brain functional asymmetry in patients with major depressive disorder related to gastrointestinal symptoms. Front. Neurosci. 15:797598. doi: 10.3389/fnins.2021.797598
PubMed Abstract | Crossref Full Text | Google Scholar
Gong, W., Guo, P., Li, Y., Liu, L., Yan, R., Liu, S., et al. (2023). Role of the gut-brain Axis in the shared genetic etiology between gastrointestinal tract diseases and psychiatric disorders. JAMA Psychiatry 80, 360–370. doi: 10.1001/jamapsychiatry.2022.4974
PubMed Abstract | Crossref Full Text | Google Scholar
Hong, J. Y., Kilpatrick, L. A., Labus, J., Gupta, A., Jiang, Z., Ashe-McNalley, C., et al. (2013). Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J. Neurosci. 33, 11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013
PubMed Abstract | Crossref Full Text | Google Scholar
Hong, J. Y., Labus, J. S., Jiang, Z., Ashe-Mcnalley, C., Dinov, I., Gupta, A., et al. (2014). Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PLoS One 9:e84564. doi: 10.1371/journal.pone.0084564
PubMed Abstract | Crossref Full Text | Google Scholar
Jin, Q., Duan, S., Li, G., Sun, L., Hu, Y., Hu, C., et al. (2019). Sex-related differences in resting-state brain activity and connectivity in the orbital frontal cortex and insula in patients with functional constipation. Neurogastroenterol. Motil. 31:e13566. doi: 10.1111/nmo.13566
PubMed Abstract | Crossref Full Text | Google Scholar
Kano, M., Dupont, P., Aziz, Q., and Fukudo, S. (2018). Understanding neurogastroenterology from neuroimaging perspective: a comprehensive review of functional and structural brain imaging in functional gastrointestinal disorders. J Neurogastroenterol Motil 24, 512–527. doi: 10.5056/jnm18072
PubMed Abstract | Crossref Full Text | Google Scholar
Ke, J., Qi, R., Liu, C., Xu, Q., Wang, F., Zhang, L., et al. (2015). Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol. Motility 27, 1796–1803. doi: 10.1111/nmo.12692
PubMed Abstract | Crossref Full Text | Google Scholar
Labus, J. S., Hubbard, C. S., Bueller, J., Ebrat, B., Tillisch, K., Chen, M., et al. (2013). Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology 145, 1253–1261. doi: 10.1053/j.gastro.2013.08.016
PubMed Abstract | Crossref Full Text | Google Scholar
Lee, I.-S., Kullmann, S., Scheffler, K., Preissl, H., and Enck, P. (2018). Fat label compared with fat content: gastrointestinal symptoms and brain activity in functional dyspepsia patients and healthy controls. Am. J. Clin. Nutr. 108, 127–135. doi: 10.1093/ajcn/nqy077
PubMed Abstract | Crossref Full Text | Google Scholar
Li, G., Zhang, W., Hu, Y., Wang, J., Li, J., Jia, Z., et al. (2021). Distinct basal brain functional activity and connectivity in the emotional-arousal network and thalamus in patients with functional constipation associated with anxiety and/or depressive disorders. Psychosom. Med. 83, 707–714. doi: 10.1097/PSY.0000000000000958
Crossref Full Text | Google Scholar
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. doi: 10.1136/bmj.b2700
PubMed Abstract | Crossref Full Text | Google Scholar
Liu, J., Cao, L., Li, H., Gao, Y., Bu, X., Liang, K., et al. (2022). Abnormal resting-state functional connectivity in patients with obsessive-compulsive disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 135:104574. doi: 10.1016/j.neubiorev.2022.104574
PubMed Abstract | Crossref Full Text | Google Scholar
Lovell, R. M., and Ford, A. C. (2012). Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4. doi: 10.1016/j.cgh.2012.02.029
PubMed Abstract | Crossref Full Text | Google Scholar
Ma, X., Li, S., Tian, J., Jiang, G., Wen, H., Wang, T., et al. (2015). Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: a resting-state fMRI study. Clin. Neurophysiol. 126, 1190–1197. doi: 10.1016/j.clinph.2014.10.004
PubMed Abstract | Crossref Full Text | Google Scholar
Mao, Y., Zhang, P., Sun, R., Zhang, X., He, Y., Li, S., et al. (2023). Altered resting-state brain activity in functional dyspepsia patients: a coordinate-based meta-analysis. Front. Neurosci. 17:4287. doi: 10.3389/fnins.2023.1174287
PubMed Abstract | Crossref Full Text | Google Scholar
Mayer, E. A., Labus, J. S., Tillisch, K., Cole, S. W., and Baldi, P. (2015a). Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 12, 592–605. doi: 10.1038/nrgastro.2015.121
PubMed Abstract | Crossref Full Text | Google Scholar
Mayer, E. A., Nance, K., and Chen, S. (2022). The gut–brain Axis. Annu. Rev. Med. 73, 439–453. doi: 10.1146/annurev-med-042320-014032
Crossref Full Text | Google Scholar
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.Group, P (2009a). Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
留言 (0)