Hypersensitivity pneumonitis (HP), also known as an extrinsic allergic alveolitis, is an immune-mediated inflammatory and granulomatous interstitial lung disease (ILD) characterized by a neutrophilic/lymphocytic inflammation of the interstitium, bronchioles, and alveoli and granuloma formation in the lung, and, in some patients, progresses to irreversible fibrosis and end-stage lung damage with respiratory failure (1, 2). HP is caused by intense or repeated inhalation of antigenic organic particles or low-molecular-weight chemicals in a variety of environmental settings in genetically susceptible individuals (1, 2). Numerous organic antigens (over 300 antigens) broadly classified into bacteria, fungi, animal proteins, plant proteins, wood dusts, low-molecular-weight chemicals, and metals have been described to cause HP (3). These antigens are often encountered in the daily life of patients suffering from disease; thus, avoiding exposure is challenging. HP is often related to a person’s occupational environments as reflected in its other names (e.g., bird fancier’s lung, famer’s lung, or hot tub lung). Saccharopolyspora rectivirgula, a thermophilic actinomycetes commonly found in moldy hay, is one of the HP-inciting antigens that cause farmer’s lung disease (one of the most common types of HP seen in agricultural workers) (4). HP may present as acute, subacute, or chronic illness with unique clinical presentations (5). Following inhalation of HP-inciting agents, patients develop severe neutrophilic inflammation in the lung. Continued antigen exposures result in a positive feedback loop, leading to increased production of interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor α (TNFα) by alveolar macrophages and increased production of interferon γ (IFNγ) by activated neutrophils and Th1 cells (6–9). Increased levels of L-selectins in macrophages and neutrophils, intracellular adhesion molecule-1 (ICAM-1), CD80 and CD86 in macrophages, CD28 in lymphocytes, and E-selectin in endothelial cells in lung tissues of HP patients correlate with the lymphocytosis and the persistence of inflammation (10–13). As the disease progresses to the subacute form, the alveolitis becomes progressively more lymphocytic, and granulomas develop in the interstitial spaces. Lymphocytes can account for 60%–90% of bronchoalveolar lavage (BAL)-recovered cells from HP patients (14, 15). Several chemokines, including CC motif chemokine ligand (CCL) 2, CCL18, CXC motif chemokine ligand (CXCL) 8, and CXCL9 are increased in the BAL fluid (BALF) of HP patients and contribute to the development of the cellular infiltrate and granulomas in HP (16–20). The chronic phase of HP can be non-fibrotic or fibrotic, and patents with fibrosis have worse prognosis and a higher risk for morbidity and mortality (21–23).
The pathogenic mechanisms involved in initiation and progression of HP are complex and remain incompletely understood. The intricate interplays between the host genetic factors, the host immune system, the environment, and the causative agents affect the disease pathogenesis. Toll-like receptors (TLRs) on/in innate immune cells recognize specific molecular patterns present in microbial pathogens, foreign particulates, and injured or altered self-tissues, and then initiate and amplify cascades of inflammatory responses (24–31). Increased expression of TLR2, TLR4, TLR6, and TLR8 in mice with HP has been reported (32, 33). Using animal models of HP, previous studies have demonstrated that TLR2, TLR4, TLR6, and TLR9 are involved in the detection of numerous HP-inciting agents and are critical contributors to lung inflammation and HP pathogenesis (34–40). TLR2, TLR6, and TLR9 have been demonstrated to be involved in mediating proinflammatory responses, neutrophil recruitment to airway, and Th17 development after acute and chronic exposures to an HP-inciting antigen S. rectivirgula (34, 35, 39). Previous studies also showed that the initial expression of the majority of proinflammatory mediators and neutrophil infiltration into the lung after exposure to HP-inciting agents are largely dependent on myeloid differentiation factor 88 (MyD88), the major signaling adaptor in the TLR pathway (38, 41–44). In addition, the receptors of IL-1 family cytokines (such as IL-1 and IL-18 that have been shown to be increased in HP patients and contribute to enhanced Th17-type responses) share their signal transduction pathway with TLRs by utilizing MyD88 as the signaling adaptor (7, 45, 46). These findings support the concept that, although there is a broad spectrum of agents that cause HP, interruption of the MyD88-dependent signaling pathway might have significant beneficial effects on attenuation of the disease progress and the signaling modulators downstream of MyD88 would be a reasonable therapeutic target for HP.
The protein kinase D1 (PKD1) is a member of the PKD family, which is composed of three structurally related serine/threonine kinases: PKD1 (PKCμ), PKD2, and PKD3 (PKCν) (47). Several previous studies have found a link between PKD1 and TLR/IL-1R signaling. TLR4-mediated p38 activation and TNFα secretion in microglial cells, TLR5-mediated p38 activation and CXCL8 production in epithelial cells, and TLR1/2-induced expression of heat shock protein 27 and CCL2 in mast cells are suppressed by Gö6976, a pharmacological inhibitor that inhibits the activity of protein kinase Cα (PKCα), PKCβ, checkpoint kinases 1 and 2 (CHK1/2), PKD1, PKD2, and PKD3 (48–52). We also found that PKD1, but not PKD2 and PKD3, is recruited to the MyD88/IL-1R-associated kinase (IRAK)/TNFR-associated factor 6 (TRAF6) signaling complex and becomes activated upon stimulation by TLR ligands (TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, TLR8, or TLR9), IL-1β, IL-18, Group B streptococci, or S. rectivirgula. This PKD1 activation by TLR/IL-1R is required for the MyD88-dependent ubiquitination of TRAF6 and activation of TGFβ-activated kinase 1 (TAK1), NF-κB, and mitogen-activated protein kinases (MAPKs), leading to the subsequent expression of proinflammatory mediators such as TNFα, IL-6, IL-12, chemokine CCL2, and costimulatory molecule CD86 (43, 53–56).
Previously, we found that S. rectivirgula induces activation of PKD1 in an MyD88-dependent manner in the mouse lung in vivo and in murine cell lines MLE12 (alveolar type II epithelial cells), MPRO (promyelocytes differentiated to neutrophils), and AMJ2-C11 (alveolar macrophages) (43). S. rectivirgula-mediated cytokine and chemokine production in these cell lines are significantly inhibited when PKD1 expression is knocked down by PKD1-specific siRNA or when PKD1 enzyme activity is inhibited by Gö6976 (43). S. rectivirgula-induced acute lung injury accompanied by neutrophilic alveolitis and increased production of proinflammatory cytokines TNFα, IL-6, IL-12, and IFNγ and chemokines CCL2 and CXCL2 in the lungs are also significantly suppressed in mice pre-treated with PKD inhibitor Gö6976 or in PKD1-insufficient mice (PKD1KO) (43, 57). In addition, PKD1-insufficient mice (compared to wild-type PKD1-sufficient mice) showed significantly reduced levels of alveolitis, surface MHC-II expression on macrophages and neutrophils infiltrated into airways and interstitial lung spaces, expression of IFNγ, IL-17A, and CXCL9 in the lungs, and granuloma formation following 5-week repeated exposures to S. rectivirgula (57). These findings imply that PKD1 is one of the critical factors required for acute and chronic pulmonary proinflammatory responses that contribute to T helper (Th) 1- and Th17-promoting milieu in the lungs and development of HP. Myeloid lineage cells, particularly macrophages and neutrophils, are crucial in the early stages of lung inflammation in HP (58, 59). They highly express TLRs, detect the presence of inciting agents, release inflammatory cytokines/chemokines to activate and attract more immune cells to the lungs, influence Th cell lineage differentiation, and express MHC-II and costimulatory molecules to activate T cells and prime the immune system towards its adaptive response (10, 60–63). In the present study, we investigated whether PKD1 in myeloid cells plays an essential role in mediating acute lung injury after exposure to S. rectivirgula. Additionally, we investigated whether PKD1 in myeloid cells contributes to the neutrophilic/lymphocytic alveolitis and the cytokine/chemokine milieu that affects the pulmonary accumulation of pathogenic Th1 and Th17 cells during the development of HP caused by repeated exposure to S. rectivirgula.
Materials and methodsMiceC57BL/6 mice and LyzCre mice (B6.129P2-Lyz2tm1(cre)lfo/J, JAX stock #018956) were purchased from The Jackson Laboratory (Bar Harbor, ME). The generation and characterization of LyzCre mice (express Cre in myeloid cells due to targeted insertion of the cre cDNA into their endogenous M lysozyme locus) were reported by Förster and colleagues (64). PKD1fl/fl mice generated as previously described (65) were kindly provided by Dr. E. Olson (University of Texas Southwestern Medical Center, Dallas, TX). PKD1fl/fl mice and LyzCre mice were backcrossed onto the C57BL/6 more than seven generations at the University of Tennessee Health Science Center (UTHSC). Subsequent cross-breeding of these PKD1fl/fl and LyzCre mice resulted in PKD1fl/fl-LyzCre (myeloid lineage cell-specific PKD1-deficient, PKD1mKO) mice. PKD1fl/fl mice were used as wild-type (WT) control and PKD1fl/fl-LyzCre+/- mice were used as PKD1mKO. Mice were bred and maintained in a pathogen-free facility at UTHSC. All animal care and housing requirements set forth by the National Institutes of Health Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources were followed. Animal protocols were reviewed and approved by the UTHSC Institutional Animal Care and Use Committee.
Saccharopolyspora rectivirgula preparation and challenge, bronchoalveolar lavage, lung interstitial cell isolation, and determination of alveolitisS. rectivirgula (ATCC®15347™) was prepared as previously described (57). Age- and gender-matched mice were exposed intranasally to S. rectivirgula (80 μg/mouse or 100 μg/mouse) or endotoxin-free saline (AdipoGen Life Sciences, San Diego, CA) for one time or three times per week for 3 weeks. Mice were euthanized at designated time points (2, 6, 24, 48, or 72 h after the last S. rectivirgula challenge). Unless otherwise indicated, control and S. rectivirgula-exposed mice were analyzed individually. To perform BAL, mice tracheas were cannulated, and the lungs were washed with 1 mL of 2 mM EDTA/PBS. The typical amount of fluid recovered was approximately 70% of the input. The recovered BALF was centrifuged, and the resulting supernatants were kept at −80°C until used for detection of cytokines and chemokines. The cells recovered from the BALF were used to determine the degree of alveolitis by counting the number of live cells using a hemocytometer or Countess II (Invitrogen) following trypan blue staining. The cellular composition of the alveolitis was determined by flow cytometric analysis. To isolate lung interstitial cells (LICs), the lungs were perfused through the right ventricle with PBS and digested with collagenase (20 U/mL, type 4, LS004186, Worthington Biochemical Co, Lakewood, NJ) and deoxyribonuclease I (40 μg/mL, LS002004, Worthington Biochemical Co) for 45 min at 37°C. Cells were freed by mechanical disruption and filtered through 40 μM nylon mesh. Discontinuous Percoll (P4937, Sigma-Aldrich, St. Louis, MO) gradient centrifugation was used to separate extraneous fibroblasts or epithelial cells. The mononuclear cells were isolated at the 40/80% interface. The number of live cells were counted using a hemocytometer or Countess II (Invitrogen) following trypan blue staining and used for flow cytometric analysis.
Isolation of spleen cells, peritoneal macrophages, thymocytes, and bone marrow neutrophilsSpleen cells were obtained as previously described (54). To isolate peritoneal macrophages, a peritoneal lavage was performed by injecting 8 mL of cold sterile PBS (Corning, Manassas, VA) into the peritoneal cavity of mice followed by a gentle massage on the peritoneal cavity and subsequent aspiration of the peritoneal fluid. Peritoneal cells were obtained from the recovered peritoneal fluid by centrifugation. After removing RBC using RBC lysis buffer, peritoneal cells were washed three times with PBS, suspended in complete medium (RPMI 1640 supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS), placed in plastic petri dishes, and then incubated for 1 h at 37°C. Suspended cells were removed. Plastic adherent cells were washed three times with prewarmed PBS and then collected by scraping. The resulting plastic adherent cells were used as peritoneal macrophages for confirming PKD1 gene deletion in myeloid lineage cells in PKD1mKO. To isolate thymocytes, thymus lobes from mice were harvested as described previously (66). Thymus cells were obtained by cutting thymus lobes into small pieces with fine scissors, crushed with a syringe plunger in cold PBS, and then filtered through a 100-μm sterile cell strainer (Fisher Scientific). Thymic cells in suspensions were washed three times with PBS and then used as thymocytes. To isolate neutrophils from bone marrow, bone marrow cells from mice were prepared as previously described (55). After removing RBC, the bone marrow cells were washed three times with PBS and then resuspended in PBS. The bone marrow cell suspension was overlaid on top of the Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and then centrifuged for 30 min at room temperature without break. After discarding upper layers, granulocytes pelleted in the bottom were washed three times with PBS and then used as neutrophils. Purity of isolated neutrophils were analyzed by Diff-Quick staining (Diff Quick Stain kit, IMEB Inc., San Marcos, CA) according to the manufacturer’s instructions and approximately 85% to 90% of the isolated cells were morphologically neutrophils/granulocytes. RPMI 1640 was purchased from Corning, Pen Strep was purchased from Gibco, and FBS was purchased from R&D Systems (Flowery Branch, GA).
Trans-well neutrophil migration assayTo evaluate the migration abilities of neutrophils, 1 mL of RPMI-1640 media or murine recombinant CXCL2 (50 ng/mL, PeproTech, cat# 250-15) was placed into the bottom chamber of each designated well in a 24-well plate and 5 × 105 bone marrow neutrophils (isolated from WT or PKD1mKO) in 200 µL of RPMI-1640 media were placed into the top chamber (Greiner Bio-One, ThinCert 3.0 μm pore, 24-well. ref#662-631). The trans-well plate was incubated at 37°C with 5% CO2 for 2 h and then neutrophils that migrated into the bottom chamber and the bottom chamber side of the membrane were collected and counted using Countess II (Invitrogen).
Genomic DNA isolation, genotyping, total RNA isolation, reverse transcription polymerase chain reaction, and real-time quantitative polymerase chain reactionGenomic DNA was isolated from mouse tail clips using a Phire Tissue Direct PCR Master Mix (ThermoFisher Scientific Inc., Vilnius, Lithuania) following the manufacturer’s protocol. To distinguish PKD1 alleles and lysozyme 2 (Lyz2) alleles, genomic DNA was analyzed by PCR performed in a thermocycler (T100 Thermal Cycler, Bio-Rad). The sequences of PCR primers used for PKD1-WT/loxP and KO were described previously (57). The sequences of PCR primers used for LyzCre are as follows: Mutant F: 5′CCCAGAAATGCCAGATTACG3′, WT F: 5′TTACAGTCGGCCAGGCTGAC3′, and Common R: 5′CTTGGGCTGCCAGAATTTCTC3′. All primers for genotyping were purchased from Integrated DNA Technologies, Inc. (IDT, Coralville, IA). DNA-free total RNA was isolated using Direct-Zol RNA Microprep kits (Zymo Research, Irvine, CA) following the manufacturer’s protocol. The relative amounts of the indicated gene transcripts were analyzed by RT-PCR as described previously (56). Sequences of RT-PCR primers used were described previously (54, 57, 67). All primers for RT-PCR were purchased from IDT. For RT real-time qPCR (SYBR Green Assay), total RNA (250 ng) was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time qPCR was performed on a QuantStudio 5 (Applied Biosystems) using the PowerUp SYBR Green Master Mix (Applied Biosystems). Primers were designed and supplied by IDT. The product size was initially monitored by agarose gel electrophoresis. Melting curves were analyzed to control for specificity of PCR reactions. The data on genes that were differentially expressed were normalized to the expression of the housekeeping gene, GAPDH. The relative units were calculated from a standard curve, plotting three different concentrations against the PCR cycle number at which the measured intensity reaches a fixed value (with a 10-fold increment equivalent to ~3.1 cycles). Fold changes comparing S. rectivirgula-treated WT mice and S. rectivirgula-treated PKD1mKO to control mice exposed to saline were calculated by comparative quantification algorithm-delta delta Ct method (fold difference = 2−ΔΔCt). Primer sequence information for qPCR is either described previously (57) or listed in Supplementary Table 1.
Western blot assay, enzyme-linked immunosorbent assay, and multiplex sandwich immunoassayLevels of specific proteins in whole cell extracts were analyzed by Western blot assay as described previously (54, 68). Blots developed in enhanced chemiluminescence reagents (GE Healthcare, UK) were either exposed onto x-ray film followed by processing through X-OMAT 2000A processor (Kodak). Blots developed with fluorochrome-conjugated secondary Abs were scanned using ODYSSEY CLx (LI-COR, Lincoln, NE). Actin was used as a loading control for all Western blot assays. Antibodies specific for actin was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Antibodies specific for PKD1 were purchased from Origene (Rockville, MD). Concentrations of IFNγ, TNFα, IL-6, and IL-17A in BALF were analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described (54, 68). All ELISA kits were purchased from Invitrogen. Concentrations of IL-1α, IL-1β, CCL2, CXCL1, and CXCL2 in BALF were analyzed using the multiplex sandwich assay kit (MCYTOMAG-70K, EMD Millipore, Billerica, MA) following the manufacturer’s protocol. The fluorescence was measured with Bio-Plex MAGPIX Multiplex Reader (Bio-Rad, Hercules, CA).
Proteome profiler mouse cytokine arrayThe relative levels of 40 cytokines and chemokines in BALF obtained from WT and PKD1mKO exposed to S. rectivirgula repeatedly for 3 weeks were evaluated using Proteome Profiler Mouse Cytokine Array Kit (ARY006, R&D Systems) following the manufacturer’s instructions. The ChemiDoc™ Touch Imaging system (Bio-Rad) was used to detect the chemiluminescent signal of the bound cytokine/chemokine, and the Image Lab (Bio-Rad) software was utilized to measure the cytokine/chemokine signal intensity of the digital image. Each spot was adjusted for adjacent background intensity and normalized to a positive control on the membrane.
Identification of interleukin-17A- and interferonγ-producing CD4+ T cells by intracellular stainingLICs (4 × 106 cells/mL) were stimulated with phorbol 12-myristat 13-acetate (PMA, 5 ng/mL) and ionomycin (500 ng/mL) for 2 h. Two hours later, 1 μL of BD GolgiPlug (BD Biosciences, San Diego, CA) was added to the culture and then incubated for four additional hours. Cells were harvested, washed with 5% FBS in PBS, stained with DAPI for 2 min on ice, washed, and then incubated with anti-CD16/32 (2.4G2) in PBS containing 5% FBS for 15 min on ice to block Fc receptors. Cells were stained with anti-βTCR (H57-597) and anti-CD4 (RM4-5) for 30 min on ice. Cells were fixed in 250 μL of BD cytofix/cytoperm solution (BD Biosciences) for 20 min on ice. Cells were washed with BD perm/wash solution (BD Biosciences) and intracellularly stained with anti-IL-17A-APC (TC11-18H10.1) and anti-IFNγ-PE (XMG1.2) for 30 min on ice. Cells were washed again with the 1× BD perm/wash solution and then subjected to flow cytometric analysis. All antibodies were purchased from BioLegend (San Diego, CA). Phorbol 12-myristate 13-acetate (PMA) and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO).
Flow cytometric analysisFor splenic immune cell profiling, spleen cells were incubated with anti-CD16/CD32 Abs and subsequently stained with fluorochrome-conjugated Abs to CD45 (30-F11), CD19 (1D3), βTCR (H57-597), CD4 (GK1.5), CD8 (53-6.7), CD11b (M1/70), Ly6G (1A8), and CD11c (N418). Cells were analyzed using a Cytek Aurora Cytometer (N7-00003, Cytek Biosciences Inc., Fermont, CA) and FlowJo flow cytometry data analysis software (FlowJo LLC, Ashland, OR). Gating strategies for splenic and bone marrow immune cell profiling are shown in Supplementary Figures 1A, B, respectively. Briefly, debris and doublets were gated out. In the single-cell gate, CD45+ population was gaited and identified as CD45+ cells. In the CD45+ cell gate, the CD19+ population (B cells), βTCR+ population (T cells), βTCR+CD4+ population (CD4+ T cells), βTCR+CD8+ population (CD8+ T cells), CD11b+ population (monocytic cells), CD11b+LyG6+ population (neutrophils), and CD11c+ population (dendritic cells) were identified. The frequency of each cell population was expressed as the percentage of CD45+ cells.
To detect neutrophils in the airway and lung interstitium after one-time S. rectivirgula exposure, BAL cells were incubated with anti-CD16/CD32 Abs, subsequently stained with fluorochrome-conjugated Abs to CD11b (M1/70), F4/80 (BM8), and Gr-1 (RB6-8C5), and then analyzed using a BD Biosciences LSR II flow cytometer (BD Biosciences, San Diego, CA) and FlowJo flow cytometry data analysis software. The gating strategy for flow cytometric analysis of neutrophils in BAL cells obtained from mice 24 h after the single S. rectivirgula exposure is shown in Supplementary Figure 2. Debris and doublets were gated out. In the single-cell gate, the CD11b+Gr1+ population was gated, and then the Gr1+F4/80- population in the CD11b+Gr1+ gate was identified as neutrophils (CD11b+Gr1+F4/80-). The frequency of CD11b+Gr1+F4/80- cells was expressed as % of BAL cells.
To determine the cellular composition and levels of certain surface markers of cells recovered from BALF and lung interstitium from mice exposed to S. rectivirgula repeatedly for 3 weeks, cells were incubated with anti-CD16/CD32 Abs; subsequently stained with fluorochrome-conjugated Abs to CD45 (I3/2.3), CD11b (M1/70), CD11c (N418), F4/80 (BM8), Gr-1 (RB6-8C5), MHCII (I-A/I-E) (M5/114.15.2), βTCR (H57-597), CD4 (RM4-5), CD8 (53-6.7), CD86 (GL-1), CD206 (C068C2), CD40 (3/23), CCR6 (29-2L17), CXCR3 (CXCR3-173), and/or CD69 (H1.2F3); and then analyzed using a BD Biosciences LSR II flow cytometer or Cytek Aurora (Cytek Biosciences) and FlowJo flow cytometry data analysis software. Gating strategies for flow cytometric analysis of BAL cells and LICs are shown in Supplementary Figures 3, 4, respectively. Debris and doublets were gated out. In the single-cell gate, the CD45+ population was identified. For myeloid lineage cells, the CD11b-CD11c- population was gated out in the CD45+ population. In the rest of CD45+ cells, the CD11c+F4/80+ population was identified as alveolar macrophages (AM), and the remaining cells were identified as the non-AM population. The non-AM population was divided into the CD11b+Gr1+ population and the non-polymorphonuclear neutrophils (PMN) population. In the CD11b+Gr1+ population, the CD11b+Gr1+F4/80- population was identified as neutrophils. In the non-PMN population, the CD11b+F4/80+ population was identified as macrophages (MAC), and the F4/80- population was identified as the non-MAC population. In the non-MAC population, the CD11c+CD11b-F4/80- population and CD11c+CD11b+F4/80- population were identified. In the non-MAC population, the CD11c+CD11b-F4/80-MHC-II+ population was identified as conventional dendritic cell type 1 (cDC1) and the CD11c+CD11b+F4/80-MHC-II+ population was identified as conventional dendritic cell type 2 (cDC2). For T cells, βTCR+ cells were identified in the CD45+ population and then the CD4+βTCR+ population was identified as CD4+ T cells, and the CD8+βTCR+ population was identified as CD8+ T cells. The frequency of each cell population was expressed as % of CD45+ cells. The surface expression levels of various markers (CD86, MHC-II, CD40, CD206, CD69, CXCR3, and CCR6) on cells were determined by their geometric mean fluorescent intensity (gMFI). The gMFI of each marker was calculated for each indicated cell population using the FlowJo flow cytometry data analysis software. All Abs were purchased from BioLegend (San Diego, CA), eBioscience, TONBO, or BD Pharmingen.
Lung histologyThe left lung lobes removed from the mice were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. The paraffin-embedded lungs were sectioned longitudinally at 5 μm and stained with hematoxylin and eosin (H&E). To determine inflammatory cell influx into the interstitial lung tissue, digital images of whole H&E-stained slides were captured using the Aperio ScanScope®XT Slide Scanner (Aperio Technologies, Inc. Vista CA).
Statistical analysisData were expressed as mean values ± SD. The differences between two groups were evaluated using two-tailed Student’s t-test. The differences between multiple groups were evaluated using one-way ANOVA with Tukey’s post-hoc test. GraphPad Prism statistical software (GraphPad Software, San Diego, CA) was used. Statistical differences with p < 0.05, p < 0.01, p < 0.001, and p < 0.0001 are indicated in the figures as *, **, ***, and ****, respectively, and considered significant.
ResultsConditional protein kinase D1 deletion in myeloid lineage cells in miceTo delete PKD1 gene in myeloid lineage cells, PKD1fl/fl mice (mice with inserted lox-p sites in PKD1 gene to flanking exons 12 through 14 that encode the kinase function) (65) were cross-bred with LyzCre mice that express Cre recombinase in myeloid lineage cells, including monocytes, mature macrophages, and granulocytes (64). Resulting myeloid lineage cell PKD1-deficient mice (PKD1fl/fl-LyzCre) were identified by genotyping (Figure 1A). The PKD1fl/fl-LyzCre mice (both PKD1fl/fl-LyzCre+/- and PKD1fl/fl-LyzCre+/+ strains) are viable and fertile and do not display any gross physical abnormalities. PKD1 gene deletion and lack of PKD1 mRNA expression in peritoneal macrophages, but not in thymocytes, of PKD1fl/fl-LyzCre was also detected (Supplementary Figure 5). To further confirm PKD1 deletion in myeloid lineage cells, neutrophils were purified from bone marrow cells and then protein levels of PKD1 were analyzed by Western blot assay. As shown in Figure 1B, levels of PKD1 protein were substantially reduced in neutrophils isolated from PKD1fl/fl-LyzCre mice. To evaluate whether PKD1 deletion in myeloid lineage cells affected the general immune cell profile, spleen cells and bone marrow cells were analyzed by flow cytometry. Proportions of CD19+ B cells, CD4+ T cells, CD8+ T cells, CD11b+ cells, CD11b+Ly6G+ cells, and CD11c+ cells in the spleens and bone marrow of PKD1mKO (PKD1fl/fl-LyzCre+/- or PKD1fl/fl-LyzCre+/+) mice were comparable to those of PKD1fl/fl mice (Figures 1C, D). A previous study has demonstrated that PKD3, one of PKD family members, is required for the neutrophil migration (69). Thus, to evaluate whether deletion of PKD1 affects chemotaxis of neutrophils, the migratory ability of neutrophils was analyzed using trans-well migration assay. As shown in Figure 1E, the migratory capability of PKD1-deficient (PKD1-/-) neutrophils toward chemokine CXCL2 was comparable to that of PKD1-intact neutrophils, indicating that PKD1-/- neutrophils do not have an intrinsic mobility defect. Taken together, these data demonstrate that PKD1 is deleted in myeloid lineage cells in PKD1fl/fl-LyzCre mice and that PKD1 deletion in myeloid lineage cells in mice did not significantly alter the general splenic and bone marrow immune cell profiles in mouse and neutrophil’s chemotaxis capacity. These results also indicate that the PKD1fl/fl-LyzCre mouse can be a suitable tool for investigating the contribution of PKD1 activation in myeloid lineage cells, including neutrophils and macrophages, to the development of acute pulmonary inflammation and HP caused by inhalation of S. rectivirgula.
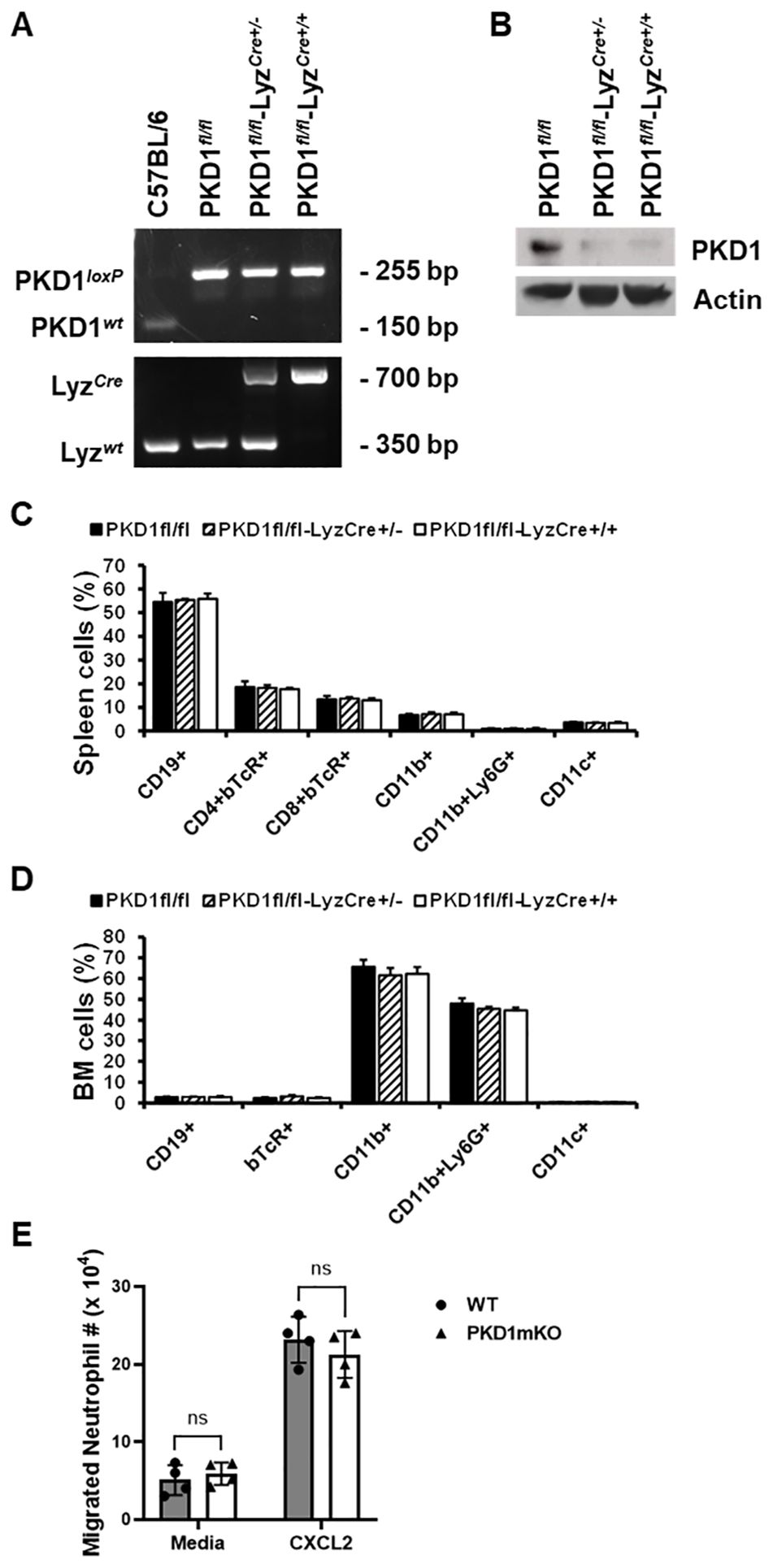
Figure 1. Genotypic and phenotypic analysis of myeloid lineage cell-specific PKD1-deficient mice. (A) Genomic DNA was isolated from tail and analyzed for PKD1 alleles and LyzCre by PCR. PCR products corresponding to PKD1 WT (151 bp), PKD1loxP (255 bp), LyzWT (350 bp), and LyzCre (700 bp) are shown. (B) Neutrophils from bone marrow were isolated. Neutrophil lysates were prepared, and protein levels of PKD1 and actin were detected by Western blot assay. Actin was used as a loading control. (C, D) For immune cell profiling, cells isolated from spleen (C) and bone marrow (D) of PKD1fl/fl (wild type; WT; n = 5), PKD1fl/fl-LyzCre+/- (PKD1mKO; n = 3), or PKD1fl/fl-LyzCre+/+ (PKD1mKO; n = 4) were analyzed by flow cytometry. Gating strategies for flow cytometric analysis of spleen cells and bone marrow (BM) cells are shown in Supplementary Figures 1A, B, respectively. Frequency of each cell population (mean % ± SD) is expressed as the percentage of CD45+ cells. Statistically significant differences determined by one-way ANOVA with Tukey’s post-hoc test. (E) One milliliter of media or murine recombinant CXCL2 (50 ng/mL) was placed into the bottom chamber of each designated well in a 24-well plate and 5 × 105 bone marrow neutrophils (isolated from PKD1fl/fl or PKD1fl/fl-LyzCre+/-) in 200 µL of media were placed into the top chamber. Two hours later, neutrophils that migrated into the bottom chamber and the bottom chamber side of the membrane were collected and counted. Data represent the mean cell # of quadruplicates ± SD. Significance was determined by two-tailed Student’s t-test. ns, not significant.
Effects of protein kinase D1 deletion in myeloid lineage cells on the acute proinflammatory responses in the lung after S. rectivirgula inhalationPKD1 is activated in the lung and plays a pivotal role in acute pulmonary inflammation and HP following inhalation of SR (43, 57). Using cell lines and PKD1-specific siRNA, we found that S. rectivirgula activates PKD1 in alveolar type II epithelial cells, neutrophils, and alveolar macrophages, and that PKD1 is indispensable for S. rectivirgula-induced expression of cytokines and chemokines in these cells (43). Neutrophils are the predominant cells infiltrated into the lung within several hours following inhalation of S. rectivirgula and play an indispensable role in both the acute lung injury and the development of HP (43, 44, 63, 70). To investigate whether lack of PKD1 activation in myeloid lineage cells, such as neutrophils and macrophages, influences inflammatory responses in the lung following exposure to S. rectivirgula, PKD1-sufficient mice (WT: PKD1fl/fl) and myeloid lineage cell PKD1-deficient mice (PKD1mKO: PKD1fl/fl-LyzCre) were exposed to S. rectivirgula by intranasal instillation. Two, six, or twenty-four hours later, BALF and lung tissues were obtained and inflammatory responses to S. rectivirgula were assessed by analyzing mRNA levels and protein levels of selected cytokines and chemokines in lungs and BALF, respectively.
At 2 h after the S. rectivirgula inhalation, lung mRNA expression levels of TNFα, CCL2, CCL4, CXCL1, and CXCL2 in WT mice and PKD1mKO were significantly increased compared to those in control saline-inhaled mice (Figure 2A). Expression levels of those cytokines and chemokines were not significantly different between WT mice and PKD1mKO. These results indicate that PKD1 activation in myeloid lineage cells is dispensable for the initial expression of TNFα, CCL2, CCL4, CXCL1, and CXCL2 in the lungs following S. rectivirgula inhalation. These results also suggest that the initial lung cellular sources of these cytokines and chemokines in response to S. rectivirgula inhalation may not be the lung-resident myeloid lineage cells. However, we cannot rule out the possibility that other signaling pathways independent of PKD1 may be involved in the expression of those proinflammatory genes in the lung myeloid lineage cells immediately following the S. rectivirgula inhalation. Lung mRNA expression levels of CCL3 in both WT mice and PKD1mKO mice 2 h after the S. rectivirgula inhalation were also significantly increased compared to those in control saline-inhaled mice. However, lung mRNA expression levels of CCL3 in PKD1mKO mice at 2 h post-S. rectivirgula inhalation were significantly lower than those in WT mice, indicating that PKD1 in myeloid lineage cells contributes to the initial expression of CCL3 in the lungs following S. rectivirgula inhalation. Lung mRNA expression levels of IL-6, CXCL5, and CXCL10 were slightly but not significantly increased in WT mice compared to those in control mice. However, mRNA expression levels of IL-6, CXCL5, and CXCL10 in PKD1mKO mice at 2 h post-S. rectivirgula inhalation were significantly increased compared to those in saline-inhaled control mice. When compared to WT mice, lung mRNA expression levels of IL-6 and CXCL10 in PKD1mKO mice at 2 h post-S. rectivirgula inhalation were slightly, but not significantly, increased (p = 0.2888 for IL-6; p = 0.2147). Lung mRNA expression levels of CXCL5 in PKD1mKO mice at 2 h post-S. rectivirgula inhalation were significantly increased compared to CXCL5 levels in WT mice. These indicate that PKD1 activation in myeloid lineage cells is dispensable for the initial expression of IL-6, CXCL5, and CXCL10 in the lungs following S. rectivirgula inhalation. These results also suggest a possibility for the presence of PKD1-dependent regulatory mechanism in myeloid lineage cells that negatively affect the initial expression of IL-6, CXCL5, and CXCL10 in the lungs following S. rectivirgula inhalation.
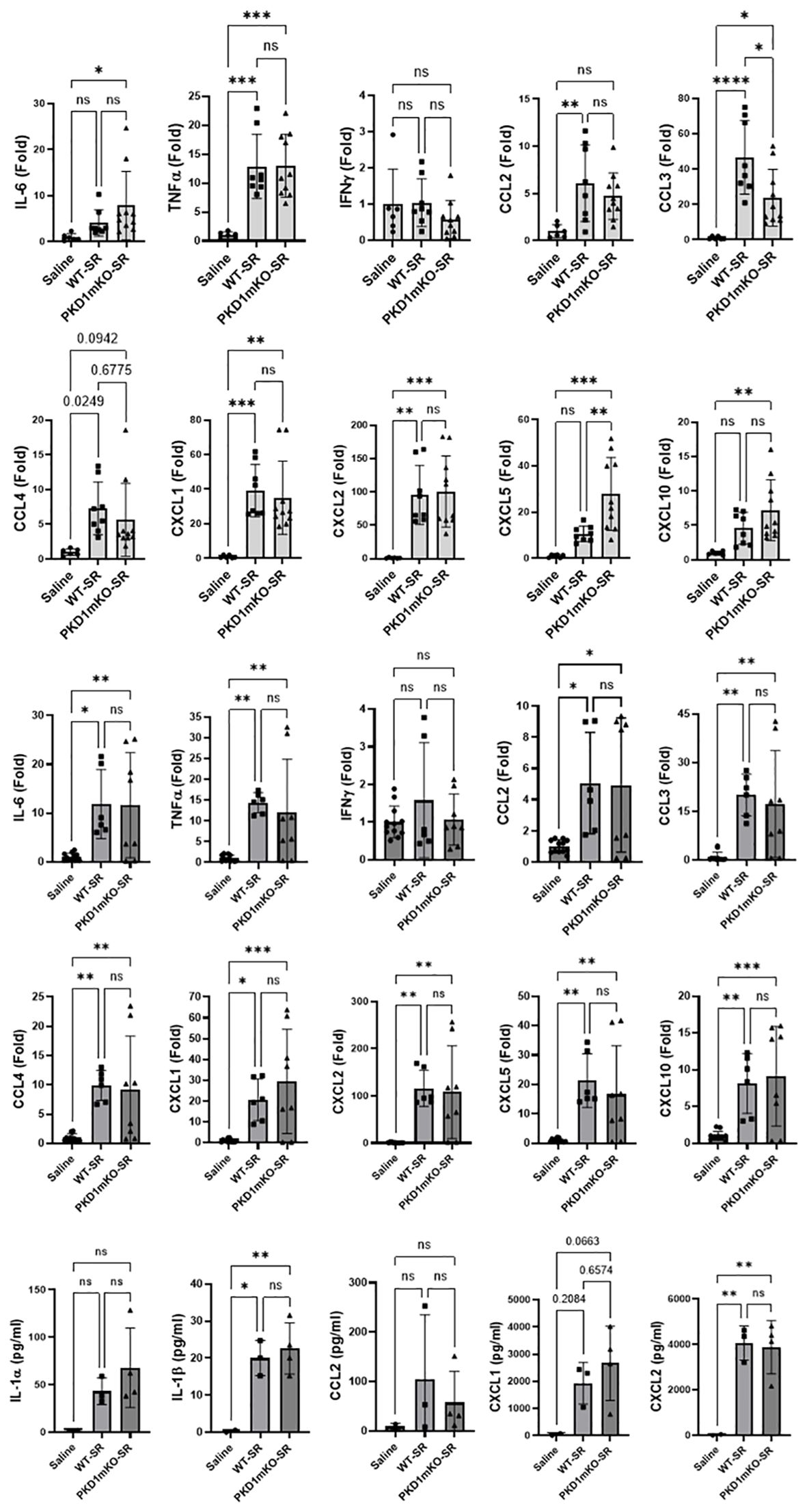
Figure 2. PKD1 in myeloid lineage cells is dispensable for the initial cytokine and chemokine expression in the lungs of mice in response to S. rectivirgula inhalation. PKD1fl/fl mice (WT) and PKD1fl/fl-LyZCre mice (PKD1mKO) were exposed intranasally to saline or S. rectivirgula (100 μg) for 2 h (A) or 6 h (B, C). (A, B) Total RNA was purified from lung lobes isolated from each individual mouse and reverse transcribed, and then mRNA levels of the indicated genes were analyzed in duplicate by real-time qPCR using SYBR Green Assay. The data on genes that were differentially expressed were normalized to the expression of the housekeeping gene [Actin for panel (A) and GAPDH for panel (B)]. Fold change comparing S. rectivirgula-exposed WT mice and S. rectivirgula-exposed PKD1mKO mice to control saline-exposed mice were calculated by comparative quantification algorithm-delta delta Ct method (fold difference = 2−ΔΔCt). Data represent the mean (Fold) ± SD. (C) Bronchoalveolar lavage (BAL) was performed. Levels of the indicated cytokines and chemokines in BAL fluid were detected by multiplex sandwich assay. Data present the mean concentration (pg/mL) ± SD. Number of mice used for each group is as follows: Saline, n = 2 to 6; WT-SR, n = 3 to 4; PKD1mKO-SR, n = 4 to 5. Statistically significant difference determined by one-way ANOVA with Tukey’s post-hoc test is indicated (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). ns, not significant.
At 6 h after the S. rectivirgula inhalation, lung mRNA expression levels of IL-6, TNFα, CCL2, CCL3, CCL4, CXCL1, CXCL2, CXCL5, and CXCL10 in both WT mice and PKD1mKO were significantly increased compared to those in control saline-inhaled mice (Figure 2B). Expression levels of those cytokines and chemokines in lungs were not significantly different between WT mice and PKD1mKO at 6 h post-S. rectivirgula inhalation. IFNγ mRNA expression was not detected at this time point. Protein levels of IL-1β and CXCL2 in BALF were significantly increased in WT and PKD1mKO at 6 h post-exposure to S. rectivirgula compared to those in mice exposed to saline (Figure 2C). Protein levels of IL-1α, CCL2, and CXCL1 in BALF were also increased in both WT and PKD1mKO exposed to S. rectivirgula compared to those in saline-exposed mice but differences were statistically insignificant. Protein levels of these cytokines and chemokines in BALF obtained 6 h after the S. rectivirgula inhalation were not statistically different between WT and PKD1mKO. Collectively, these early time point (2 h and 6 h post-challenge) results indicate that PKD1 activation in myeloid lineage cells is dispensable for the initial proinflammatory responses in the lungs following S. rectivirgula inhalation.
At 24 h after the S. rectivirgula inhalation, the expression levels of proinflammatory cytokines IL-6, TNFα, and IFNγ and chemokines CCL2, CCL3, CCL4, CXCL1, CXCL2, and CXCL10 in the lungs of WT mice were significantly increased (Figure 3A). We were not able to detect increased expression of CXCL5, CXCL9, and CXCL11 in the lungs of WT mice at 24 h after the S. rectivirgula exposure. When compared to WT, PKD1mKO mice showed significantly reduced mRNA expression levels of TNFα, IL-6, IFNγ, CCL2, CCL3, CCL4, CXCL1, CXCL2, and CXCL10 in the lungs in response to S. rectivirgula exposure. The mRNA expression levels of those cytokines and chemokines in the lungs of PKD1mKO exposed to S. rectivirgula for 24 h were not significantly different from those in lungs of control mice exposed to saline. Protein levels of IL-1α, IL-1β, IL-6, TNFα, IFNγ, CCL2, CXCL1, and CXCL2 in BALF were significantly increased in WT mice at 24 h after the exposure to S. rectivirgula compared to those in mice exposed to saline (Figure 3B). Compared to those in WT mice, protein levels of IL-1β, IL-6, TNFα, IFNγ, CCL2, and CXCL2 in BALF from S. rectivirgula-exposed PKD1mKO mice were significantly decreased. Although it showed substantial reduction in S. rectivirgula-exposed PKD1mKO, the protein levels of CXCL1 in BALF were not significantly different between WT and PKD1mKO exposed to S. rectivirgula one time for 24 h (p = 0.1017). When compared to those in saline-treated control mice, the protein levels of IL-6, TNFα, IFNγ, CCL2, and CXCL1 in BALF from SR-exposed PKD1mKO were not significantly different. However, the protein levels of IL-1β in PKD1mKO exposed to S. rectivirgula were significantly higher than those in saline-treated control mice. Increasing trends for the protein levels of IL-1α (p = 0.0878) and CXCL2 (p = 0.1091) in PKD1mKO following S. rectivirgula exposure, compared to those in saline-treated control, were also observed. These results indicate that PKD1 in myeloid lineage cells is involved in cytokine and chemokine production in the lungs during the later phase of acute lung insult by S. rectivirgula exposure. Taken together, our results demonstrate that although multiple signaling pathways and multiple types of lung cells are involved in acute pulmonary inflammation caused by exposure to S. rectivirgula, PKD1 in myeloid lineage cells plays a significant role for the acute pulmonary proinflammatory responses following one-time exposure to S. rectivirgula.
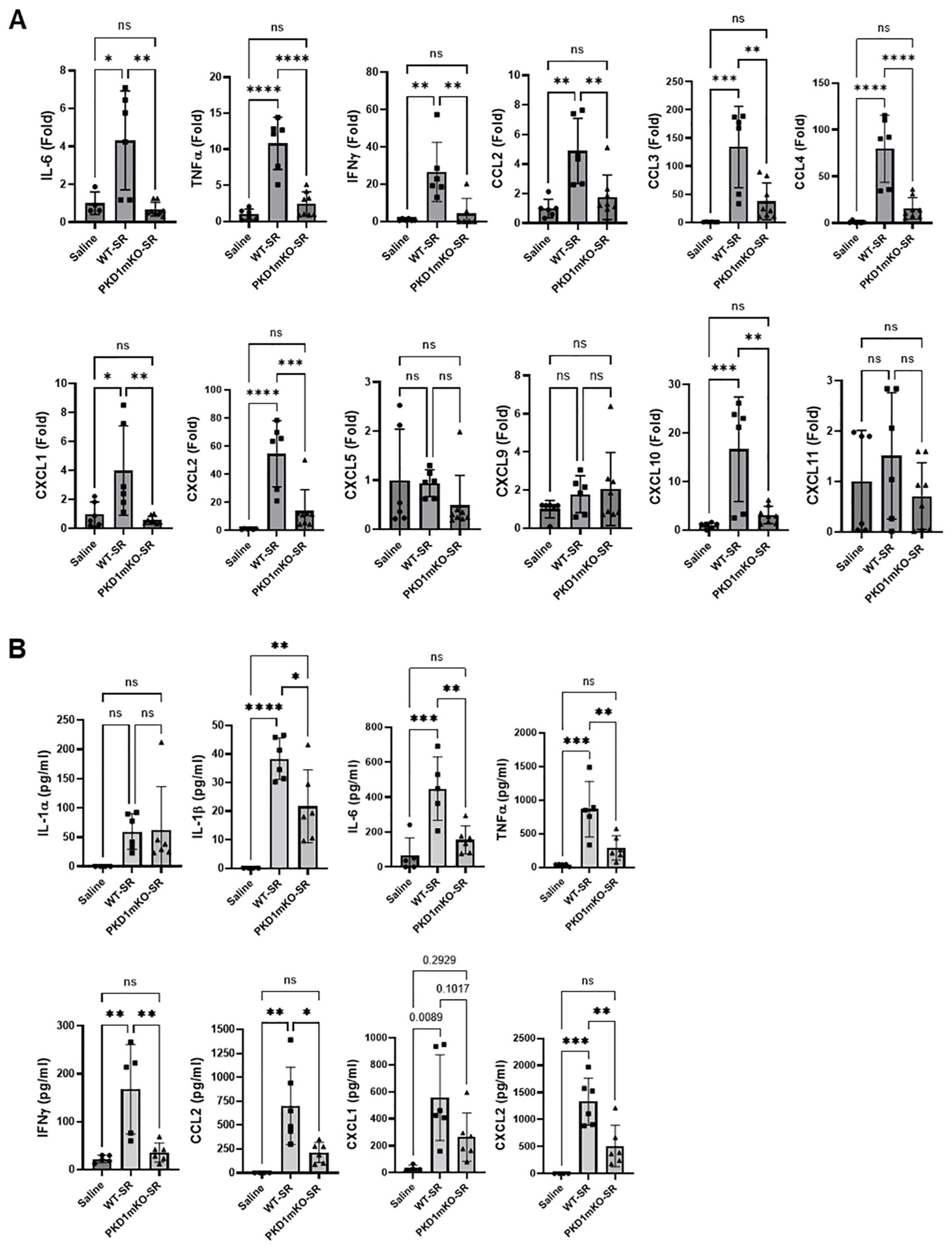
Figure 3. Contribution of PKD1 in myeloid lineage cells to the proinflammatory responses in the lung of mice following one-time exposure to S. rectivirgula. PKD1fl/fl mice (WT) and PKD1fl/fl-LyZCre mice (PKD1mKO) were exposed intranasally to saline or S. rectivirgula (80 μg) for 24 (h) (A) Total RNA was purified from lung lobes isolated from each individual mouse and reverse transcribed, and then mRNA levels of the indicated genes were analyzed in duplicate by real-time qPCR using SYBR Green Assay. The data on genes that were differentially expressed were normalized to the expression of the housekeeping gene, GAPDH. Fold change comparing S. rectivirgula-exposed WT mice and S. rectivirgula-exposed PKD1mKO mice to control saline-exposed mice were calculated by comparative quantification algorithm-delta delta Ct method (Fold difference = 2−ΔΔCt). Data represent the mean (Fold) ± SD. (B) Bronchoalveolar lavage (BAL) was performed. Levels of the indicated cytokines and chemokines in BAL fluid were detected by either ELISA (IL-6, TNFα, and IFNγ) or multiplex sandwich assay (IL-1α, IL-1β, CCL2, CXCL1, and CXCL2). Data represent the mean concentration (pg/mL) ± SD. Number of mice used for each group is as follows: Saline, n = 3 to 5; WT-SR, n = 3 to 6; PKD1mKO-SR, n = 4 to 6. Statistically significant difference determined by one-way ANOVA with Tukey’s post-hoc test is indicated (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). ns, not significant.
Development of acute neutrophilia following single-time exposure to S. rectivirgula is significantly suppressed in myeloid lineage cell protein kinase D1-deficient miceSignificantly reduced expression of CCL2, CCL3, CCL4, CXCL1, and CXCL2 in the lungs of PKD1mKO 24 h after the single exposure to S. rectivirgula predict that PKD1 in myeloid lineage cells may contribute to the leukocyte influx into the lung by upregulating these chemokines following exposure to S. rectivirgula. Therefore, we investigated whether deletion of PKD1 in myeloid lineage cells affects leukocyte infiltration into the lung following S. rectivirgula exposure. As shown in Figure 4A (left panel), at 24 h post-exposure to S. rectivirgula, WT mice exhibited significantly increased total BAL cell numbers (alveolitis) compared to those in control mice exposed to saline. Histological sections of lungs from S. rectivirgula-exposed WT mice also showed the presence of extensive leukocyte infiltration in the lungs compared to those from saline-exposed mice (Figure 4B). As previously reported (43, 44, 57, 63, 70), neutrophils were the predominant cell type recovered from the airways in WT mice exposed to S. rectivirgula (Figure 4A, middle panel). Approximately 78% of cells recovered from BALF were neutrophils determined by flow cytometric analysis. In contrast, significantly less BAL cells were recovered from the PKD1mKO mice at 24 h after the S. rectivirgula exposure compared to the S. rectivirgula-exposed WT mice (Figure 4A, left panel). The number of total cells recovered from the BALF from S. rectivirgula-exposed PKD1mKO mice were approximately 35% of those in S. rectivirgula-exposed WT mice. Histological sections of lungs from S. rectivirgula-exposed PKD1mKO mice also showed substantially less leukocyte infiltration in the lungs compared to those from S. rectivirgula-exposed WT mice (Figure 4B). Like the S. rectivirgula-exposed WT mice, the major cell type recovered from the airways of PKD1mKO mice was neutrophils (approximately 67%) (Figure 4A, middle panel). Although frequencies of neutrophils in BAL cells from S. rectivirgula-exposed PKD1-mKO mice were slightly lower compared to those of S. rectivirgula-exposed WT mice, the difference was not significant (p = 0.0704). The number of neutrophils recovered from the BALF from PKD1mKO mice were approximately 31% of those in S. rectivirgula-exposed WT mice (Figure 4A, right panel). Although they are not statistically significant, the numbers of total cells (p = 0.1849) and neutrophils (p = 0.1055) recovered from BALF of S. rectivirgula-exposed PKD1mKO were substantially higher than those in saline-exposed mice. Taken together, our results demonstrate that PKD1 in myeloid lineage cells plays a significant role in S. rectivirgula-induced acute neutrophilic alveolitis.
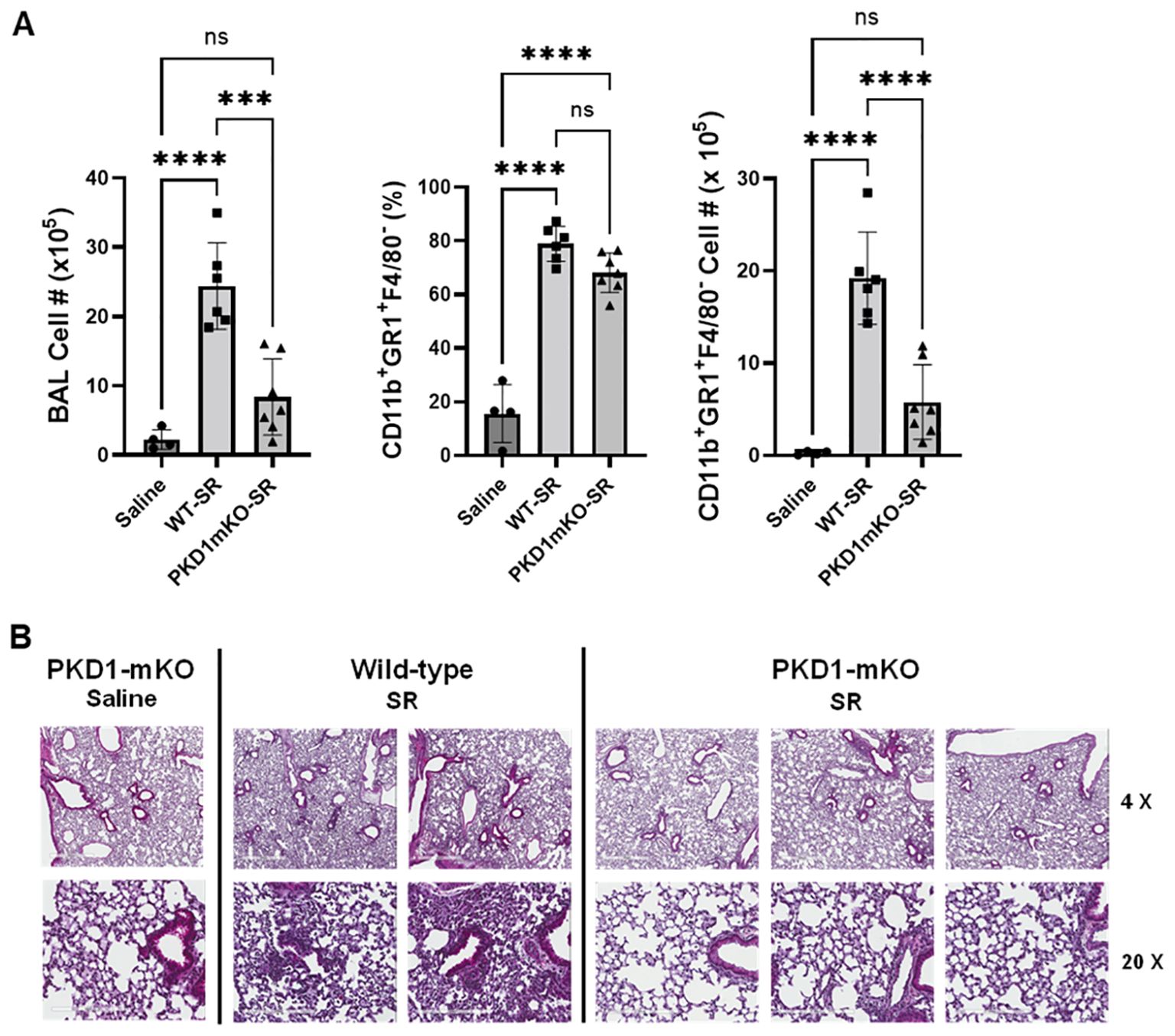
Figure 4. PKD1 in myeloid lineage cells contributes significantly to the neutrophilic alveolitis developed following single exposure to S. rectivirgula. PKD1fl/fl mice (WT) and PKD1fl/fl-LyZCre mice (PKD1mKO) were exposed intranasally to saline or S. rectivirgula (80 μg) for 24 h. (A) BAL cells were counted using trypan blue exclusion and presented as the mean cell number ± SD (left panels). Total neutrophil cell count was derived by staining BAL cells with Abs to Gr-1, CD11b, and F4/80 followed by flow cytometric analysis. Gating strategy for flow cytometric analysis is shown in Supplementary Figure 2. Neutrophils were identified as CD11b+Gr1+/F4/80-. The frequency of neutrophils is expressed as % in BAL cells, and data represent the mean (%) ± SD (middle panels). The number of neutrophils is presented as the mean cell number ± SD (right panels). Statistical differences were determined by one-way ANOVA with Tukey’s post-hoc test and significant differences are indicated (***p < 0.001; ****p < 0.0001). ns, not significant. (B) Representative H&E staining of the left lung lobe sections from mice exposed to saline or SR for 24 h are shown. The Aperio ScanScope®XT Slide Scanner system was used to capture whole-slide digital images. Each column represents the lung collected from an individual mouse. The images presented are 4× magnification (scale bar = 600 μm) and 20× magnification (scale bar = 200 µm). Number of mice used for each group is as follows: Saline, n = 3 to 11; WT-SR, n = 3 to 6; PKD1mKO-SR, n = 4 to 7.
Protein kinase D1 in myeloid lineage cell contributes to leukocyte infiltration into the bronchial space and granuloma formation following repeated exposures to S. rectivirgulaRepeated exposures to HP-inciting antigens cause recurring leukocyte infiltrations into the airways and interstitial lung space and lead to the formation of granulomas in both HP patients and animal models of HP (2, 3, 70, 71). To determine whether PKD1 in myeloid lineage cells contributes to the immune cell influx into the bronchial space and lungs following repeated exposures to S. rectivirgula, WT mice and PKD1mKO mice were exposed to S. rectivirgula three times (once a day)/week for 3 weeks as previously described (57). As shown in Figure 5A, leukocyte infiltration in bronchial space was significantly increased in both WT and PKD1mKO compared to the control saline-exposed mice. However, S. rectivirgula-induced leukocyte infiltration in bronchial space was significantly suppressed in PKD1mKO compared to WT (approximately 62% of WT-SR). Cellular composition of alveolitis following 3-week exposures to S. rectivirgula was not significantly different between WT mice and PKD1mKO mice (Table 1). To determine whether PKD1 in myeloid lineage cells contributes to leukocyte infiltration and accumulation, and the development of granulomas in the lungs, we examined lung tissue sections from WT mice and PKD1mKO mice that had been repeatedly exposed to S. rectivirgula for 3 weeks. As shown in Figure 5B and Supplementary Figure 6, while mice exposed to saline showed normal lung architecture, both WT mice and PKD1mKO mice repeatedly exposed to S. rectivirgula for 3 weeks exhibited alveolitis and granuloma formation. However, compared to WT mice, deletion of PKD1 in myeloid lineage cells resulted in substantially less leukocyte accumulation and reduced granuloma formation following repeated exposures to S. rectivirgula (Figure 5B; Supplementary Figure 6). Of note, the frequencies of CD45+ cells in isolated LICs were significantly lower in PKD1mKO mice compared to WT mice (Supplementary Table 2). Cellular composition of LICs following 3-week exposures to S. rectivirgula was not significantly different between WT mice and PKD1mKO mice (Supplementary Table 2). Collectively, these results demonstrate that PKD1 in myeloid lineage cells contributes significantly to leukocyte infiltration and accumulation in the lungs, and subsequent granuloma formation following repeated exposures to S. rectivirgula.
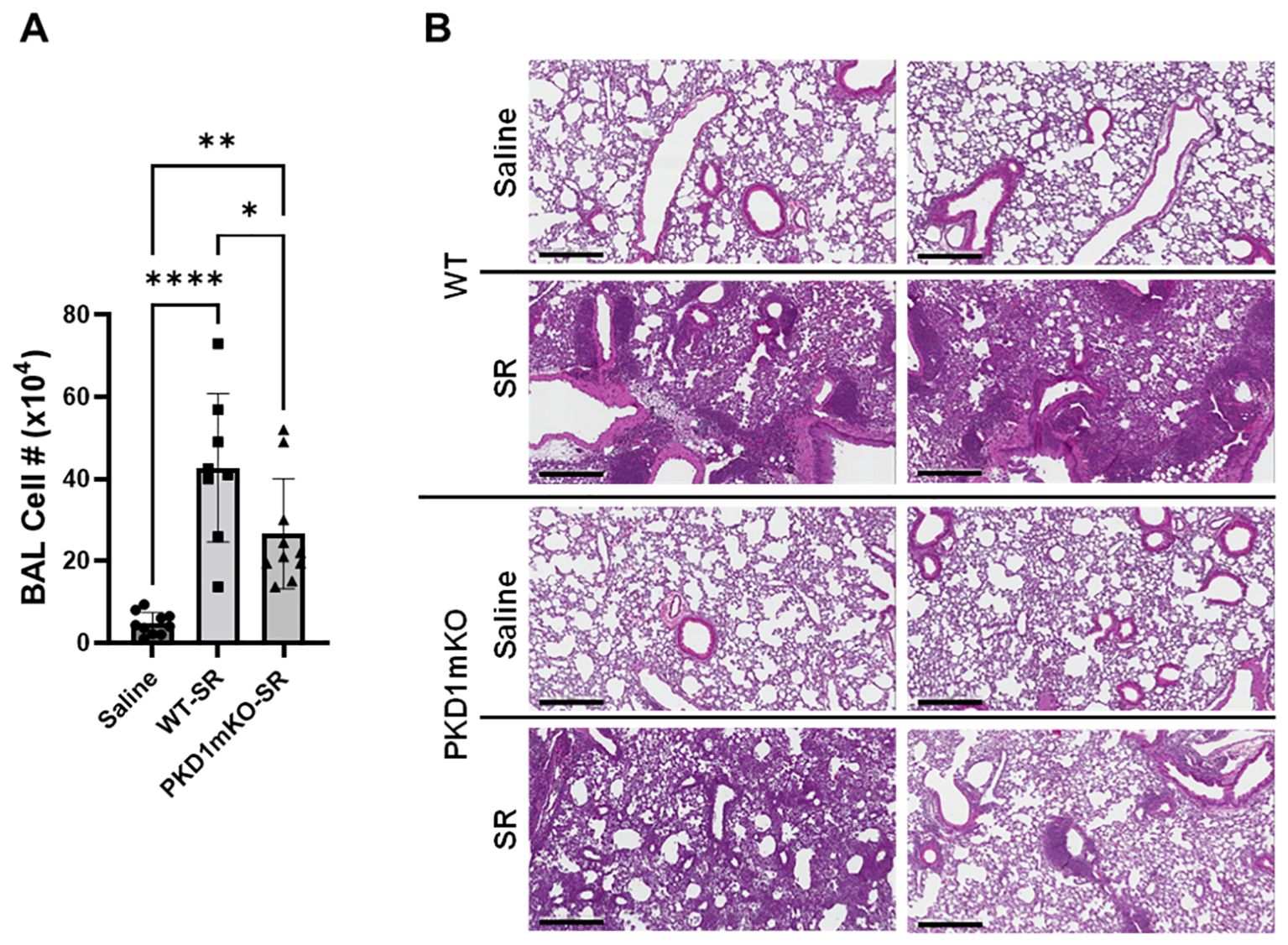
Figure 5. Deletion of PKD1 in myeloid lineage cells results in significant reduction in alveolitis and granuloma formation following repeated exposures to S. rectivirgula. PKD1fl/fl mice (WT) and PKD1fl/fl-LyZCre mice (PKD1mKO) were exposed intranasally to saline or S. rectivirgula (100 μg) three times per week for 3 weeks. (A) Seventy-two hours after the last SR exposure, BAL was performed, and the BAL cells recovered. BAL cells were counted to determine the degree of alveolitis using trypan blue exclusion. Data represent the mean cell number ± SD (n = 8 to 10 mice/group). Significance was determined by one-way ANOVA with Tukey’s post-hoc test. Statistically significant differences are indicated (*p < 0.05; **p < 0.01; ****p < 0.0001). (B) Forty-eight hours after the last S. rectivirgula exposure, the left lung lobes were removed from the mice. Representative H&E staining of the left lung lobe sections are shown. The Aperio ScanScope®XT Slide Scanner system was used to capture whole-slide digital images. Each panel represents the lung collected from an individual mouse. Number of mice used for each group is as follows: Saline, n = 4; WT-SR, n = 4; PKD1mKO-SR, n = 3. The scale bar = 300 µm.
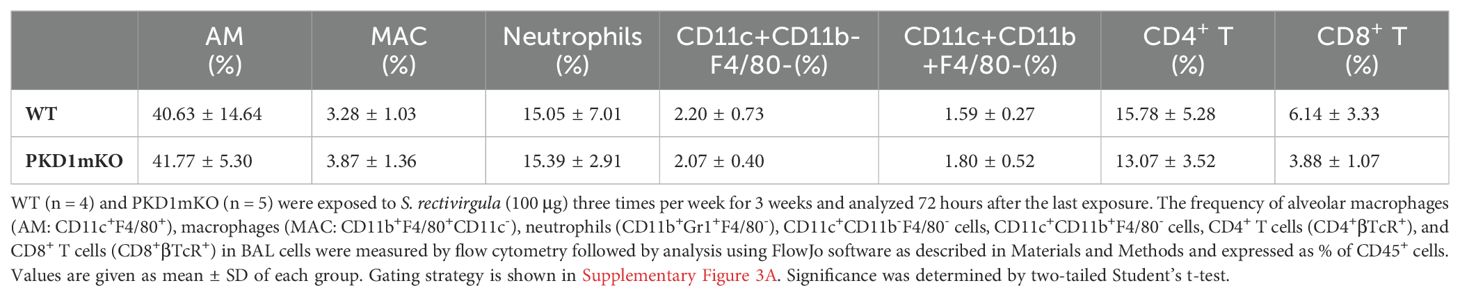
Table 1. Cellular composition of alveolitis following 3-week exposures of WT and PKD1mKO mice to S. rectivirgula.
Protein kinase D1 in myeloid lineage cells contributes to the expression of major histocompatibility complex class II in neutrophils and macrophages infiltrated into the bronchial space and lung interstitium following repeated exposures to S. rectivirgulaIn the previous studies, we found that PKD1 contributes significantly to the increased surface expressio
留言 (0)