In 2022, there were > 600,000 reported deaths from malaria worldwide (1). Over 95% of malaria deaths globally occurred in Africa in children under 5 years (1). Cerebral malaria (CM) is a severe form of the disease where coma not attributable to another cause occurs in an individual with Plasmodium falciparum parasitemia (2, 3). Infected erythrocytes attach to the endothelial cells of the microvasculature, something known as parasite sequestration (4–7). The use of artesunate and preventative techniques have decreased deaths from malaria, however, additional adjunctive treatments have failed to further reduce mortality and morbidity (8, 9). Up to half of individuals who survive CM have neurologic sequelae such as epilepsy, behavioral dysregulation, motor delays, and cognitive impairments (10, 11). Further exploration is needed to determine how cerebral malaria results in profound neurologic injury given the parasite remains in the vasculature and does not enter the brain parenchyma. Once determined, the impact of the deployment of targeted treatments may be undertaken (12).
Transcranial Doppler (TCD) ultrasound is a portable, safe, and non-invasive imaging technique that is readily available in many African hospitals which measures cerebral blood flow velocities (CBFV) in all major vessels of the cerebral arterial circulation (13, 14). Emerging research in CM utilizing TCD has demonstrated five distinct phenotypes of abnormal CBFV and waveform patterns. These phenotypes include hyperemia, low flow, microvascular obstruction, vasospasm, and isolated posterior circulation high flow (15). These phenotypes demonstrate that TCD can be used to characterize changes in the neurovasculature children with CM. Additionally, O’Brien et al. noted that these changes in TCD findings are likely due to primary pathologic changes and not other typical physiologic alterations (15). For example, fever, seizures, and hypercapnia were not associated with hyperemia whereas non-invasive markers of increased intracranial pressure (ICP) were not associated with low flow. Further work is needed to determine aspects of TCD measurements or waveforms that provide additional understanding of pathophysiologic underpinnings of these phenotypes.
The Gosling pulsatility index (PI) is a TCD parameter calculated as the difference between systolic CBFV and diastolic CBFV divided by the mean CBFV [CBFVsys – CBFVdia/CBFVmean] (16). An increased PI could be the result of increases in systolic flow velocity and/or decreases in diastolic flow velocity. Inversely, a decrease in PI is seen when systolic flow velocity decreases and/or diastolic flow velocity increases. The former is commonly seen as a result of increased ICP where diastolic flow, due to decreased driving pressure during this phase of the cardiac cycle, is preferentially decreased. Asil et al. found that, in patients with MCA infarction, the PI of the MCA increased over the first three days and was correlated with the significance of midline shift on the third day after stroke (17). Studies have demonstrated that a PI ranging from 1.3 to 1.5 correlates with some degree of intracranial hypertension (18). In patients with traumatic brain injury, PI values significantly decrease after decompressive craniectomy (19). Alternatively, the PI is reduced in vascular changes such as vasospasm where autoregulation results in decreased resistance to flow, and an increased diastolic flow velocity. As such, PI has clinical applications in the assessment of cerebral circulation (20).
While a single PI value provides a measure of underlying cerebrovascular physiology during the examination, it does not capture dynamic changes over time that could provide more insight into underlying pathologic changes. A novel use of PI has been developed, referred to as Day-to-Day (DTD) PI change, which evaluates the variability in PI from one day to the subsequent day (21). As a measurement of spread between the mean, variability demonstrates the level of stability of PI rather than simply a high or low average value. In a study of 42 children with traumatic brain injury, Jordan et al. found that variability of DTD PI change was larger in children with worse neurocognitive outcomes, irrespective of injury severity (21). Presumably the high variability of DTD PI change indicates a loss or defect in autoregulatory functioning of the cerebral vasculature which leads to worse neurocognitive outcomes due to periods of inappropriate perfusion.
Therefore, we hypothesized that the variability of DTD PI change is a potential measure that will further understanding of the alterations to the cerebral vasculature in pediatric cerebral malaria patients. We therefore performed a retrospective analysis of 122 children in sub-Saharan-Africa with CM and described the association between variability of DTD PI change and brain volume score, neurologic outcome, and TCD derived CM phenotypes.
2 Materials and methodsAfter Institutional Review Board approval and execution of a Data-Use Agreement, a de-identified data set from parent study was transferred to a secure database. The parent study enrolled children with cerebral malaria who were treated at Queen Elizabeth Central Hospital in Blantyre, Malawi, Kalembe Lembe Children’s Hospital in Kinshasa, Democratic Republic of the Congo (DRC) and Lodja District Referral Hospital in Lodja, DRC between January 2018 and June 2021. Inclusion criteria included: age 6 months – 12 years, met the World Health Organization definition of cerebral malaria (Plasmodium falciparum parasitemia, Blantyre Coma Score (BCS) ≤2, and no other discernible cause of encephalopathy). Exclusion criteria included developmental delay, diagnosis of sickle cell disease, severe malnutrition, and advanced HIV disease. Children with developmental delay were excluded because the outcome measurement is validated on children without developmental delay. Children with sickle cell disease have a high frequency of abnormal TCD examinations as a population. Additionally, the impact of severe malnutrition and/or advanced HIV status on TCD examinations is unknown. Therefore, these children were excluded. This study utilizes the same inclusion and exclusion criteria with an additional exclusion criterion. To calculate variability of DTD PI change, at least three sequential PI measurements are required; therefore, participants in the parent study without three sequential PI measurements were excluded.
Data was imported into Excel for cleaning. R software (R Core Team, 2022) was used for the data analysis. Descriptive statistics were used to explore patient and disease characteristics. The participant’s DTD PI change and subsequent analysis was calculated using the following steps.
Step 1 – DTD PI change is defined as the difference in PI measurement from one day to the next and is calculated using the following formula (using day1 and day2 as an example):
(PIday2 – PIday1) / PIday1 = DTD PI change.
The DTD PI change for other continuous days was calculated similarly.
Step 2 – These values are averaged to derive their Mean DTD PI change during the hospital stay. This value represents the mean difference between each consecutive day’s measurement (21, 22).
Step 3 –The variability of DTD PI change, defined as the dispersion of PI measurements from their mean values was then calculated using the variation function. As a measurement of spread between the mean, variation demonstrates the level of stability of PI rather than simply a high or low average value. Values were calculated for both MCAs in each participant.
Step 4 – Simple linear regression is used to explore the association of neurocognitive outcomes with variation of DTD PI change, and the association with Brain Volume Score (BVS).
Step 5 – Lastly, the variability of DTD PI change is explored by TCD phenotypes.
Brain Volume Score (BVS) is a classification of brain size based on neuroimaging. BVS ranges from 1, marked atrophy, to 8, effacement of the cisterns and sulci with herniation. A score of 3 indicates normal brain volume (23). Neurocognitive outcomes were classified as normal, sequelae, or dead as determined by the Pediatric Cerebral Performance Categorization (PCPC) score at hospital discharge. The PCPC scoring system was developed to measure morbidity after critical illness in the pediatric population (24, 25) The scores progress from 1 to 6 to represent increasing levels of impairment. Children with a PCPC score of 1 were classified as having a ‘normal’ outcome, a score of 2–5 was classified as ‘sequelae’ due to the neurologic impairment, and a score of 6 was classified as ‘dead’.
3 ResultsThe participants with 3 or more sequential TCD (n = 122) were predominantly male (51.6%) with an average age of approximately 5 years old (5 ± 2.5 years). Additionally, 68 (55.7%) participants received a magnetic resonance imaging scan. Of these 68 participants, a majority had a BVS of 5 or 6 (27 (40%) and 17 (35%), respectively). Of the 122 participants, 95 (77.9%) had a good neurologic outcome (no neurologic sequelae), and 27 (22.1%) had a poor outcome (neurologic sequelae or died). See Table 1 for full demographic information.
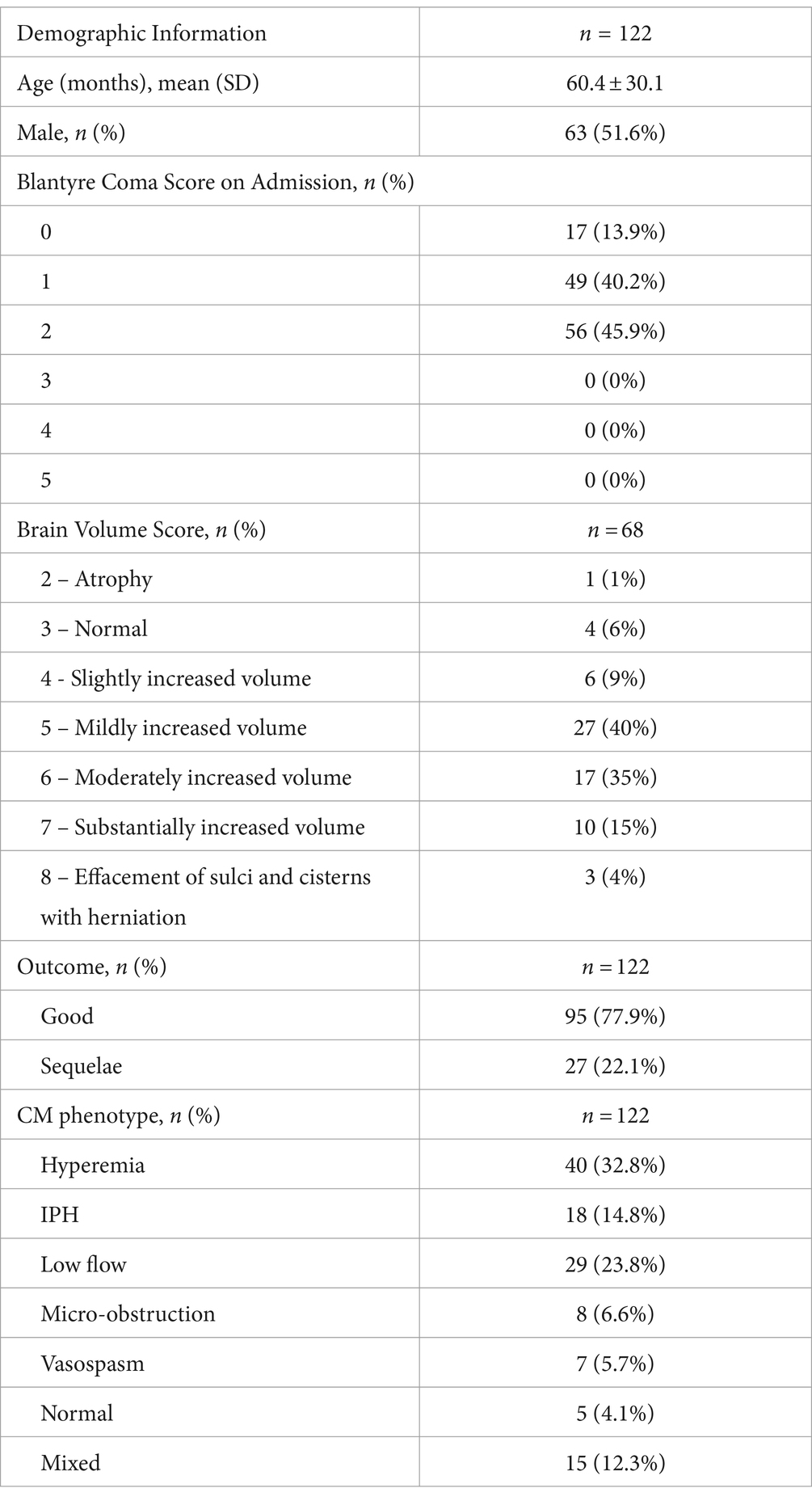
Table 1. Demographic information.
Analysis of the outcome groups revealed that patients who had poor neurologic outcome had higher variability of DTD PI change in the right MCA (0.14 ± 0.21) compared to those with a good neurologic outcome (0.1 ± 0.1), with a difference of 0.03 (95% CI [−0.13, 0.05]). In the left MCA, the variability of the DTD PI change was also higher in those with poor outcomes (0.17 ± 0.41) compared to those with good neurologic outcomes (0.11 ± 0.19), with a difference of 0.06 (95% CI [−0.23, 0.11]). See Table 2 for more information.
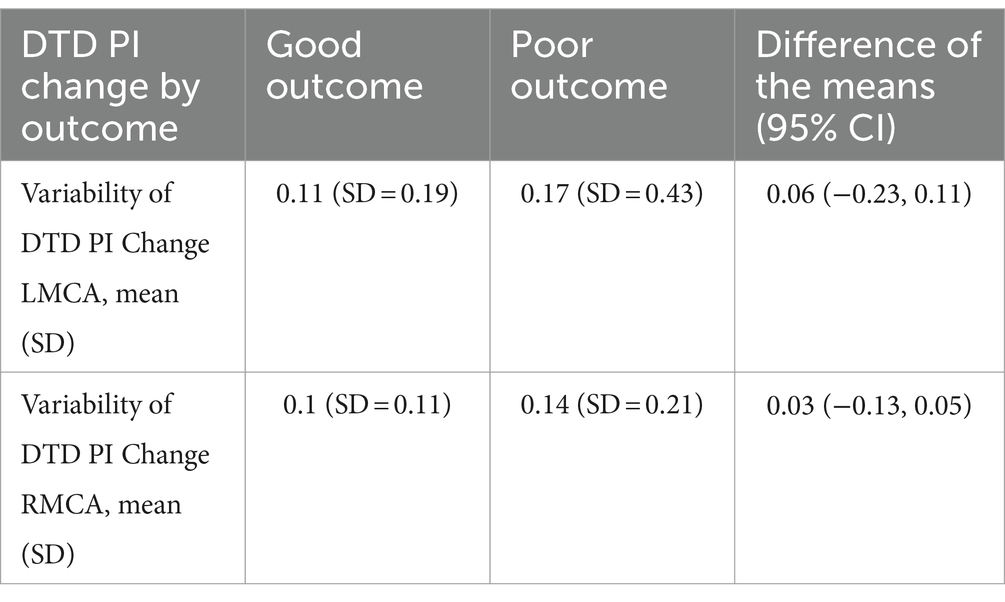
Table 2. Day-to-Day PI Change by Neurologic Outcome.
When evaluating variability of DTD PI change by BVS, participants with a higher BVS score had a higher variability of DTD PI change. This difference in variability of DTD PI change was seen in both left and right MCAs. In the left MCAs, the most frequent BVS of 5 equated to a lower DTD PI change compared to a BVS of 8 (0.053 ± 0.077 and 0.898 ± 1.197, p = 0.012, respectively). In the right MCAs, this pattern again appeared in participants with a BVS of 5 compared to BVS of 8 (0.075 ± 0.1 and 0.502 ± 0.516, p = 0.189 respectively). See Table 3 for more information.
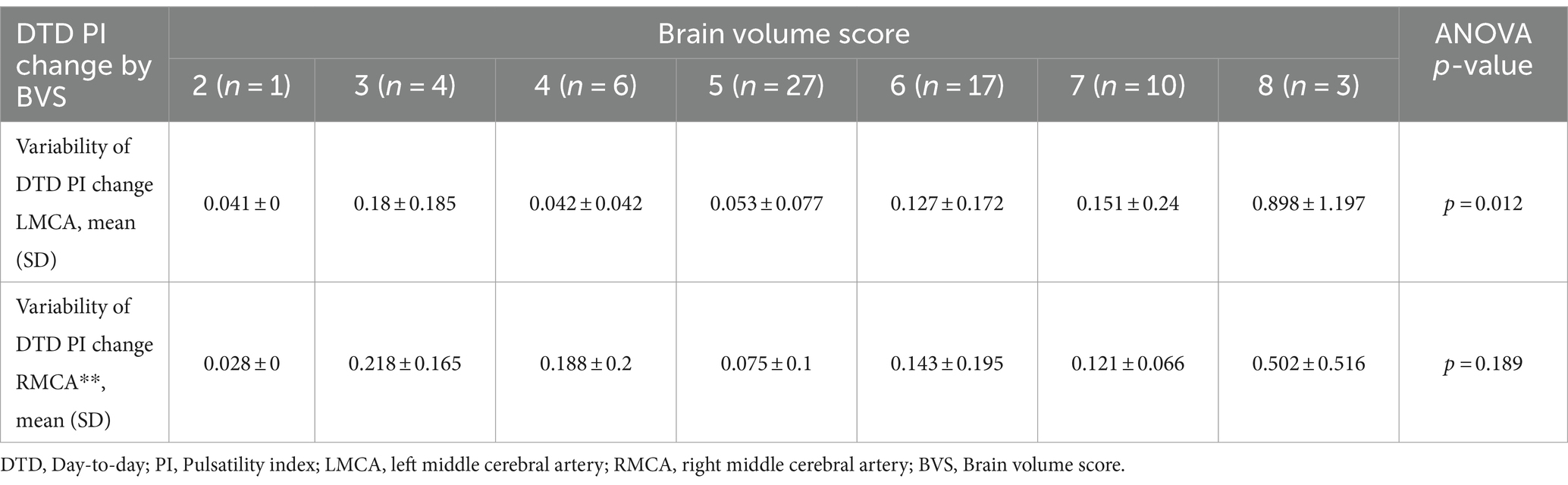
Table 3. Day-to-day PI change by BVS.
Lastly, the variability of the DTD PI change demonstrated discreet differences in the previously described CM phenotypes. The largest variability of DTD PI change was seen in the microvascular-obstruction group for both the LMCA (0.478 ± 0.833, p = 0.019) and RMCA (0.237 ± 0.386, p = 0.273). The smallest variability of DTD PI change was seen in the group in the normal phenotype group for both the LMCA (0.048 ± 0.038, p = 0.019) and RMCA (0.054 ± 0.064, p = 0.273). See Table 4 for information on all phenotypes.
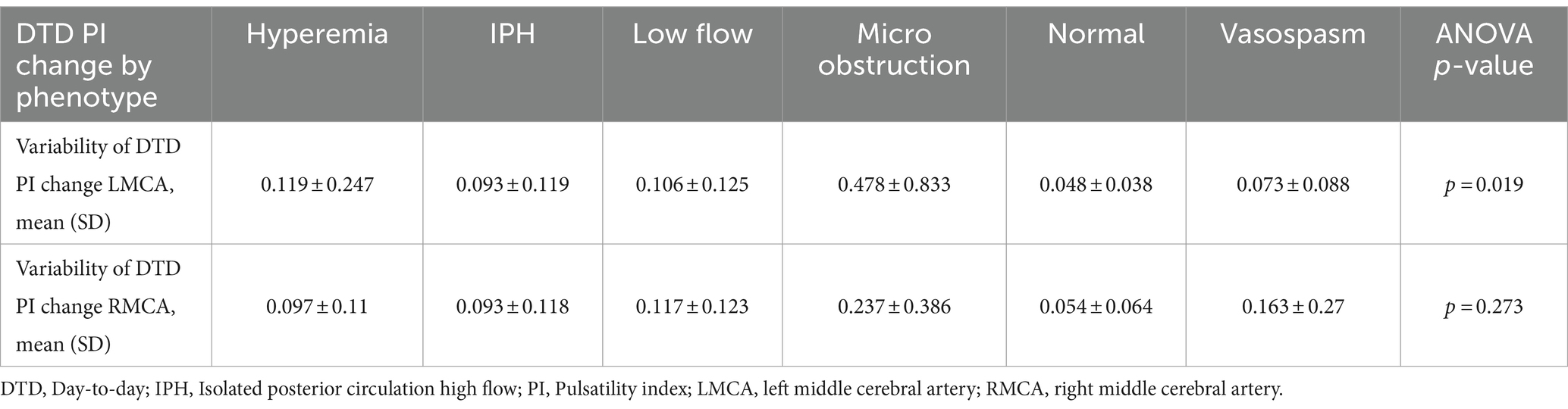
Table 4. Day-to-day PI change by phenotype.
4 DiscussionIn a group of 122 pediatric participants with CM who had daily TCD measurements of their bilateral MCAs, the variability of DTD PI change was evaluated in the context of neurologic outcome, BVS, and TCD phenotype. Individuals with the normal phenotype have the lowest variability of DTD PI change indicating low neurovascular involvement. These participants also had better outcomes indicating perhaps that they had less significant disease at the time of presentation or may have had an alternate diagnosis. Participants with hyperemia, isolated posterior hyperemia, vasospasm or low flow may be experiencing abnormal vascular tone. This dysregulated vascular tone may account for the lower variability of DTD PI change compared to a more volatile phenotype such as micro-obstruction. In the micro-obstructive phenotype, the primary pathophysiologic mechanism is hypothesized to be sequestered red cells occluding the microcirculation. Artesunate results in rapid parasite clearance, which may cause a highly abnormal PI to quickly return to normal as parasitized RBCs are cleared and result in a high DTD PI change.
Participants with a higher BVS had higher variability of DTD PI change. While brain volume is likely influenced by the factors noted above, identifying alternate measures that are more easily obtainable than MRI in resource limited settings where malaria is endemic is important as BVS is an important prognostic marker. Therefore, the use of TCD may be able to provide similar information portably, non-invasively, and in real time. Future studies with larger sample sizes should assess this relationship.
TCD has been used to evaluate the cerebrovasculature in several cohorts of children with CM (12, 15). Multiple different TCD abnormalities were described in children with CM with studies classified into one of five distinct phenotypes. In the current study, each of the phenotypes had a distinct variability of DTD PI change (Table 4). In this participant group, 22.1% of the participants died or had neurologic sequelae after treatment for CM. These participants demonstrated a higher variability of DTD PI change. DTD PI variability can be increased in the setting of brain parenchymal changes (such as cerebral edema, cerebral hemorrhage) that increase ICP, in alterations to vascular tone, or in venous congestion. In CM, a significant systemic inflammatory response occurs, cytokines are released that damage the blood–brain barrier, and a secondary vasogenic edema has been reported to occur (26). Parasite sequestration may result in cerebral blood flow disruption with cytotoxic edema. Lastly, venous congestion occurs as infected red blood cells (RBC) obstruct microvasculature and impair forward flow and venous drainage. The poor outcome of these individuals with high variation of DTD PI change may be due to these factors that in isolation or together lead to intracranial hypertension.
This study describes the first application of DTD PI change in this population and begins to lay the foundation for clinical changes. As a portable and low-cost imaging modality that is available in many low and middle resourced countries, TCD is a valuable tool in clinical care (13, 14). This study adds to the early research on DTD PI change and its use in pediatric neurologic disorders as well as demonstrates its feasibility (21). While additional research is needed to develop formal clinical guidelines, this study demonstrates the importance of continued research in this area to determine feasibility of more frequent TCD measurements, the optimal timing of the measurements, and ideal interval between measurements.
4.1 LimitationsThis relatively small sample size of 129 patients and retrospective design limits our ability to generalize these findings. Children with less than 3 days of sequential TCD measurements were necessarily excluded further limiting the sample size. Thus, variability of DTD PI change was not available in those who recovered quickly or died soon after admission, thereby, further limiting the sample. TCD was only performed while Blantyre Coma Score was <3. Additionally, the data is a convenience sample of patients who presented with C.M. who had TCDs performed as a component of their care. Lastly, as some findings were not statistically significant, additional research is needed before clinical recommendations can be made. Future research should focus on larger sample size, more frequent TCD studies, account of age, sex, and time of day, and continued TCD studies on all participants including those that recover or die quickly.
5 ConclusionDTD PI change may help to prognosticate the neurologic outcomes in children with CM. The pathophysiologic underpinnings of increased DTD PI variability in those with poor outcomes should be explored as TCD could then be used as a point of care neuromonitoring tool and inform treatment strategies to improve outcomes.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the University of Alabama at Birmingham Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsJJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NO’B: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – review & editing. PL: Formal analysis, Methodology, Writing – review & editing. DM: Data curation, Writing – review & editing. RE: Data curation, Writing – review & editing. JM: Data curation, Writing – review & editing. LM: Data curation, Writing – review & editing. BG: Data curation, Writing – review & editing. TP: Data curation, Writing – review & editing. SJ: Data curation, Writing – review & editing. MG: Data curation, Writing – review & editing. TT: Data curation, Writing – review & editing. KR-R: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by University of Alabama at Birmingham, School of Nursing, Dean’s Scholar Award, Day-to-Day Pulsatility Index Change in Pediatric Brain Injury: A Proof-of-Concept Study. PI: J. Jordan, Dates of Award 11/11/2022–07/01/2025.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. World Health Organization (WHO) . World malaria report 2022. Geneva: World Health Organization (2023).
2. Thakur, KT, Vareta, J, Carson, KA, Kampondeni, S, Potchen, MJ, Birbeck, GL, et al. Cerebrospinal fluid Plasmodium falciparum histidine-rich protein-2 in pediatric cerebral malaria. Malar J. (2018) 17:125. doi: 10.1186/s12936-018-2272-y
PubMed Abstract | Crossref Full Text | Google Scholar
3. Mallewa, M, Vallely, P, Faragher, B, Banda, D, Klapper, P, Mukaka, M, et al. Viral CNS infections in children from a malaria-endemic area of Malawi: a prospective cohort study. Lancet Glob Health. (2013) 1:e153–60. doi: 10.1016/S2214-109X(13)70060-3
PubMed Abstract | Crossref Full Text | Google Scholar
4. Seydel, KB, Milner, DA, Kamiza, SB, Molyneux, ME, and Taylor, TE. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis. (2006) 194:208–5. doi: 10.1086/505078
PubMed Abstract | Crossref Full Text | Google Scholar
5. Taylor, TE, Fu, WJ, Carr, RA, Whitten, RO, Mueller, JS, Fosiko, NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. (2004) 10:143–5. doi: 10.1038/nm986
PubMed Abstract | Crossref Full Text | Google Scholar
6. Ponsford, MJ, Medana, IM, Prapansilp, P, Hien, TT, Lee, SJ, Dondorp, AM, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis. (2012) 205:663–71. doi: 10.1093/infdis/jir812
PubMed Abstract | Crossref Full Text | Google Scholar
7. van der Heyde, HC, Nolan, J, Combes, V, Gramaglia, I, and Grau, GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. (2006) 22:503–8. doi: 10.1016/j.pt.2006.09.002
Crossref Full Text | Google Scholar
8. Murray, CJL, Rosenfeld, LC, Lim, SS, Andrews, KG, Foreman, KJ, Haring, D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. (2012) 379:413–31. doi: 10.1016/S0140-6736(12)60034-8
Crossref Full Text | Google Scholar
9. Enwere, G . A review of the quality of randomized clinical trials of adjunctive therapy for the treatment of cerebral malaria. Trop Med Int Health. (2005) 10:1171–5. doi: 10.1111/j.1365-3156.2005.01505.x
PubMed Abstract | Crossref Full Text | Google Scholar
10. Birbeck, GL, Molyneux, ME, Kaplan, PW, Seydel, KB, Chimalizeni, YF, Kawaza, K, et al. Blantyre malaria project epilepsy study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. (2010) 9:1173–81. doi: 10.1016/S1474-4422(10)70270-2
Crossref Full Text | Google Scholar
11. Langfitt, JT, McDermott, MP, Brim, R, Mboma, S, Potchen, MJ, Kampondeni, SD, et al. Neurodevelopmental impairments 1 year after cerebral malaria. Pediatrics. (2019) 143:e20181026. doi: 10.1542/peds.2018-1026
PubMed Abstract | Crossref Full Text | Google Scholar
12. O’Brien, NF, Mutatshi Taty, T, Moore-Clingenpeel, M, Bodi Mabiala, J, Mbaka Pongo, J, Ambitapio Musungufu, D, et al. Transcranial Doppler ultrasonography provides insights into neurovascular changes in children with cerebral malaria. J Pediatr. (2018) 203:116–124.e3. doi: 10.1016/j.jpeds.2018.07.075
PubMed Abstract | Crossref Full Text | Google Scholar
13. Aaslid, R, Markwalder, TM, and Nornes, H. Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. (1982) 57:769–74. doi: 10.3171/jns.1982.57.6.0769
PubMed Abstract | Crossref Full Text | Google Scholar
14. Adelson, PD, Srinivas, R, Chang, Y, Bell, M, and Kochanek, PM. Cerebrovascular response in children following severe traumatic brain injury. Childs Nerv Syst. (2011) 27:1465–76. doi: 10.1007/s00381-011-1476-z
PubMed Abstract | Crossref Full Text | Google Scholar
15. O’Brien, NF, Fonseca, Y, Johnson, HC, Postels, D, Birbeck, GL, Chimalizeni, Y, et al. Mechanisms of transcranial Doppler ultrasound phenotypes in paediatric cerebral malaria remain elusive. Malar J. (2022) 21:196. doi: 10.1186/s12936-022-04163-0
PubMed Abstract | Crossref Full Text | Google Scholar
16. Michel, E, and Zernikow, B. Gosling’s Doppler pulsatility index revisited. Ultrasound Med Biol. (1998) 24:597–9. doi: 10.1016/S0301-5629(98)00024-6
Crossref Full Text | Google Scholar
17. Asil, T, Uzunca, I, Utku, U, and Berberoglu, U. Monitoring of increased intracranial pressure resulting from cerebral edema with transcranial Doppler sonography in patients with middle cerebral artery infarction. J Ultrasound Med. (2003) 22:1049–53. doi: 10.7863/jum.2003.22.10.1049
PubMed Abstract | Crossref Full Text | Google Scholar
18. Moreno, JA, Mesalles, E, Gener, J, Tomasa, A, Ley, A, Roca, J, et al. Evaluating the outcome of severe head injury with transcranial doppler ultrasonography. Neurosurg Focus. (2000) 8:e8:1–7. doi: 10.3171/foc.2000.8.1.1702
PubMed Abstract | Crossref Full Text | Google Scholar
19. Bor-Seng-Shu, E, Figueiredo, EG, Fonoff, ET, Fujimoto, Y, Panerai, RB, and Teixeira, MJ. Decompressive craniectomy and head injury: brain morphometry, ICP, cerebral hemodynamics, cerebral microvascular reactivity, and neurochemistry. Neurosurg Rev. (2013) 36:361–70. doi: 10.1007/s10143-013-0453-2
PubMed Abstract | Crossref Full Text | Google Scholar
20. Wielicka, M, Neubauer-Geryk, J, Kozera, G, and Bieniaszewski, L. Clinical application of pulsatility index. Med Res J. (2020) 5:201–10. doi: 10.5603/MRJ.a2020.0016
Crossref Full Text | Google Scholar
21. Jordan, J, Ladores, S, Kong, M, Smith, T, Li, P, and Reuter-Rice, K. Association between Day-to-Day Pulsatility index change and neurocognitive outcomes in pediatric traumatic brain injury. Neurotrauma Reports. (2022) 3:369–76. doi: 10.1089/neur.2022.0035
PubMed Abstract | Crossref Full Text | Google Scholar
22. Jordan, J, and Reuter-Rice, K. Day-to-day change in pulsatility index describes anterior cerebral circulation disturbance and functional outcomes in pediatric traumatic brain injury. J Neurosci Nurs. (2020) 52:224–9. doi: 10.1097/JNN.0000000000000533
PubMed Abstract | Crossref Full Text | Google Scholar
23. Seydel, KB, Kampondeni, SD, Valim, C, Potchen, MJ, Milner, DA, Muwalo, FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. (2015) 372:1126–37. doi: 10.1056/NEJMoa1400116
PubMed Abstract | Crossref Full Text | Google Scholar
24. Fiser, DH . Assessing the outcome of pediatric intensive care. J Pediatr. (1992) 121:68–74. doi: 10.1016/S0022-3476(05)82544-2
Crossref Full Text | Google Scholar
25. Fiser, DH, Tilford, JM, and Roberson, PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Crit Care Med. (2000) 28:1173–9. doi: 10.1097/00003246-200004000-00043
留言 (0)