Myasthenia gravis (MG) is an autoimmune disease that affects the postsynaptic membrane of neuro muscular junctions, resulting in fluctuant weakness in various muscle groups such as the extraocular, limb, respiratory, and bulbar muscles (1). The incidence rate of MG is 0.3–2.8 cases per 100,000 people, which is estimated to affect 700,000 people worldwide (2). The onset of MG may occur at any age, while females are significantly earlier than males. The peak incidence of female patients is before 40 years old, while the male patients is after 50 old years (3). The primary antibodies involved in the pathogenesis of MG are directed against the nicotinic acetylcholine receptor (AChR) and anti-muscle-specific tyrosine kinase (MuSK). AChR antibodies account for approximately 85% of cases, whereas MuSK antibodies account for approximately 6% (4). In approximately 15% of patients with generalized MG and 50% of those with ocular MG, both MuSK and AChR antibodies are negative, which are referred to double serum negative MG (dSnMG) (5). In the dSnMG group, 2–27% of patients have antibodies directly targeting low-density lipoprotein receptor associated protein 4 (LRP4) (6). In general LPR4 antibody-positive accounted for 2%, and triple seronegative antibodies accounted for 5% (7).
Although there are several treatment options available for patients with MG (8–10), oral steroids remain the first-line treatment and one of the most commonly used immunosuppressants (11–13). However, 5–20% patients with MG are nonresponsive to steroids, and high doses can lead to several adverse effects (13–16). Therefore, it is crucial to appropriately add non-steroid immunosuppressants for these patients (17). Besides, MuSK antibody-positive patients (MuSK-MG) frequently present exacerbation and myasthenia crisis (18, 19), and current guideline recommend Rituximab as an early-line treatment (20). Rituximab targets CD20 on the surface of B lymphocytes (21), which is different from other non-steroid immunosuppressants. Therefore, we focus on more about the AChR-MG and dSnMG patients (5).
Currently, there is a consensus on the treatment goal for patients with MG, which emphasizes achieving minimal manifestation status (MMS) or better with no more than 5 mg of prednisone (22). To achieve this treatment goal, it is important to incorporate non-steroid immunosuppressants into the treatment plan for patients with MG. Recent German guidelines recommend the glucocorticoids monotherapy or in combination with other non-steroidal immunosuppressants as first-line treatment for patients with mild to moderate disease activity (23).
However, determining the appropriate time to add non-steroid immunosuppressants to the treatment for patients with MG is controversial. A previous study combined steroid and non-steroid immunosuppressants in the initial treatment (24). However, this approach may present some challenges. First, approximately 20% of patients experienced spontaneous remission lasting for an average of 5 years (25–27). Second, some patients are responsive to lower doses of steroid [Muscle Study (28)]. Therefore, the early initiation of non-steroid immunosuppressants may result in overtreatment.
Consequently, identifying patients who would benefit from the early add-on of nonsteroid immunosuppressants is a critical issue. Accordingly, we conducted a retrospective study to explore the predictive factors for the add-on of non-steroid immunosuppressants, with the aim to provide valuable information for clinical practice.
Materials and methods Patient and data collectionWe retrospectively collected the clinical and follow-up data of patients with MG who were admitted to the Department of Neurology, Huashan Hospital, from May 15, 2015 to December 29, 2020. The patients were immunotherapy-naïve at baseline visit and had a minimum follow-up period of 1 year. The diagnostic criteria for MG are that the patient presents with symptoms of fluctuating weakness and has at least one positive result on the neostigmine test, serologic and electrophysiological tests (29). Patients were accessed by MG composite (MGC) scale before and after 30 min neostigmine 0.5 mg administration. The neostigmine test was defined to be positive if there was a decrease of ≥3 points in the MGC scale score after the injection compared to before (30). Patients were defined as RNS-positive when the decrease in CAMP from the first to the fourth or fifth CAMP exceeded 10% during repetitive nerve stimulation (RNS) at 2–5 Hz (31). MuSK-MG patients were excluded due to early add-on treatment with rituximab.
We evaluated the patients using the Myasthenia Gravis Foundation of American (MGFA) (32) stage, quantitative MG score (QMG) (33), quality of life (QoL) (34) score and activities of daily living (ADL) (35) scale at the first visit. The minimal symptom expression (MSE) was defined as an ADL score of 0 or 1 after 6 months from the first visit (36). The treatment strategy for steroid administration involved an initial dose of prednisone at 20 mg once a day, which was increased to 30 mg once a day after 1 week. Gradual reduction of steroid was initiated once the disease was stable. Non-steroid immunosuppressant treatment referred to oral azathioprine or tacrolimus, and was added to the steroid treatment when neurologists considered the MG symptom was not controlled well or reducing steroids was difficult.
The relapse is defined as recurrence of MG symptoms or a substantial increase in MG medications after the patient achieving MMS or better (37). The term “worsened MG” was defined as a QMG score increase of ≥4 points or ADL score increase of ≥2 points, compared to the baseline score (32). Early onset refers to the occurrence before 50 years old, while late onset indicates after the age of 50 (7). Obesity was defined as a body mass index (BMI) ≥ 28, and overweight was defined as a BMI ≥ 24 (38).
The included patients were categorized into two groups on the basis of whether non-steroid immunosuppressants were added after steroid treatment: the steroid group and the combination treatment group, as shown in Figure 1.
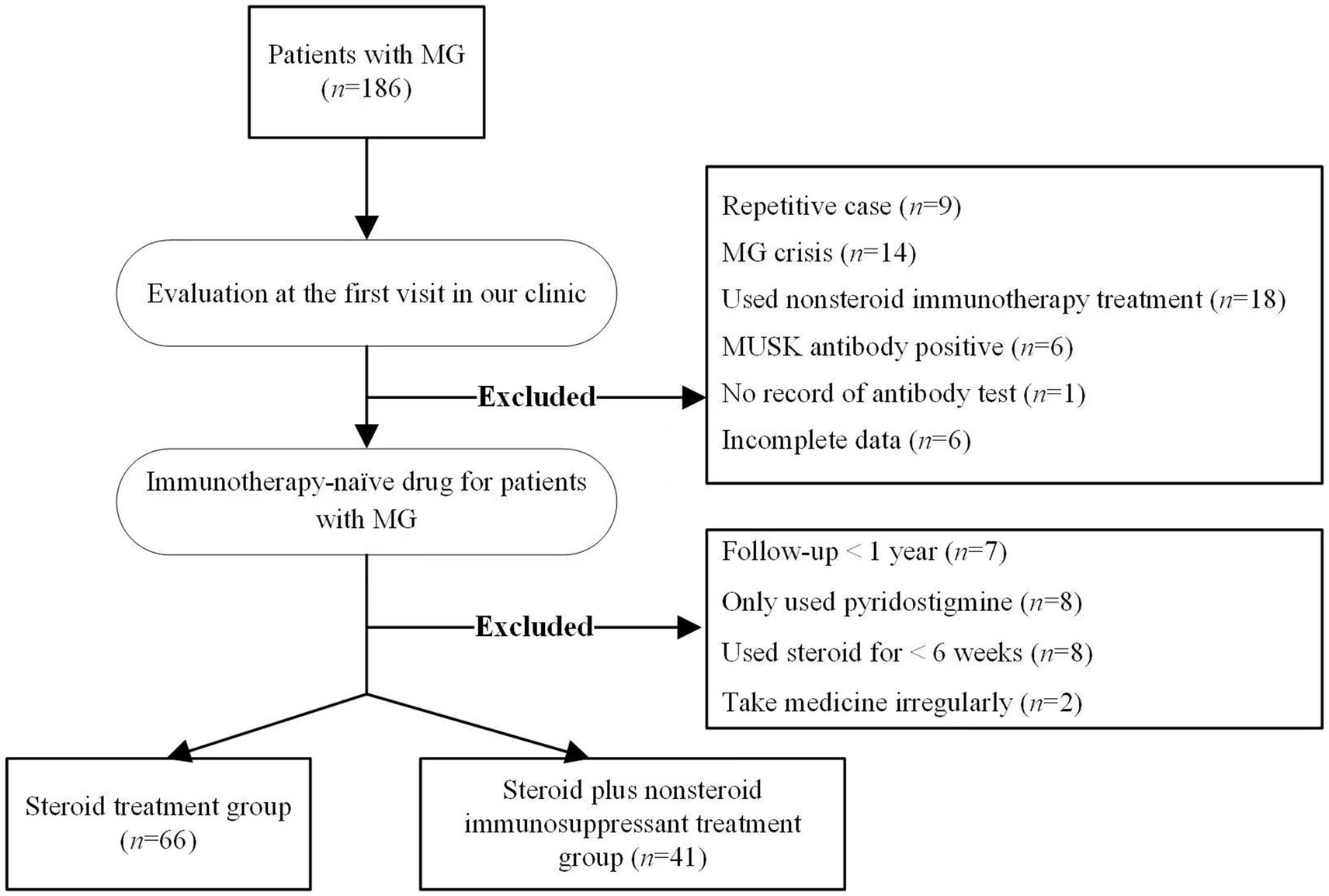
Figure 1. Flowchat for the selection of included patients.
Serum antibody testingSerum samples were collected from patients for antibody testing. The anti-AChR antibody titers were evaluated with an enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (RSR Limited, Cardiff, UK), in which positivity was defined as at least 0.45 nmol/L. Serum MuSK antibody titers were evaluated by a commercial MuSK radioimmunoassay (RIA) antibody kit (RSR Ltd., UK). The results are expressed as nmol/L of MuSK protein bound, and positivity was defined as >0.05 nmol/L (7).
Statistical analysisNormally distributed measurements are described as the mean ± standard deviation (SD), while skewed data are presented as median (interquartile range, IQR). The counting data are described using frequency (percentage). The t-test or nonparametric test was used for the comparison of continuous measurements. Ordinal data were analyzed using a nonparametric test, while chi-square test was used for categorical data. The association between the clinical variable and outcome was analyzed using the log-rank test and univariate and multivariate Cox regression analyses. Factors with p < 0.1 in univariate analysis or considered clinically significant were included in the multivariate Cox regression model.
Statistical analysis was conducted using the R programming language (version 4.0.3, The R Foundation, Vienna, Austria). A two-sided p < 0.05 was taken to indicate statistical significance.
ResultsA total of 186 patients with MG were assessed. After applying the inclusion and exclusion criteria, 107 patients with MG were ultimately included (Figure 1). The mean onset age of MG was 38.4 ± 19.50 years, 41.12% (44/107) were males, and 58.88% (63/107) were female. Early onset of MG accounted for 78.50% (84/107), and 26.17% (28/107) had ocular MG while 73.83% (79/107) had generalized MG. Among the included patients, 93.45% (100/107) were positive for anti-AChR antibodies 6.55% (7/107) were dSnMG. During the follow-up period, 38.3% (41/107) of patients were transitioned to add-on non-steroid immunosuppressants. The transition time was 43–1,455 days after the initiation of steroid treatment, with a median transition time of 160 days. The nonsteroidal immunosuppressants used in this study included azathioprine and tacrolimus. Of the 41 patients who required add-on immunosuppressants, 46.3% (19/41) used azathioprine, 48.8% (20/41) used tacrolimus, and 4.9% (2/41) switched to tacrolimus because of adverse reactions to azathioprine.
A comparative analysis of demographic and clinical variables between the steroid group and the add-on non-steroid immunosuppressant group revealed no significant differences regarding onset age, sex, BMI, early or late onset, complicated with autoimmune disorders, onset MGFA type, MGFA type at first visit, onset symptom, AChR-ab (positive vs. negative), relapse, thymoma, thymic surgery prior to baseline visit, worsening of disease, Qol-15 score, ocular, limb QMG score at first visit, and total and each single ADL score at first visit. However, significant differences were observed in terms of disease duration, history of thymoma surgery, QMG axial muscle score, QMG bulbar muscle score, and QMG respiratory muscle score (Table 1).
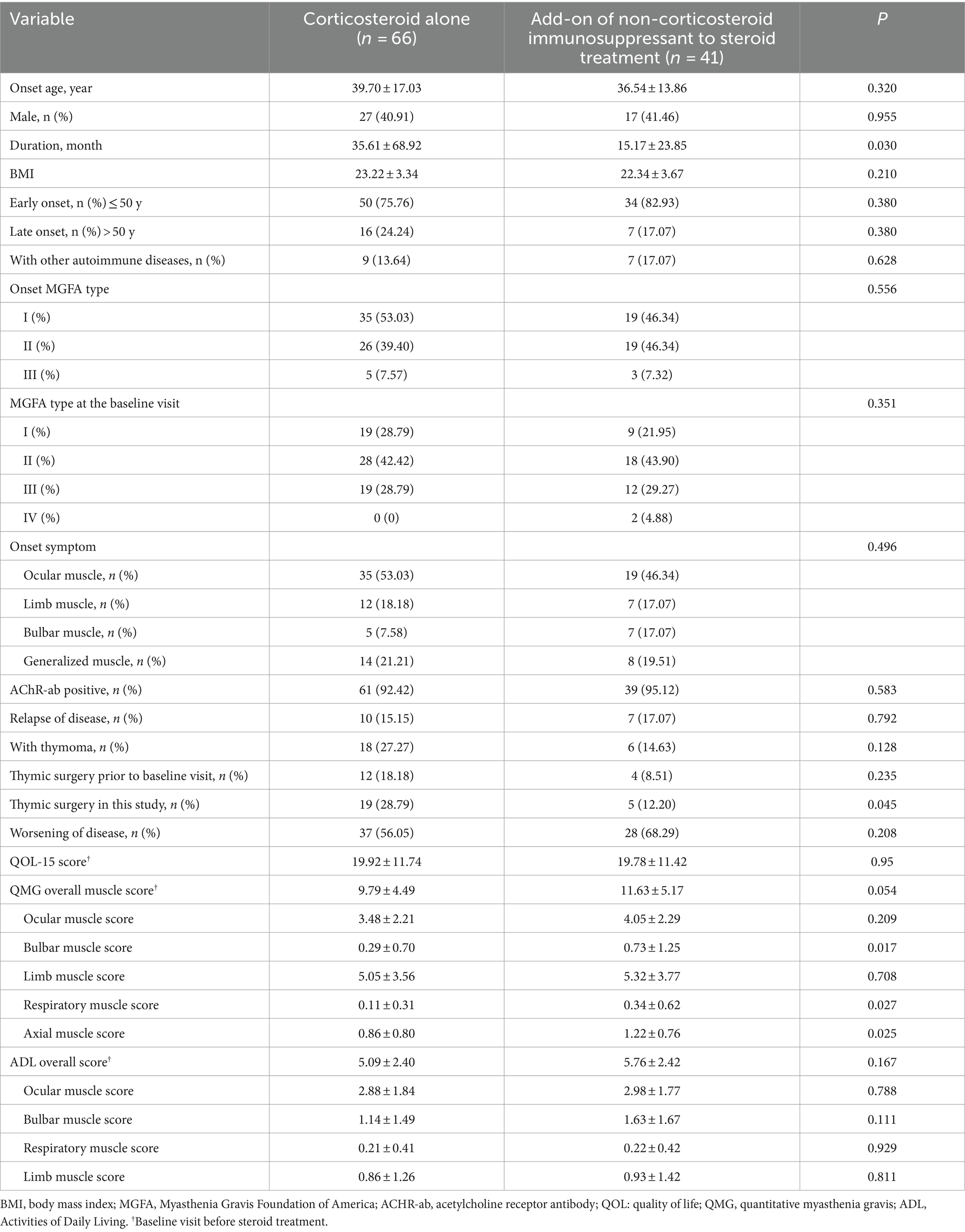
Table 1. Baseline features of the included patients.
Univariate analysis indicated that MGFA type at baseline visit before steroid administration, MSE within 6 months, BMI, QMG bulbar muscle score, QMG respiratory muscle score, QMG axial muscle score, QMG total score, and ADL bulbar muscle score were associated with the add-on of non-steroid immunosuppressant (Table 2). Multivariate analysis confirmed that BMI, MSE within 6 months, QMG bulbar muscle score, disease duration, and relapse were associated with the outcome of adding non-steroid immunosuppressants (Table 3).
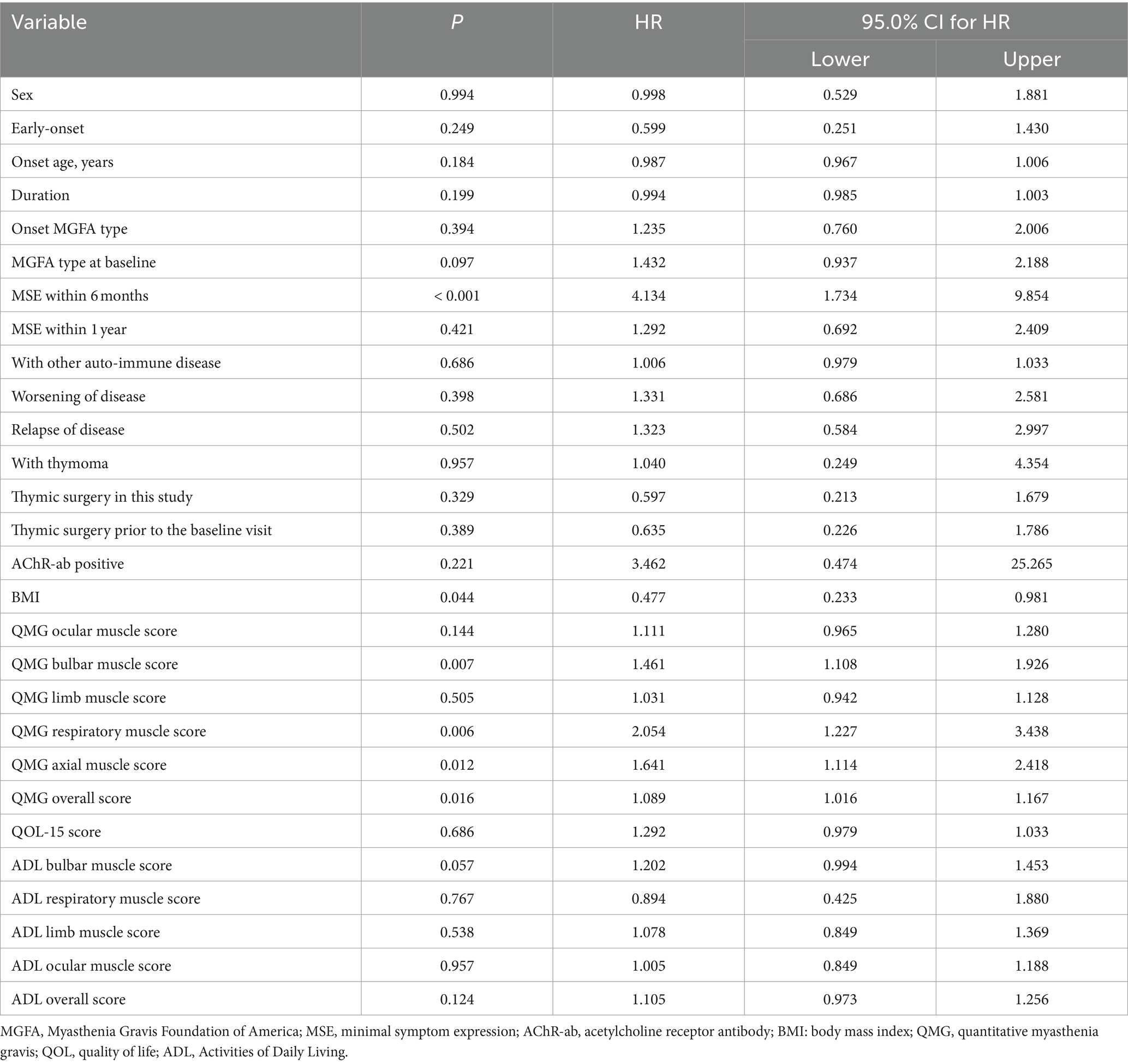
Table 2. Univariate analysis for the add-on of non-steroid immunosuppressants to steroid treatment.
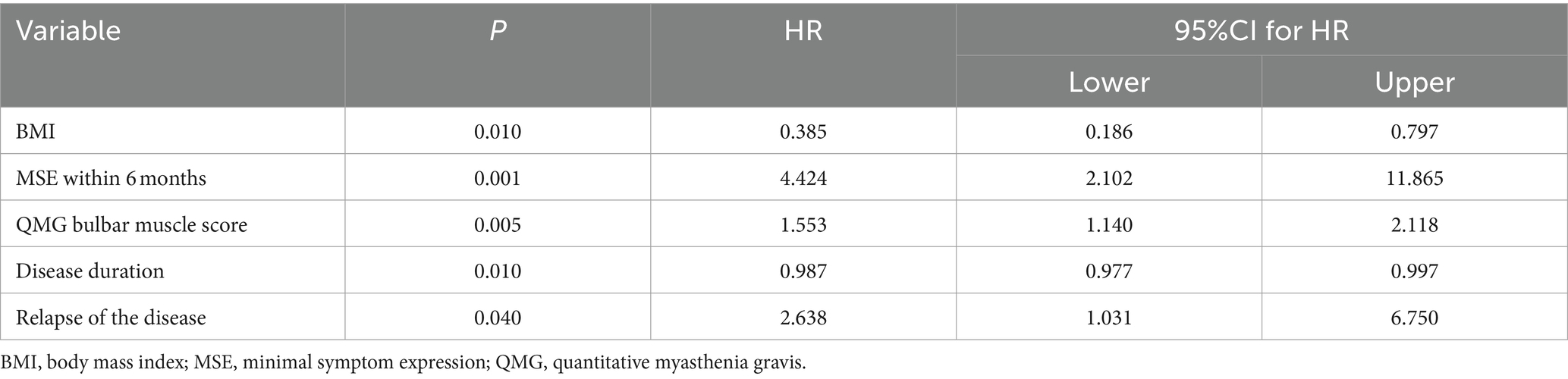
Table 3. Multivariate analysis for the add-on of non-steroid immunosuppressants to steroid treatment.
For categorical features, we also used the K-M plot to illustrate the differences in survival outcome between patients with different relapse statuses, BMI categories, and MSE status at 6 months (Figure 2). As shown in Figure 2A, the average time from initial steroid treatment to the add-on of non-steroid immunosuppressants was significantly shortened in patients with MG with relapse (log-rank p < 0.05). It was demonstrated that patients with MG achieved MSE within 6 months, which required a longer time for adding non-steroid immunosuppressants (Figure 2B, log-rank p < 0.05). For patients who did not achieve the MSE at 6th month, the survival curve declined much more rapidly compared to those who did. We can observe a sharp decline starting from the very beginning of the follow-up, which gradually reached a relative stable status after about 800 days. Overweight patients were more likely to add non-steroid immunosuppressant than non-overweight patients (Figure 2C, log-rank p < 0.05).

Figure 2. Survival curves for time of addition of non-steroid immunosuppressive treatment in patients with different characteristics (The horizontal axis represents follow-up time, and the vertical axis represents survival rate).
DiscussionMG is a heterogeneous disease with various treatment options available (39). Despite the increasing use of immunotherapy, steroids remain the first-line treatment for MG (11, 17). A previously published study on 116 patients with MG treated with steroids found that 80.2% achieved remission or remarkably improved (13). Moreover, several studies have reported that low doses of steroids are effective in some patients with MG (Muscle study (28)). Previous studies have also revealed that symptoms of MG can be improved by a single use of steroids (11). However, it has also been reported that 5–20% of patients with MG are nonresponsive to steroid treatment (14–16).
Treatment with non-steroid immunosuppressants plays an important role in inadequate response or unresponsive patients (40). Recent studies have demonstrated that azathioprine, mycophenolate mofetil and tacrolimus are widely used and may be effective as monotherapy for patients with MG (9, 10, 41). Many studies indicated adding non-steroidal immunosuppressive agents may help reducing the dosage of steroids (37, 42). However, the decision of when and whether to add immunosuppressants remains a topic of debate. One study combinated of steroids and non-steroid immunosuppressants in the initial MG treatment (24). In this study, we explored the factors associated with the addition of non-steroid immunosuppressants to steroid therapy. Our results indicated that MSE within 6 months, BMI, QMG bulbar muscle score, disease duration, and relapse of illness are influencing factors that suggest the need for the addition of non-corticosteroid immunosuppressants to corticosteroids in patients with MG.
Our results suggested that high BMI (≥24 kg/m2) is a risk factor for the add-on of nonsteroidal immunosuppressants. Indeed, obesity is a known risk factor for autoimmune diseases such as asthma, rheumatoid arthritis, and psoriasis (43, 44). Obese patients have low sensitivity to steroid treatment, and weight loss might improve the response to steroid treatment (45). A cross-sectional study focusing on the association between obesity and patients with MG has indicated an increased risk of obesity in patients with MG, regardless of corticosteroid treatment (46). Based on our findings, we supposed that obese patients with MG may be less sensitive to corticosteroid therapy, which requires an early combination of non-corticosteroid immunosuppressants. Obesity may affect sensitivity to steroid treatment through various mechanisms. First, increased body fat could lead to increased levels of inflammation (47), affecting the body’s ability to respond to steroid treatment. Obesity can also change the distribution of fat (61), leading to changes in steroid absorption and metabolism, potentially affecting the effectiveness of steroid therapy. Additionally, obesity is often associated with insulin resistance, which interferes with the ability of steroids to target receptors (48), thus reducing the effectiveness of steroid treatment. However, further research is needed to better understand the association between obesity and MG.
In clinical practice, the QMG score is an important tool for evaluating disease severity and treatment response in patients with MG (49). Indeed, Li et al. (50) suggested that patients who achieved MMS may have lower baseline QMG scores than those who did not, and a low QMG score (QMG score ≤ 16 points) may be regarded as a potential predictor of reaching MMS. A study on the prediction of postoperative crisis in patients with thymoma showed that bulbar symptoms were a risk factor for developing postoperative crisis (OR = 7.24), although without controlling potential covariates (51). Su et al. (53) found that the onset of bulbar symptoms is a risk factor for disease relapse during the glucocorticoid reduction and withdrawal period for patients with MG who reached MMS. Therefore, with a high baseline QMG score, particularly for a high bulbar QMG score, it is crucial to add a non-steroid immunosuppressant to reduce relapse and achieve MMS, which corresponds to our study. Besides, the respiratory function or respiratory QMG score might be an influence factor for the need of add-on non-steroid immunosuppressant therapy. A recent study showed that respiratory involvement was more common in those taking prednisone (52). In our study, although there was no significant difference in multivariate analysis, it is necessary to add a non-steroid immunosuppressant for those with a high baseline respiratory QMG score in univariate analysis.
Recent research has indicated that ADL is highly associated with QMG scores (r = 0.726) (53). Previous studies have shown that 70% of patients with MG reach the most severe state of disease in the first year (13). The median time for female and male patients to achieve maximum improvement is 6 and 5 months for female and male patients, respectively (13). Therefore, we selected MSE within 6 months and MSE within 1 year as potential influencing factors, and found that patients with MSE within 6 months are less likely to receive non-steroid immunosuppressants, which might provide more evidence for the decision of whether early non-immunosuppressant treatment is needed.
Our results also suggested that disease duration before immunotherapy is associated with MG prognosis. However, there is inconsistency in the current understanding (54). Some studies have indicated that long disease duration is a risk factor for poor prognosis for postoperative crisis of thymoma (51), while others have shown that a long disease duration is associated with remission in medication therapy (54). Our results showed that a long disease duration before steroid treatment is negatively associated with the add-on of non-steroid immunosuppressants. This study may indicate that patients with a long disease duration before immunotherapy were relatively stable. Besides, ocular patients with MG, who have a disease duration of more than 2 years, currently are considered in a relatively stable status (27). Another study indicated that generalized MG exhibited a longer duration than ocular MG, along with a higher proportion of patients receiving immunosuppressant therapy (52). This implies that the subtype classification of MG could also be a significant factor influencing the need for increased immunosuppressive therapy.
Previous studies demonstrated that 18–34% of patients with MG will experience a relapse (13, 37, 55). A study has reported that patients who have a shorter duration of corticosteroid reduction, specifically less than 11.5 months, are more prone to relapse (53). It has been suggested that a proportion of patients with MG are dependent on steroid treatment. A study of nine patients with steroid-dependent MG showed that cyclosporine can improve symptoms and prevent relapse after steroid reduction or withdrawal (56), suggesting that cyclosporine can reduce steroid dependence in patients with MG. Another comparative study, which compared rapid and slow steroid reduction with the use of azathioprine, found that the rate of disease relapse within 15 months was not affected, suggesting that azathioprine can reduce the steroid dependency in patients with MG (57). These findings suggest that the add-on of non-steroid immunosuppressants to steroid treatment may be necessary for patients with MG experiencing a relapse. Furthermore, these results imply that patients with disease relapse may not be responsive to or reliant on steroid treatment.
Recently, new treatment options as potential steroid-sparing agents have garnered significant attention. Among these, FcRn inhibitors and complement inhibitors are expected to play a central role in the treatment of MG (58–60). A recent real-world experience study demonstrated that both efgartigimod and eculizumab show significant efficacy in improving patient symptoms and reducing steroid dependence, indicating a promising direction for future treatments (59).
Limitation of the studyThere are several limitations that are worth considering. First, MuSK-MG was not included, which may limit the generalizability of the findings. Second, the retrospective nature of this study, combined with a relatively small sample size, raises concerns regarding potential selection bias among the participants. Furthermore, the limited follow-up duration may have resulted in the oversight of patients who are reliant on steroids, thereby affecting the comprehensiveness of the data. These factors should be taken into account when interpreting the results of this study.
ConclusionIn conclusion, our findings suggest that patients with MG who achieve MSE within 6 months, without experiencing relapse, and have a normal or lean weight may respond well to treatment with a monotherapy of steroid. However, for patients who do not meet these criteria, it may be necessary to consider the add-on of nonsteroidal immunosuppressant treatment.
Data availability statementThe data supporting the findings of this study can be obtained from the corresponding author upon reasonable request.
Ethics statementThe studies involving humans were approved by the Ethics Committee of Huashan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were primarily isolated as part of our previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsJM: Formal analysis, Writing – original draft. DC: Formal analysis, Writing – original draft. FY: Data curation, Writing – original draft. JS: Data curation, Writing – original draft. SL: Supervision, Writing – review & editing. HZ: Data curation, Writing – review & editing. JX: Supervision, Writing – review & editing. ZW: Methodology, Writing – review & editing. ZL: Resources, Writing – review & editing. CZ: Conceptualization, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work has been supported by grants from China’s National Natural Science Foundation (No. 82071410 and 82001335), National Key Research and Development Program of China (2022YFC3501303).
AcknowledgmentsWe thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sanders, DB, Wolfe, GI, Benatar, M, Evoli, A, Gilhus, NE, Illa, I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. (2016) 87:419–25. doi: 10.1212/wnl.0000000000002790
PubMed Abstract | Crossref Full Text | Google Scholar
2. Deenen, JC, Horlings, CG, Verschuuren, JJ, Verbeek, AL, and van Engelen, BG. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis. (2015) 2:73–85. doi: 10.3233/JND-140045
PubMed Abstract | Crossref Full Text | Google Scholar
4. Lazaridis, K, and Tzartos, SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. (2020) 11:212. doi: 10.3389/fimmu.2020.00212
PubMed Abstract | Crossref Full Text | Google Scholar
5. Vinciguerra, C, Bevilacqua, L, Lupica, A, Ginanneschi, F, Piscosquito, G, Rini, N, et al. Diagnosis and Management of Seronegative Myasthenia Gravis: lights and shadows. Brain Sci. (2023) 13:1286. doi: 10.3390/brainsci13091286
PubMed Abstract | Crossref Full Text | Google Scholar
6. Zhang, B, Tzartos, JS, Belimezi, M, Ragheb, S, Bealmear, B, Lewis, RA, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. (2012) 69:445–51. doi: 10.1001/archneurol.2011.2393
PubMed Abstract | Crossref Full Text | Google Scholar
9. Narayanaswami, P, Sanders, DB, Thomas, L, Thibault, D, Blevins, J, Desai, R, et al. Comparative effectiveness of azathioprine and mycophenolate mofetil for myasthenia gravis (PROMISE-MG): a prospective cohort study. Lancet Neurol. (2024) 23:267–76. doi: 10.1016/S1474-4422(24)00028-0
PubMed Abstract | Crossref Full Text | Google Scholar
10. Vinciguerra, C, D'Amico, A, Bevilacqua, L, Rini, N, D'Apolito, M, Liberatoscioli, E, et al. Effectiveness and safety of Mycophenolate Mophetil in myasthenia gravis: a real-life multicenter experience. Brain Sci. (2024) 14:774. doi: 10.3390/brainsci14080774
PubMed Abstract | Crossref Full Text | Google Scholar
11. Cosi, V, Citterio, A, Lombardi, M, Piccolo, G, Romani, A, and Erbetta, A. Effectiveness of steroid treatment in myasthenia gravis: a retrospective study. Acta Neurol Scand. (1991) 84:33–9. doi: 10.1111/j.1600-0404.1991.tb04899.x
Crossref Full Text | Google Scholar
12. Huscher, D, Thiele, K, Gromnica-Ihle, E, Hein, G, Demary, W, Dreher, R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. (2009) 68:1119–24. doi: 10.1136/ard.2008.092163
PubMed Abstract | Crossref Full Text | Google Scholar
13. Pascuzzi, RM, Coslett, HB, and Johns, TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol. (1984) 15:291–8. doi: 10.1002/ana.410150316
PubMed Abstract | Crossref Full Text | Google Scholar
14. Curtis, JR, Westfall, AO, Allison, J, Bijlsma, JW, Freeman, A, George, V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. (2006) 55:420–6. doi: 10.1002/art.21984
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lindberg, C, Andersen, O, and Lefvert, AK. Treatment of myasthenia gravis with methylprednisolone pulse: a double blind study. Acta Neurol Scand. (1998) 97:370–3. doi: 10.1111/j.1600-0404.1998.tb05968.x
Crossref Full Text | Google Scholar
16. Mann, JD, Johns, TR, and Campa, JF. Long-term administration of corticosteroids in myasthenia gravis. Neurology. (1976) 26:729–40. doi: 10.1212/wnl.26.8.729
Crossref Full Text | Google Scholar
17. Imai, T, Suzuki, S, Tsuda, E, Nagane, Y, Murai, H, Masuda, M, et al. Oral corticosteroid therapy and present disease status in myasthenia gravis. Muscle Nerve. (2015) 51:692–6. doi: 10.1002/mus.24438
PubMed Abstract | Crossref Full Text | Google Scholar
18. Guptill, JT, Sanders, DB, and Evoli, A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. (2011) 44:36–40. doi: 10.1002/mus.22006
PubMed Abstract | Crossref Full Text | Google Scholar
20. Narayanaswami, P, Sanders, DB, Wolfe, G, Benatar, M, Cea, G, Evoli, A, et al. International consensus guidance for Management of Myasthenia Gravis: 2020 update. Neurology. (2021) 96:114–22. doi: 10.1212/WNL.0000000000011124
PubMed Abstract | Crossref Full Text | Google Scholar
21. Vakrakou, AG, Karachaliou, E, Chroni, E, Zouvelou, V, Tzanetakos, D, Salakou, S, et al. Immunotherapies in MuSK-positive myasthenia gravis; an IgG4 antibody-mediated disease. Front Immunol. (2023) 14:1212757. doi: 10.3389/fimmu.2023.1212757
PubMed Abstract | Crossref Full Text | Google Scholar
22. Imai, T, Utsugisawa, K, Murai, H, Tsuda, E, Nagane, Y, Suzuki, Y, et al. Oral corticosteroid dosing regimen and long-term prognosis in generalised myasthenia gravis: a multicentre cross-sectional study in Japan. J Neurol Neurosurg Psychiatry. (2018) 89:513–7. doi: 10.1136/jnnp-2017-316625
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wiendl, H, Abicht, A, Chan, A, Della Marina, A, Hagenacker, T, Hekmat, K, et al. Guideline for the management of myasthenic syndromes. Ther Adv Neurol Disord. (2023) 16:17562864231213240. doi: 10.1177/17562864231213240
Crossref Full Text | Google Scholar
24. Zhang, Y, Li, F, Zhu, H, Yu, H, Wang, T, and Yan, X. Less is not necessarily more: low-dose corticosteroid therapy and long-term prognosis in generalized myasthenia gravis after thymectomy. Neurol Sci. (2022) 43:3949–56. doi: 10.1007/s10072-022-05897-0
PubMed Abstract | Crossref Full Text | Google Scholar
25. Grob, D. Course and management of myasthenia gravis. J Am Med Assoc. (1953) 153:529–32. doi: 10.1001/jama.1953.02940230001001
Crossref Full Text | Google Scholar
26. Kennedy, FS, and Moersch, FP. Myasthenia gravis: a clinical review of eighty-seven cases observed between 1915 and the early part of 1932. Can Med Assoc J. (1937) 37:216–23.
PubMed Abstract | Google Scholar
27. Oosterhuis, HJ. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatry. (1989) 52:1121–7. doi: 10.1136/jnnp.52.10.1121
Crossref Full Text | Google Scholar
29. Gilhus, NE, Tzartos, S, Evoli, A, Palace, J, Burns, TM, and Verschuuren, JJGM. Myasthenia gravis. Nat Rev Dis Primers. (2019) 5:30. doi: 10.1038/s41572-019-0079-y
Crossref Full Text | Google Scholar
30. Sciacca, G, Reggio, E, Mostile, G, Nicoletti, A, Drago, F, Salomone, S, et al. Clinical and CN-SFEMG evaluation of neostigmine test in myasthenia gravis. Neurol Sci. (2018) 39:341–5. doi: 10.1007/s10072-017-3194-0
PubMed Abstract | Crossref Full Text | Google Scholar
31. AAEM Quality Assurance Committee. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert‐Eaton myasthenic syndrome. Muscle Nerve. (2001) 24:1239–47. doi: 10.1002/mus.1140
Crossref Full Text | Google Scholar
32. Jaretzki, A, Barohn, RJ, Ernstoff, RM, Kaminski, HJ, Keesey, JC, Penn, AS, et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory Board of the Myasthenia Gravis Foundation of America. Neurology. (2000) 55:16–23. doi: 10.1212/wnl.55.1.16
Crossref Full Text | Google Scholar
33. Barohn, RJ, McIntire, D, Herbelin, L, Wolfe, GI, Nations, S, and Bryan, WW. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. (1998) 841:769–72. doi: 10.1111/j.1749-6632.1998.tb11015.x
PubMed Abstract | Crossref Full Text | Google Scholar
34. Twork, S, Wiesmeth, S, Klewer, J, Pöhlau, D, and Kugler, J. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual Life Outcomes. (2010) 8:129. doi: 10.1186/1477-7525-8-129
PubMed Abstract | Crossref Full Text | Google Scholar
35. Wolfe, GI, Herbelin, L, Nations, SP, Foster, B, Bryan, WW, and Barohn, RJ. Myasthenia gravis activities of daily living profile. Neurology. (1999) 52:1487–9. doi: 10.1212/wnl.52.7.1487
Crossref Full Text | Google Scholar
36. Uzawa, A, Ozawa, Y, Yasuda, M, Onishi, Y, Akamine, H, and Kuwabara, S. Minimal symptom expression achievement over time in generalized myasthenia gravis. Acta Neurol Belg. (2023) 123:979–82. doi: 10.1007/s13760-022-02162-1
PubMed Abstract | Crossref Full Text | Google Scholar
37. Su, S, Lei, L, Fan, Z, Zhang, S, Wen, Q, Wang, J, et al. Clinical predictors of relapse in a cohort of steroid-treated patients with well-controlled myasthenia gravis. Front Neurol. (2022) 13:816243. doi: 10.3389/fneur.2022.816243
PubMed Abstract | Crossref Full Text | Google Scholar
38. Zhou, BFCooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96.doi: 10.1046/j.1440-6047.11.s8.9.x
PubMed Abstract | Crossref Full Text | Google Scholar
39. Evoli, A, Spagni, G, Monte, G, and Damato, V. Heterogeneity in myasthenia gravis: considerations for disease management. Expert Rev Clin Immunol. (2021) 17:761–71. doi: 10.1080/1744666x.2021.1936500
PubMed Abstract | Crossref Full Text | Google Scholar
40. Zhou, L, Liu, W, Li, W, Li, H, Zhang, X, Shang, H, et al. Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China. Ther Adv Neurol Disord. (2017) 10:315–25. doi: 10.1177/1756285617721092
留言 (0)