Digoxin, the oldest known cardiovascular drug, is still used today to treat heart failure and atrial fibrillation. Because it has a narrow therapeutic index and multiple interactions, it frequently causes toxicity with a wide range of symptoms and cardiac arrhythmias. More importantly, elevated serum digoxin levels have been linked to a higher risk of death in patients with heart failure or atrial fibrillation, even without signs or symptoms of toxicity. This article reviews the current state of digoxin use, its pharmacologic principles, and the mechanisms, clinical presentation, and management of toxicity.
KEY POINTSDigoxin, a reversible sodium-potassium adenosine triphosphatase inhibitor, has inotropic and vagomimetic properties that make it useful for treating refractory heart failure with reduced ejection fraction and for controlling the heart rate in atrial fibrillation.
The drug has a narrow therapeutic index, and toxicity is common, especially in patients with impaired kidney function, polypharmacy, or electrolyte derangements.
Digoxin toxicity can present with a wide range of nonspecific gastrointestinal and central nervous system symptoms and several cardiac arrhythmias. Hence, it can be difficult to diagnose and easy to miss.
Treatment of digoxin toxicity includes supportive management and digoxin-specific antibody fragments that can be used if the patient has life-threatening cardiac arrhythmias or electrolyte abnormalities.
Digoxin, extracted from the foxglove plant (Digitalis purpurea and Digitalis lanata), is the oldest cardiovascular drug still used today. As far back as 1785, when Dr. William Withering reported using foxglove to treat edematous states (“dropsy”), physicians have known about its beneficial effects—and its toxicity.1 Here is Dr. Withering:
The Foxglove when given in very large and quickly-repeated doses, occasions sickness, vomiting, purging, giddiness, confused vision, objects appearing green or yellow; increased secretion of urine, with frequent motions to part with it, and sometimes inability to retain it; slow pulse, even as slow as 35 in a minute, cold sweats, convulsions, syncope, death.
For more than 2 centuries, the drug was the mainstay of treatment for heart failure, as it increases both the force of the heart’s contractions and the urine volume. It also has a parasympathetic effect, giving it a role in controlling the ventricular rate in patients with atrial fibrillation. Although digoxin use is decreasing (prescriptions for it dropped by 46.4% in the United States from 2007 to 2014, for example2), it is still widely used.
Before laboratory assays were widely available to measure the serum digoxin concentration, physicians would titrate the drug to clinical response (increase in urine output or reduction of cardiac silhouette in the chest radiograph) or until side effects such as nausea, altered color perception, or electrocardiographic changes ensued. Digoxin toxicity was therefore common, and its presentation was widely taught in medical schools. Not until the 1970s, when a radioimmunoassay to measure serum digoxin concentrations became available, were doses titrated to a target therapeutic range, and thereafter toxicity became less frequent.
Nonetheless, digoxin is still causing toxicity, having a narrow therapeutic index, multiple interactions, and variability of serum levels with changes in renal clearance. And not only does digoxin toxicity produce a wide range of morbidity, but, more importantly, elevated serum levels are associated with increased mortality. Therefore, cardiovascular and internal medicine physicians still need to be familiar with the presentation of digoxin toxicity, its mechanisms and predisposing factors, and its medical management.
DIGOXIN’S CLINICAL USESDigoxin is approved by the US Food and Drug Administration for treating heart failure with reduced ejection fraction (HFrEF) and for rate control in atrial fibrillation. Table 1 shows the dosing recommendations for digoxin based on the American Heart Association (AHA) and American College of Cardiology (ACC) guidelines.3–6
TABLE 1Dosing recommendations for digoxin therapy
Heart failure with reduced ejection fractionThe 2022 AHA/ACC guidelines4 recommend digoxin for patients with HFrEF who have symptoms despite guideline-directed medical therapy and for patients who cannot tolerate guideline-directed medical therapy, to decrease hospitalizations for decompensated heart failure. However, digoxin gets only a class 2b (weak) recommendation, based on level B-R evidence (moderate quality, based on randomized trials or meta-analysis of such trials). With its inotropic properties, digoxin is useful specifically for patients with end-stage HFrEF who cannot tolerate afterload-reduction agents because of hypotension. In this population, digoxin can increase the cardiac index and offset neurohormonal imbalances present in heart failure.
Data on digoxin in heart failureThe DIG trial. The AHA/ACC recommendation is based on results from the Digitalis Investigation Group (DIG) trial,7 published in 1997. Patients in this trial had left ventricular ejection fractions of 45% or less and normal sinus rhythm, and were already on diuretics and angiotensin-converting enzyme inhibitors (the mainstay of heart failure therapy in 1997). They were randomized in a double-blind fashion to receive digoxin or placebo. The digoxin group did not have a lower mortality rate, but they did have a lower rate of hospitalizations for heart failure (risk ratio [RR] 0.72, 95% confidence interval [CI] 0.66–0.79, P < .001).
Of note, 11.9% of the patients in the digoxin group developed suspected digoxin toxicity vs 7.9% in the placebo group, representing a number needed to harm of 25. Of those in the digoxin group with suspected toxicity, 16.5% were hospitalized. There was no difference in the rate of ventricular arrhythmias between groups, but the digoxin group did have higher rates of supraventricular tachyarrhythmias (2.5% vs 1.2%, RR 2.10, 95% CI 1.45–3.07, P < .001) and second- or third-degree atrioventricular block (1.2% vs 0.4%, RR 2.87, 95% CI 1.56–5.28, P < .001).7
A post hoc analysis of the DIG trial8 suggested that patients with higher serum digoxin concentrations (≥ 1 ng/mL) had higher rates of cardiovascular mortality (hazard ratio [HR] 1.26, P < .001) and all-cause mortality (HR 1.23, P < .002) compared with patients with lower concentrations and those on placebo. In contrast, patients with low concentrations had lower mortality rates compared with those on placebo; hence the dosing recommendations in current guidelines.
Get with the Guidelines. With the advent over the past 3 decades of multiple drugs that reduce mortality, digoxin use for heart failure has decreased significantly. Data from more than 117,000 patients with HFrEF enrolled in the Get with the Guidelines registry between 2005 and 2014 showed that, over time, prescriptions for digoxin decreased substantially, from 33.1% of all patients with HFrEF in 2005 to 10.7% in 2014 (P < .0001), a 68% relative reduction.9
Goldberger and Alexander10 similarly showed that office visits for digoxin therapy for heart failure in the United States declined by 91%, from more than 2.5 million visits in 1997 to fewer than 500,000 in 2012.
Atrial fibrillationThe 2023 AHA/ACC and Heart Rhythm Society guidelines for the management of patients with atrial fibrillation5 state that digoxin can be considered as a rate-control agent, albeit not as a first-line agent and usually in conjunction with beta-blockers or nondihydropyridine calcium channel blockers. The class of recommendation is 2a (moderate), level of evidence B-R.
Digoxin can be particularly helpful in patients with atrial fibrillation associated with severe left ventricular dysfunction and heart failure. Likewise, it is helpful in patients who cannot tolerate other rate-control drugs, patients with hypotension or borderline low blood pressure who cannot tolerate beta-blockers or calcium channel blockers for rate control, or patients in whom cardioversion is contraindicated due to risk of stroke. It is not recommended in patients with preexcitation and atrial fibrillation.5
Data on digoxin in atrial fibrillationIn several studies conducted over the past decade, 23%11 to 33%12 of patients with atrial fibrillation were receiving digoxin at baseline, and several suggested that digoxin may be associated with higher mortality rates. For example:
TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in Atrial Fibrillation),11 using data from more than 100,000 patients with atrial fibrillation in the Veterans Health Administration healthcare system between 2003 and 2008, showed that those treated with digoxin had higher mortality rates than those not treated with digoxin, even after adjusting for drug adherence:
After multivariate adjustment: HR 1.26, 95% CI 1.23–1.29, P < .001
After propensity matching: HR 1.21, 95% CI 1.17–1.25, P < .001.
The ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation)13 similarly showed that patients with digoxin levels of 1.2 ng/mL or greater had a higher risk of death (adjusted HR 1.56, 95% CI 1.20–2.04, P < .001) compared with those not on digoxin. For sudden cardiac death, the number needed to harm was 180 for the first year of use and 56 at 2 years.
Vamos et al14 performed a meta-analysis of 37 trials of digoxin therapy for both heart failure and atrial fibrillation and found a higher risk of death in patients taking digoxin (HR 1.17, 95% CI 1.05–1.29, P < .01). The increase was higher in patients taking digoxin for atrial fibrillation (HR 1.23) than in those taking it for heart failure (HR 1.11).
DIGOXIN HAS INOTROPIC AND OTHER EFFECTSEmbedded in the cell membrane of cardiac myocytes is an important molecule: sodium-potassium adenosine triphosphatase (ATPase). This molecule pumps sodium ions out of the cell and potassium ions in, so that there is more sodium outside than in the cell, and more potassium inside than out. At the same time, another pump, the sodium-calcium exchanger, takes advantage of this sodium gradient to let sodium ions back into the cell while pumping calcium out.
Digoxin inhibits sodium-potassium ATPase, so that there is more sodium inside the cell and therefore less of a sodium gradient. In turn, the sodium-calcium exchanger cannot pump as much calcium out of the cell, resulting in higher intracellular concentrations of calcium. The excess calcium binds with troponin C and other contractile proteins that rely on calcium coupling, thus leading to an enhanced myocardial inotropic response and increased force of contraction.
Digoxin’s effect on cardiac contractility is seen primarily in patients with decreased left ventricular function, in whom digoxin improves left ventricular ejection fraction and decreases pulmonary capillary wedge pressure. These effects are not seen in patients with normal left ventricular ejection fraction.15
But there are dangers. More calcium inside the cell leads to inactivation of L-type calcium channels (the main route for calcium entry into cardiomyocytes), which shortens the duration of the action potential and refractory period of cardiomyocytes, a mechanism that favors reentry arrhythmias. Less potassium and more sodium in the cell lead to increased diastolic repolarization and automaticity, which may favor supraventricular arrhythmias and lead to rapid spontaneous rhythms of Purkinje fibers.15 At higher digoxin concentrations, the sarcoplasmic reticulum becomes overloaded with calcium and can spontaneously release enough calcium to depolarize the cell, resulting in extrasystoles, bigeminy, and a higher risk of ventricular fibrillation.
In the autonomic nervous system, digoxin decreases the sympathetic response and increases the parasympathetic response, mainly by stimulating the central vagal nucleus. It also restores baroreceptor sensitivity, which is attenuated in low-output heart failure, and as such improves heart rate variability and decreases catecholamine release.
In conjuction with digoxin’s inotropic effect, these neurohormonal changes lead to favorable hemodynamic changes in heart failure. Decreased preload and afterload with increased contractility lead to reduced chamber dilation and wall stress, thereby reducing myocardial oxygen consumption. The vagal (parasympathetic) effects of digoxin result in a lower sinus rate, decreased automaticity and conduction velocity, and a prolonged refractory period of the atrioventricular node, which makes it effective for rate control in atrial fibrillation.15
PHARMACOKINETICS AND DOSINGDigoxin has an oral bioavailability of about 70%. In some individuals, gut microflora can metabolize digoxin and decrease its bioavailability. Twenty-five percent of serum digoxin is albumin-bound, and its volume of distribution is large (5–10 L/kg) due to extensive binding to muscle tissue. The drug penetrates the blood-brain and placental barriers and cannot be removed from plasma with dialysis. Serum digoxin levels are typically checked at least 6 hours after an oral dose.16
The onset of action after an oral dose is at about 2 hours, and the peak effect is at 6 hours. Given intravenously, the onset of action is within 5 to 30 minutes, with maximum effect within 1.5 to 4 hours. Digoxin is excreted primarily by the kidneys; its half-life is 36 to 48 hours in patients with normal kidney function, but up to 6 to 8 days in anuric patients.16
When used for heart failure, digoxin is given in daily oral doses, without the need for a loading dose, and it reaches a steady-state plateau concentration after 4 to 5 half-lives, roughly 6 to 8 days.16
When digoxin is used for rate control in atrial fibrillation, intravenous loading is usually required for faster onset of action. In this setting, an initial intravenous dose of 0.25 to 0.5 mg is given over several minutes, followed by 0.25 mg every 6 hours for a total of 0.75 to 1.5 mg over 24 hours (10–12 μg/kg of lean body weight). For patients with low body weight (ie, 45–70 kg), digoxin loading should be limited to 0.75 to 1.0 mg in the first 24 hours.5,16
MANY DRUGS INCREASE DIGOXIN LEVELSDigoxin has several drug interactions that can predispose to toxicity (Table 2).17,18
TABLE 2Drug interactions that increase the risk of digoxin toxicity
P-glycoprotein inhibitors. P-glycoprotein is a drug efflux pump that mediates secretion of digoxin in the kidney, liver, and gut. Drugs that inhibit P-glycoprotein raise the serum level of digoxin and can lead to toxicity. These include several antiarrhythmics such as amiodarone, quinidine, dronedarone, nondihydropyridine calcium channel blockers, propafenone, and flecainide, as well as other drugs such as clarithromycin, cyclosporine, and itraconazole.17,18 Quinidine can double the serum digoxin concentration, and amiodarone increases it by 60%.
Digoxin dosing should be reduced, typically to half of the previous dose, when it is given concomitantly with most P-glycoprotein inhibitors. Digoxin levels should be checked 1 week after starting these drugs.
Some antibiotics can decrease initial degradation of digoxin by gut microflora and thereby increase its absorption. In about 10% of patients, digoxin undergoes sequential hydrolysis in the proximal gastrointestinal tract. Macrolides and tetracycline increase serum digoxin levels by inhibiting this mechanism, and digoxin levels should be closely monitored when giving these antibiotics.
Diuretics can increase serum digoxin concentrations by decreasing the glomerular filtration rate and causing hypokalemia, which increases digoxin’s potential for arrhythmias.
INTERACTIONS WITH CATIONSHyperkalemia reduces digoxin’s binding affinity for sodium-potassium ATPase. On the other hand, hypokalemia reduces repolarizing potassium currents in the action potential, leading to increased diastolic depolarizations and automaticity and thus enhancing the arrhythmogenic effects of digoxin.
Hypercalcemia and hypomagnesemia contribute to calcium overload in the sarcoplasmic reticulum and therefore promote spontaneous depolarizations.17
CLINICAL PRESENTATION OF TOXICITYThe annual incidence of digoxin toxicity is difficult to accurately define, but older reports claim it to be as high as 13% to 25% of all patients who are prescribed the drug.17 Risk factors for digoxin toxicity have been widely studied (Table 3).17–20 Because it produces a wide variety of symptoms, digoxin toxicity is easy to suspect, but proving that the symptoms are due to digoxin toxicity is harder. Toxicity is more common with levels higher than 2.0 ng/mL.
TABLE 3Risk factors for digoxin toxicity
A study of all patients who were admitted to a Boston hospital who were taking digoxin during an 8-month period in 1969–1970 reported a prevalence of toxicity of 23%.21 In patients with confirmed toxicity, the mean serum digoxin concentration was 2.3 ng/mL (± 1.6 ng/mL standard deviation), compared with 1.0 ± 0.5 ng/mL in patients without toxicity. Of note, there was significant overlap in serum levels between patients with or without toxicity, as some patients are unusually sensitive to the drug.
Digoxin toxicity has a variety of symptoms (Table 4).15,19,20,22,23 Cardiac arrhythmias are the most frequent side effect (90% of patients), followed by gastrointestinal symptoms (55%) and central nervous system symptoms (12%).21
TABLE 4Clinical manifestations of digoxin toxicity
Cardiac manifestations of digoxin toxicity include virtually any type of arrhythmia and are the most serious and potentially lethal complications of toxicity. Digoxin toxicity can lead to all degrees of atrioventricular block and result in clinically significant bradycardia that can be refractory to pacing, as well as sinus arrest and sinus exit block through its action on the sinus node. Ventricular ectopy is an early sign of digoxin toxicity but is not always present. Bidirectional ventricular tachycardia (Figure 1) and nonparoxysmal junctional tachycardia (> 80 beats per minute) are suggestive of but not specific to digoxin toxicity.22 Enhanced automaticity can lead to supraventricular tachycardia as well as ventricular tachycardia and fibrillation.17,23
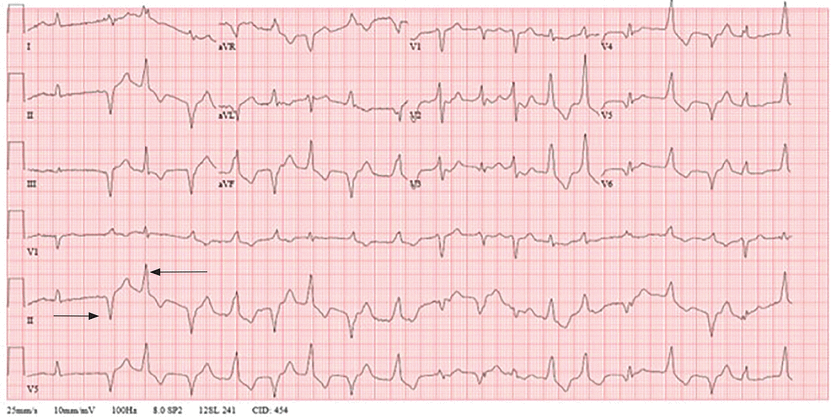
 Figure 1
Figure 1 Bidirectional ventricular tachycardia in a patient with digoxin toxicity. The QRS axis alternates with each QRS complex (see rhythm strip for lead II).
Other electrocardiographic changes of digoxin toxicity include PR prolongation, shortening of the QT and QTc intervals, and a change in ventricular repolarization resulting in nonspecific ST-segment depressions classically described as “sagging” depressions (Figure 2). These changes do not imply toxicity and can be present with therapeutic drug levels.17

 Figure 2
Figure 2 Electrocardiogram showing “sagging” ST depressions, most notably in leads V3–V6 and lead II, and ventricular ectopy in a patient with digoxin toxicity with a serum level of 8.0 ng/mL (normal range 0.6–1.2).
Gastrointestinal. Nausea, anorexia, and fatigue are common, with anorexia present in up to 61% of individuals.21 In rare instances, excessive smooth muscle contraction of visceral arteries can lead to mesenteric ischemia manifested with abdominal pain, diarrhea, and gastrointestinal bleeding.
Central nervous system. Visual disturbances can be present and are described as flashing lights, halos, and green-yellow perception impairment. Mental status changes such as confusion, hallucinations, or apathy can be present, especially in the elderly.15
Toxicity can also manifest with hyperkalemia (because less potassium is being pumped into the cells via sodium-potassium ATPase, resulting in elevated extracellular potassium).15 Attributing these symptoms to digoxin toxicity is often difficult, as some of them are also commonly attributed to cardiovascular disease.
The evaluation of a patient with suspected digoxin toxicity should include an electrocardiogram to assess for arrhythmias and changes associated with digoxin toxicity. Laboratory evaluation should include measurement of digoxin levels as well as renal function and electrolyte disturbances such as hypokalemia, hypercalcemia, and hypomagnesemia, as these are often predisposing factors for toxicity.
MEASURING DIGOXIN LEVELSSerum digoxin levels are usually measured with immunoassays that measure total digoxin levels, including bound and unbound molecules. When starting therapy, measuring the digoxin level is usually recommended after achieving a steady state, 1 to 2 weeks after initiating therapy.
Because patients with elevated digoxin concentrations (> 1.2 ng/mL) may have no signs of toxicity, and because elevated levels have been linked to increased mortality, measuring serum digoxin levels is recommended to titrate dosing to a goal therapeutic range (Table 1). Serum levels should ideally be obtained at least 6 hours after the last dose to avoid falsely elevated results, as complete redistribution of digoxin into body tissues takes several hours.
Immunoassays also identify digoxin-like immunoreactive substances, endogenous molecules equivalent to digitalis that cross-react with many of the older available immunoassays. Digoxin-like immunoreactive substances have been found in neonates and older children, adults with renal insufficiency, hepatic disease, and hypertension, transplant recipients, and pregnant women, increasing the risk for falsely positive results in these populations.24 Older immunoassays for digoxin have also been known to interact with spironolactone, digoxin-fab, the Chinese medicine Chan Shu, and herbal supplements with oleander and lily of the valley extracts.24 The presence of any of these substances in serum can cause falsely elevated levels of serum digoxin when using available immunoassays.
MANAGEMENT OF DIGOXIN TOXICITYDigoxin-specific antibody fragments (digoxin-fab) were first developed in 1967 for immunoassays to measure digoxin levels, and were first used to treat acute digoxin toxicity in 1976. Before they became readily available in the 1980s, treatment of digitalis toxicity included stopping the drug and giving supportive therapy with hydration, correcting electrolyte abnormalities, and treating cardiac arrhythmias; mortality rates were as high as 20% to 30%.25 Quantitative serum digoxin measurements and antibodies to treat digoxin toxicity have reduced the digoxin-associated mortality rate to 3.7% (in-hospital) to 10% (30-day) in the past decade.2
Supportive therapySupportive therapy with intravenous fluids should be given if the patient has dehydration due to nausea and vomiting. Activated charcoal can be used for patients with acute intoxication if digoxin was ingested less than 2 hours before presentation.
If symptomatic bradycardia is present, atropine can help improve the heart rate temporarily by decreasing vagal tone, but its effects are usually transitory. Transvenous pacing can often result in iatrogenic arrhythmias in the setting of digoxin toxicity and therefore should be avoided unless bradycardia is refractory to atropine.26
Electrolyte abnormalities such as hypokalemia and hypomagnesemia should be corrected, as these can potentiate toxicity. Hyperkalemia should be corrected without using calcium salts, as these can worsen intracellular hypercalcemia and worsen spontaneous cardiac depolarizations.
Digoxin-fabMild cases of toxicity might resolve if digoxin therapy is simply stopped, but severe cases with bradycardia or ventricular arrhythmias generally require the use of digoxin-fab. It is indicated in patients with life-threatening tachy- or bradyarrhythmias, hyperkalemia (serum potassium > 6 mmol/L), or hemodynamic instability with end-organ dysfunction with elevated serum digoxin concentrations (> 2 ng/mL) that suggest digoxin is the cause.27
Digoxin-fab is an ovine (sheep) monovalent immunoglobulin with 100 to 1,000 times more affinity for digoxin than digoxin’s binding site in sodium-potassium ATPase.27 It rapidly binds free digoxin in the serum and creates a gradient for intracellular digoxin to move into the serum, where it is subsequently bound by antibodies.
Digoxin-fab is eliminated by the kidneys and liver; it has a half-life of 19 to 30 hours, but this can increase up to 10 times in patients with renal dysfunction. The onset of action to reversal of digoxin toxicity after acute ingestion is around 30 to 45 minutes.27 The main adverse effect is a hypersensitivity reaction to sheep protein.
Dosage and administration. One vial contains 38 to 40 mg of digoxin-fab, which binds approximately 0.5 mg of digoxin. In the case of acute ingestion in which the total ingested dose is known, the number of required vials is calculated by dividing the total body load (ingested dose × 0.8) by 0.5 and rounding up to the nearest digit.28 If steady-state serum digoxin levels are known in a stable patient with chronic toxicity, the dose of vials can be calculated by dividing the product of the serum concentration (in ng/mL) and the patient’s weight (in kg) by 100 and rounding up to the nearest digit (Figure 3).29

 Figure 3
Figure 3 Treatment of severe digoxin toxicity with digoxin-fab.
aThe calculated number of vials should be rounded up to the nearest digit. Each vial contains 38 to 40 mg of digoxin-fab.
Based on information from reference 29.
If the digoxin level is not known or cannot be accurately measured due to recent ingestion (< 6 hours), 2 vials can be given, with repeated dosing if there is no apparent clinical response.29 This approach can also be used if a patient has relative hemodynamic instability and waiting for serum digoxin levels is impractical.
The use of digoxin-fab can precipitate rebound heart failure or atrial fibrillation due to the sudden binding of free serum digoxin. If this is a clinical concern, half of the calculated dose can be given instead.
In general, vials should be administered over 30 minutes, unless a patient is in imminent cardiac arrest, in which case 10 vials (or 5 vials for pediatric patients) can be given empirically over several minutes.
Digoxin-fab causes redistribution of digoxin from tissues into serum, and digoxin bound to antibodies is also recognized by immunoassays, both of which can result in rising levels of serum digoxin if these are checked after digoxin-fab administration. In general, digoxin levels should not be used for clinical decision-making up to 3 weeks after using digoxin-fab, since assays will measure antibody-bound digoxin as well as unbound digoxin in serum. Because antibody half-life increases up to 10-fold in patients with renal dysfunction, these patients might require closer monitoring and even measurement of digoxin-binding antibodies before digoxin therapy is restarted.27
A significant limitation of digoxin-fab is its cost. Although the direct cost to patients varies widely based on insurance coverage,30 the only commercially available digoxin-fab in the United States (DigiFab, BTG Pharmaceuticals, Conshohocken, PA) currently costs about $5,000 per 40-mg vial of intravenous powder for injection.
Digoxin-fab is used in about 20% of cases of reported digoxin toxicity.31 Its utility has been elucidated mostly by case series, which report a response rate of 50% to 90%.32,33 While its use may show a nonsignificant trend toward lower rates of mortality at 30 days and overall, the efficacy of digoxin-fab is unclear due to the lack of high-quality evidence and the fact that it is used more frequently in patients with underlying comorbidities (mainly heart failure) or with acute intoxication, likely representing a sicker population. Nonetheless, and despite its declining use over several decades, digoxin-fab is the mainstay of treatment for severe digoxin toxicity. Further research into the appropriate dosing and administration of these antibodies is required, given the paucity of high-quality evidence on the management of digoxin toxicity.
A CHANGING LANDSCAPEThe landscape of digoxin use has changed over the past decades. Multiple associations with digoxin use and increased mortality in heart failure and atrial fibrillation, especially with higher serum concentrations, have raised concerns about the use of this medication. Due to digoxin’s narrow therapeutic window, dependence on renal clearance, and multiple drug interactions, digoxin toxicity occurs often and remains an important clinical entity despite a decreasing trend in digoxin use.
Toxicity has a wide range of symptoms and cardiovascular effects that can result in potentially fatal arrhythmias and death, and therefore digoxin use must be monitored carefully, with knowledge of the drug’s pharmacokinetic profile. The availability of digoxin-specific antibody fragments has allowed prompt treatment of severe cases of digoxin toxicity associated with life-threatening arrhythmias.
DISCLOSURESThe authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.
留言 (0)