A 62-year-old man with a medical history of hypertension and a family history of hypertrophic obstructive cardiomyopathy in his sister presented to the emergency room for a second episode of syncope in the previous few weeks.
The first episode occurred 4 weeks earlier. The patient had consumed a large amount of alcohol the day before the episode and had been working outside in the summer heat that morning. He experienced positional lightheadedness and, after taking a shower, noted a slow, thready pulse. Vital signs assessed by emergency medical services were within normal limits. He was taken to the emergency room by ambulance and admitted for syncope workup. Telemetry during admission was unremarkable. Echocardiography was obtained given the patient’s family history of hypertrophic obstructive cardiomyopathy. It showed increased left ventricular septal wall thickness and impaired left ventricular diastolic function with no signs of obstruction. The patient was discharged with a working diagnosis of neurally mediated syncope.
Before the second episode, the patient was sitting in a chair visiting a family member at the hospital when he suddenly lost consciousness with no prodrome. No convulsions, urinary or fecal incontinence, or tongue biting were noted. He was taken to the emergency room in a wheelchair where he was initially noted to be confused. His only medication was finasteride.
Further review of the patient’s history revealed that 7 years earlier he developed tingling and numbness of the first 3 digits of both hands. He was diagnosed with carpal tunnel syndrome and underwent 2 surgeries on each hand. Despite repeat interventions, carpal tunnel syndrome recurred in both hands.
The patient also noted that he had ankle edema for the previous 18 months, which his primary care physician attributed to calcium channel blocker use. Amlodipine was discontinued, but the ankle swelling persisted.
DIFFERENTIAL DIAGNOSIS1. Which underlying cause of this patient’s episodes of syncope would be most consistent with his presentation?
Neurally mediated syncope is the most common form of syncope1 and could have been a cause of this patient’s episodes, especially with the premonitory symptoms and history of exertion and dehydration during the first episode. However, the second syncopal episode lacked a prodrome or history suggestive of neurally mediated syncope. Orthostatic syncope can occur with volume depletion and finasteride use and is typically related to change in posture.2,3 During his second episode, the patient had been sitting for some time before he lost consciousness.
He experienced some confusion immediately following the second episode, but seizure was less likely given that the episode was witnessed by multiple family members and no seizure-like activity was noted. His brief confusion could have been attributed to a slight delay in cerebral reperfusion as he was taken to the emergency room in a sitting (and therefore upright) position. And, though he had a family history of hypertrophic cardiomyopathy, which can be hereditary and cause left ventricular outflow obstruction, outflow obstruction is typically precipitated by factors that decrease preload or afterload, such as strenuous exertion, dehydration, or vasodilator use. The patient was dehydrated during his first syncope episode, but echocardiography at the time did not suggest obstruction, and hence syncope caused by hypertrophic cardiomyopathy was less likely. Also, the first episode happened after the patient had showered (and therefore occurred after and not during exertion), which is also less typical of syncope caused by ventricular outflow obstruction. The abrupt and unprovoked nature of this patient’s second syncopal episode was most suspicious for arrhythmia, and further workup to rule out malignant arrhythmia was warranted.
INITIAL EVALUATION AND MANAGEMENTThe results of initial laboratory testing at the emergency room following the patient’s second episode of syncope are presented in Table 1.
TABLE 1Initial laboratory test results
Orthostatic vital signs were negative for orthostatic hypotension (blood pressure with the patient in the supine position was 121/71 mm Hg with a heart rate of 68 beats per minute vs 124/74 mm Hg and 78 beats per minute while standing). No bruits were noted on physical examination.
Electrocardiography showed sinus rhythm with normal voltage. Computed tomography of the brain showed no acute intracranial process. Due to the presence of bilateral lower extremity edema noted on examination, elevated D-dimer, and unexplained syncope, computed tomography of the chest with pulmonary embolism protocol was done. It showed mild mediastinal and bilateral hilar adenopathy (< 1 cm) and no evidence of pulmonary embolism. Bilateral lower extremity ultrasonography was negative for thrombosis.
Repeat echocardiography findings were similar to those from echocardiography done at the time of the first syncope epidsode, with mild left ventricular septum hypertrophy and a dilated left atrium. Nonsustained polymorphic ventricular tachycardia was noted on telemetry (Figure 1).
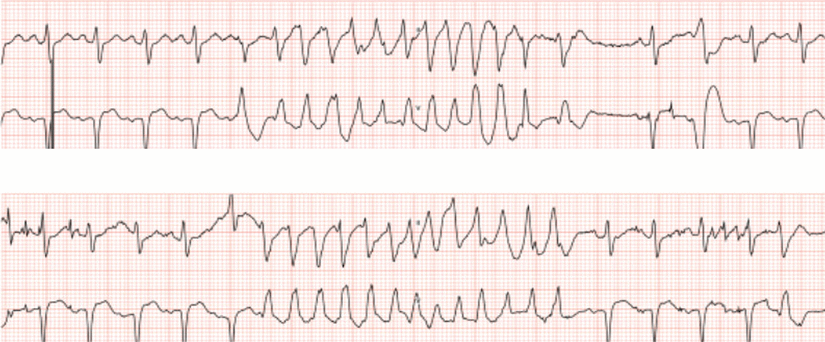
 Figure 1
Figure 1 Polymorphic ventricular tachycardia episodes captured on continuous telemetry.
Left heart catheterization was done due to concern for myocardial ischemia given the patient’s chest pain, shortness of breath on exertion, and persistently elevated high-sensitivity troponin (56 ng/mL in the emergency room and 68 ng/mL on repeat laboratory evaluation). The results showed no coronary artery stenosis.
Cardiac magnetic resonance imaging (MRI) showed subendocardial to mid-myocardial delayed gadolinium enhancement with multiple foci, predominantly within the basal inferolateral and basal septum. A dualchamber implantable cardioverter-defibrillator was placed before the patient was discharged.
CASE CONTINUEDBecause the patient’s cardiac MRI report suggested that the imaging findings were most consistent with cardiac sarcoidosis, the patient was referred to the sarcoidosis clinic after discharge. Bronchoscopy was offered to evaluate for cardiac sarcoidosis, but the patient declined. Additional testing was ordered:
Positron emission tomography (PET) and computed tomography of the whole body showed no evidence of focal uptake to suggest a fluorodeoxyglucose F18 (FDG)–avid neoplastic process or active granuloma disease such as sarcoidosis
Cardiac PET showed evidence of active inflammation with a large amount (> 5 segments) of inflamed myocardium with focal-on-diffuse myocardial FDG uptake.
Further testing and resultsGiven the patient’s history of recurrent bilateral carpal tunnel syndrome, unexplained anemia, proteinuria with edema, and renal insufficiency, workup to rule out cardiac amyloidosis was done:
Kappa free light chains: 1,099.7 mg/L (reference range 3.3–19.4)
Lambda free light chains: 2.3 mg/L (5.7–26.3)
Kappa-to-lambda free light chain ratio: 478.13 (0.26–1.65)
Serum protein electrophoresis monoclonal (M) protein concentration: 1.99 g/dL (0)
24-hour urine protein: 4.11 g (< 0.15), M protein present
Lactate dehydrogenase: 282 U/L (135–225)
Beta-2 microglobulin: 3.4 mg/L (< 3.1)
LABORATORY WORKUP INTERPRETATION2. For which type of disorder do these laboratory results raise suspicion?
This patient’s laboratory results showed significantly elevated kappa free light chains, an abnormal kappa-to-lambda ratio, and paraproteinemia. These findings were most suspicious for a plasma cell dyscrasia. The presence of M-protein confirmed that a monoclonal gammopathy was present. The extremely elevated (≥ 100) ratio of involved to uninvolved serum free light chains was also indicative of an underlying plasma cell disorder, and combined with 10% or greater clonal bone marrow plasma cells or biopsy-proven plasmacytoma, would be diagnostic for multiple myeloma.4
Though an elevated lactate dehydrogenase level, free light chains, and M-spike can sometimes be seen in B-cell lymphomas, T-cell lymphomas are not typically associated with monoclonal gammopathies.5
3. Which type of amyloidosis does this patient most likely have?
Immunoglobulin light chain (AL) amyloidosis
Serum amyloid A (AA) amyloidosis
Transthyretin (ATTR) amyloidosis
AL amyloidosis, or primary amyloidosis, occurs when misfolded immunoglobulin light chains are deposited in tissues of patients with an underlying plasma cell dyscrasia.6 As this patient had an underlying plasma cell dyscrasia, AL amyloidosis was most likely.
In AA amyloidosis, the deposited protein is derived from the acute-phase reactant serum AA protein. This condition is commonly found with long-standing inflammatory disorders such as autoimmune disease or chronic infection.7
ATTR amyloidosis results from the misfolding of transthyretin, a protein involved in transporting thyroxine- and retinol-binding protein.8 ATTR amyloidosis can occur due to pathologic deposits of transthyretin protein in patients with hereditary mutations in the transthyretin gene (hereditary ATTR amyloidosis) or with no known mutation (wild-type ATTR amyloidosis).
DIAGNOSTIC TESTING4. Which diagnostic modality is the gold standard test for differentiating cardiac sarcoidosis and cardiac amyloidosis?
Echocardiography
Cardiovascular MRI
FDG-PET
Myocardial biopsy
Amyloidosis and sarcoidosis are both infiltrative cardiomyopathies caused by interstitial deposition of pathological tissue.9 Cardiac amyloidosis can present with ventricular wall thickening and a granular sparkling appearance of the septum on echocardiography.10 The granulomatous lesions and thinning from fibrous scars seen in cardiac sarcoidosis can cause wall motion abnormalities, diastolic dysfunction, and abnormal myocardial wall thickness in a noncoronary distribution.11 Cardiovascular MRI also can be useful for differentiating amyloidosis and sarcoidosis: amyloidosis more often presents with global subendocardial late gadolinium enhancement, while a wider variety of late gadolinium enhancement distributions is seen in sarcoidosis (eg, nodular, circumferential, subepicardial, or subendocardial types). However, amyloidosis and sarcoidosis can mimic each other on both echocardiography and cardiovascular MRI, and neither test can definitively diagnose either condition.12
FDG-PET in cardiac sarcoidosis shows focal areas of increased FDG uptake corresponding to the increased glucose consumption of macrophages within granulomatous lesions or resting perfusion defects from compression of the microvasculature due to inflammation or fibrosis.11 Amyloidosis, on the other hand, can have variable FDG avidity, and FDG-PET is not routinely used for diagnosis.13
Myocardial biopsy is the gold standard for establishing a diagnosis and should be pursued when the diagnosis cannot be fully substantiated through other modalities.14
FURTHER INVESTIGATIONS AND FINAL DIAGNOSISCardiovascular technetium-99m pyrophosphate scintigraphy was obtained and was negative for ATTR amyloidosis. Bone marrow biopsy showed 30% plasma cells, and Congo red staining was positive for amyloid deposition in the periosteal soft tissue. Flow cytometry of marrow aspirate showed an abnormal plasma cell population with trisomy 9, gain at the CCND1 locus, and deletion at the RB1 locus.
Atypical presentation of AL cardiac amyloidosis was suspected, and cardiac biopsy was pursued. Biopsy of the right ventricle showed no sarcoplasmic inclusions or vascularizations but was positive for amyloid on thioflavin S stain examined under fluorescence microscopy. Immunohistochemical staining performed for amyloid typing showed amyloid deposits positive for kappa light chain and negative for lambda light chain and transthyretin.
A dual diagnosis of systemic AL amyloidosis with cardiac involvement (Mayo 2012 stage 3; European modification Mayo 2004 stage IIIa)15,16 and Revised International Staging System17 stage 2, standard-risk immunoglobulin G kappa multiple myeloma was made. The patient was started on induction therapy with daratumumab, cyclophosphamide, bortezomib, and dexamethasone. He has not had any further episodes of ventricular tachycardia.
AL AMYLOIDOSISAL amyloidosis is a rare disease with an estimated global incidence of 10 cases per million population.18 AL amyloidosis occurs when soluble light chains are misfolded and convert into insoluble fibrillar aggregates that deposit in tissues throughout the body.19 The most commonly affected organs in AL amyloidosis include the heart, kidney, nervous system, gastrointestinal tract, liver, spleen, and lungs as well as soft tissue. Clinical manifestations vary widely depending on organ involvement and can include shortness of breath, orthopnea, peripheral edema, arrhythmia, peripheral neuropathy, autonomic dysfunction, macroglossia, carpal tunnel syndrome, waxy skin, easy bruising, hepatomegaly, fatigue, weight loss, and early satiety.20
Diagnosis of amyloidosis is often delayed due to the nonspecific presentation of the disease.21 Clinicians may erroneously attribute early signs and symptoms to other, more common pathologies. However, features that should trigger suspicion of amyloidosis include nephrotic-range proteinuria not attributable to diabetes, heart failure and left ventricular hypertrophy in the absence of aortic stenosis or hypertension, peripheral or autonomic neuropathy of unclear etiology, hepatomegaly with increased alkaline phosphatase, macroglossia, bilateral carpal tunnel syndrome, and periorbital purpura.20
Effective therapies for AL amyloidosis are becoming available, with a recent trial showing improved rates of complete hematologic response and survival free from major organ deterioration or hematologic progression with the addition of daratumumab to bortezomib, cyclophosphamide, and dexamethasone.22 Early diagnosis of AL amyloidosis is essential to halt disease progression and maximize patients’ chances of longer survival and recovery of organ function.
TAKE-HOME POINTSCardiac AL amyloidosis is a rare disease with varying presentations that can mimic other pathologies on imaging. Early recognition of the clinical manifestations of amyloidosis is crucial for facilitating timely intervention and preventing complications such as life-threatening arrhythmic events and advanced heart failure.
DISCLOSURESDr. Anwer disclosed consulting, teaching and speaking, and acting as principal investigator and co-principal investigator for Bristol-Myers Squibb Co. and consulting for Caribou Bio. Dr. Lin and Dr. Sheu report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.
留言 (0)