Diabetes technology is evolving rapidly and is changing the way both patients and clinicians approach the management of diabetes. With more devices gaining US Food and Drug Administration approval and insurance coverage expanding, these new technologies are being widely adopted by people living with diabetes. We provide a summary of the commonly available devices in the market today that clinicians will likely encounter. This includes continuous glucose monitors (CGMs); connected insulin pens, caps, and buttons; and insulin pumps. Clinicians’ awareness of and familiarity with this technology will enhance its accessibility for patients with diabetes.
KEY POINTSCGMs measure interstitial glucose and transmit data wirelessly to a receiver. Evidence supports their use in patients on insulin therapy or who are at a high risk of hypoglycemia.
Connected insulin pens, caps, and buttons act as bridges between traditional insulin pens and insulin pumps. The technology allows patients to calculate how much insulin to take, accounting for carbohydrate intake, glucose level, and, in some models, previous insulin dose.
Insulin pumps deliver rapid-acting insulin continuously via the subcutaneous route either independently (open loop) or in association with CGMs (automated insulin delivery).
Technology in diabetes management has come a long way. Devices measure glucose levels continuously (continuous glucose monitors, or CGMs), make it easier for patients to calculate insulin doses (smart pens, caps, buttons), and deliver insulin based on an algorithm (hybrid closed-loop insulin pumps). These devices help patients manage their diabetes in a manner consistent with their goals and lifestyle.
CGMs are increasingly available, providing information that clinicians can use to adjust medication doses or recommend lifestyle modifications. Insulin pump initiation and management traditionally has been the domain of endocrinologists. The introduction of newer, more simplified pumps, such as those that deliver basal insulin only or mealtime insulin only, however, may allow for increased management in primary care settings. Further, nonspecialty clinicians who are aware of pump basics can respond appropriately in urgent situations.
This review of the basics of various diabetes management devices is intended to enhance clinicians’ comfort level in helping patients use these technologies. It is especially advisable to develop a working knowledge of CGMs, which are widely available. We acknowledge that there are other devices available in the market that are beyond the scope of this review, and more devices may become available through FDA approval after this article is published.
CONTINUOUS GLUCOSE MONITORSA CGM system consists of a sensor, transmitter, and receiver. All models measure glucose in the interstitial fluid, with most doing so via a filament-like sensor inserted through the skin (Figure 1); one model is implanted subcutaneously via a tiny incision.1 Data from the sensor are transmitted wirelessly to the receiver, either a cell phone or a dedicated reader.2 In some models, the sensor and transmitter are 1 combined piece that is disposable. Other systems have reusable transmitters that may need to be charged.
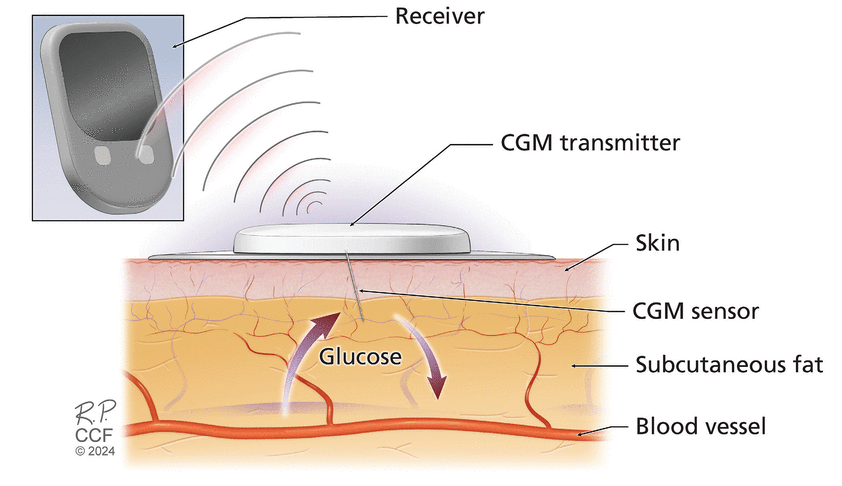
 Figure 1
Figure 1 Schematic illustration of components of a continuous glucose monitor (CGM) system.
Professional CGMs are owned by a hospital or a practice and are worn temporarily by the patient. Patient-owned, or personal, CGMs are becoming more common; these are classified as intermittently scanned or real-time.3 Most CGMs (eg, Freestyle Libre 2, Freestyle Libre 3, Dexcom G6, and Dexcom G7) do not need calibration with fingerstick blood glucose monitors, although some, like Eversense E3 and Guardian Connect, need 1 to 2 calibrations daily to enhance sensor accuracy (Table 1).4–13
TABLE 1Continuous glucose monitors
Intermittently scanned CGM devicesIntermittently scanned devices measure glucose levels and capture data continuously throughout the day. To save the data, the devices’ sensors must be manually scanned with a smartphone or reader at least every 8 hours.1
The Freestyle Libre 2 is a coin-shaped intermittently scanned device with an easy applicator for applying the sensor to the body. The patient needs to scan the sensor with a compatible reader or smartphone. The sensor lasts for 14 days and has a 1-hour warm-up time (the time from applying the sensor to display of the first available glucose reading). Advantages of the device are its affordability and availability, but if the patient does not scan the sensor at least every 8 hours, the resulting gaps in data transmission make interpretation more difficult.
The Freestyle Libre 2 Plus is a recently announced modification of the Libre 2 system. Significant changes include 15-day wear time, higher vitamin C threshold (1,000 mg) before interactions (falsely elevated glucose readings) occur, and integration with insulin pumps, ie, the ability of the sensor to communicate with an insulin pump.4 This modification also has a lower mean absolute relative difference (MARD) of 8.2%. MARD is the difference of the CGM reading from the reference or standard-of-care measurement; a lower MARD indicates greater accuracy. The Freestyle Libre 2 Plus transmits CGM data via Bluetooth to the insulin pump without a scan, but the patient needs to scan the sensor to display a blood glucose reading on the display device.
Real-time CGMsReal-time CGMs, which also capture glucose data continuously throughout the day, need no participation from the patient except when the sensor needs to be replaced. Data are transmitted wirelessly to the patient’s smartphone or reader. While many sensors work with smartphones, it is important to verify compatibility with the patient’s smartphone. If a sensor is incompatible, then the standalone reader would need to be prescribed. Most manufacturers specify the compatibility of their devices on their websites.
The Freestyle Libre 3 sensor lasts for 14 days. This real-time CGM can be used with the Freestyle Libre 3 application on a smartphone, but also works with a standalone reader. Freestyle Libre 3 has an improved MARD compared with Freestyle Libre 2.5 The Freestyle Libre 3 uses Bluetooth, improving its connectivity and data transfer compared with Libre 2, which uses near-field communication, a wireless technology that requires a distance of 4 cm or less between devices for connection to occur.
The latest models of the Dexcom system are G6 and G7.6,7Dexcom G6 requires 2 replaceable parts to function: a sensor that needs to be replaced every 10 days and a transmitter that needs to be replaced every 3 months. Its warm-up time—how long until a glucose reading is available after the CGM is inserted—is 2 hours.6 A smartphone or reader is needed to collect data.8Dexcom G7, cleared by the US Food and Drug Administration (FDA) in December 2022,9 has several advantages over its predecessor. It has a shorter warm-up period (30 minutes), the MARD is lower (8.2% vs 9.0%), and the sensor and transmitter have been integrated into a single device that requires replacement every 10 days, with a 12-hour grace period.
Eversense E3 is unique among CGMs in that its sensor is implanted in the patient’s subcutaneous tissue for long-term (up to 6 months) glucose monitoring. The sensor communicates via a rechargeable transmitter that is attached to the skin over the sensor using an adhesive patch and wirelessly (Bluetooth) transmits data to a mobile device. The glucose data are displayed on a smartphone application; no dedicated reader is available. A trained healthcare professional can manage the insertion and replacement process during an office visit.10,11
Guardian Connect consists of a sensor and transmitter that act as a standalone CGM. It works exclusively with smartphone applications; the sensor lasts for 7 days and needs to be calibrated twice daily (ie, at least every 12 hours) with a fingerstick glucose monitor to maintain accuracy. The transmitter needs to be charged weekly and is typically used for 1 year or longer.12,13
Which patients are candidates for a CGM?The distinct advantage CGMs have over traditional fingerstick glucose checks is fewer severe hypoglycemic and hyperglycemic episodes.3 CGMs were developed for patients who were using insulin pumps or needed multiple daily insulin injections. Now, with increased availability of CGM devices and their ability to support meaningful lifestyle changes, patients with diabetes can benefit from continuous glucose monitoring regardless of medication regimens, glucose patterns, or risks of hypoglycemia. Even patients with a “smoldering” hemoglobin A1c of 7.5% to 8.5% may benefit from a CGM. While hemoglobin A1c is a good tool for assessing glycemic control, it does not provide detailed analyses such as postprandial spikes or undetected early morning hypoglycemia during sleep.14 CGMs can be useful in this setting, even in seemingly stable patients with diabetes, to guide medication and behavioral changes.
The patient’s preference, comfort and ease with technology, willingness to engage with the device, caregiver support, interacting medications, and insurance coverage, along with device affordability, are important factors in determining which CGM is appropriate.3,15
Except for Eversense E3, CGM sensors must be removed for computed tomography scans and magnetic resonance imaging.16 The Eversense E3 transmitter must be removed, however. Certain drugs can interfere with readings: vitamin C can affect readings from Freestyle Libre CGMs, particularly at high doses17; acetaminophen at doses greater than 4 g daily along with hydroxyurea can affect readings from Guardian Connect and Dexcom G6 and G716,18; and tetracycline and mannitol can affect Eversense E3 readings.11,19
PENS, CAPS, AND BUTTONSSmart insulin pens and associated smart caps and buttons can help address hurdles such as missed doses and suggest correction doses for hyperglycemia. These devices can act as a bridge for patients on insulin who are interested in insulin pumps but are not ready to adopt them yet.
Smart insulin pensMost people with diabetes face challenges regarding timing of injections and administering the correct dosage of insulin. Bluetooth- or near-field communication–enabled smart insulin pens communicate with smartphone applications to monitor insulin administered at different times of day. Smart insulin pens keep track of insulin doses administered and active insulin on board; many have an insulin bolus calculator that enables the user to calculate mealtime insulin doses based on the amount of carbohydrates they are about to consume and their glucose level prior to the meal. Specialized short-acting insulin cartridges like Humalog and NovoLog need to be prescribed separately for use with these devices.
The InPen is a good example of a smart insulin pen available in the United States. It can communicate with CGM applications (eg, Guardian Connect, Dexcom G6, Dexcom G7) to keep track of both blood glucose levels and administered insulin.20,21 It can also alert the user to a missed basal or bolus insulin dose, thus preventing large fluctuations in blood glucose levels.
Smart caps and buttonsThe Bigfoot Unity diabetes management system offers smart caps, devices that attach to commercially available short- or long-acting disposable insulin pens. In this system, the patient scans the Freestyle Libre 2 sensor with a smart cap that is attached to the insulin pen. The cap captures the glucose data and suggests a correction dose based on predetermined settings defined by the healthcare team and programmed into the mobile application. The cap also records the timing of insulin administration to prevent stacking of insulin doses, and will not recommend an additional correction dose within 3 hours of a previous dose.22
The Bigfoot Unity application combines the glucose data from the CGM with insulin doses administered and makes it available at a single location for the patient and clinician. The application, which has alarms set for hypoglycemia and missed insulin doses, is currently FDA-cleared for both type 1 and type 2 diabetes.22 The pen caps, white for rapid-acting insulin and black for long-acting insulin, are rechargeable. This system historically has required clinics or hospitals to subscribe to the company to use its devices; however, it is projected to be available through pharmacies in the near future.
The Tempo Smart Button device attaches to a prefilled custom-made manufacturer-branded insulin pen. When the patient presses the button to deliver insulin, it shares data via Bluetooth with a smartphone application, recording the type of insulin, dose, and time of administration. Each button lasts for up to 8 months. The smartphone application integrates insulin dosing data, glucose data obtained from Dexcom G6 or G7, and food, exercise, and sleep data. The application also delivers customized alerts to patients to view their glucose data on a daily or weekly basis and provides alerts on missed doses.23
Which patients are candidates for smart pens, caps, or buttons?A person living with diabetes who needs multiple daily insulin injections and has access to a smartphone and suitable applications might consider smart pens, caps, or buttons as an alternative to insulin pumps. These devices are most useful for patients who struggle with dose calculations or who miss or forget doses and may benefit from missed-dose alerts.24,25 These options also might be preferable for patients who are less comfortable with technology.
PUMPS AND CONTINUOUS INSULIN INFUSIONInsulin pumps, used for continuous subcutaneous insulin infusion, deliver rapid-acting insulin at a preset per-hour dose throughout the day for basal needs. Patients must self-administer mealtime boluses with the pump itself based on the amount of carbohydrates in their upcoming meal. Most currently available pumps have a reservoir that stores the insulin and tubing that connects the reservoir to a cannula inserted into the skin. Examples of available pumps are the t:slim X2, Mobi, MiniMed, and iLet ACE. Omnipod DASH and Omnipod 5 are examples of tubeless, or patch, pumps.
Insulin pumps can be broadly divided into systems based on their level of automation and interaction with CGMs.
Manual or open loopThese systems deliver basal insulin based on predetermined settings without regard to CGM readings. In such systems, the CGM device and insulin pump act as separate entities and do not interact.
Automated insulin deliveryWith these systems, CGMs and insulin pumps are programmed to share information, allowing for insulin administration based on CGM data.
Low glucose suspend systems are insulin pumps that shut off insulin delivery to minimize hypoglycemia. They do this in 1 of 2 ways:
Suspend at low glucose value. The MiniMed Paradigm series, no longer available for purchase in the Unites States, suspends insulin delivery and alerts the user when a preprogrammed low glucose threshold is reached; the MiniMed 670G, 770G, and 780G pumps can suspend insulin delivery in manual mode (in manual mode, the pump does not take into account the glucose values from the CGM and delivers insulin via basal rates independently)
Suspend at predicted low glucose value. Pumps such as the t:slim X2 with Basal-IQ predict the onset of hypoglycemia and suspend insulin delivery before it occurs; the MiniMed 670G, 770G, and 780G pumps can also do this in manual mode.
Hybrid closed-loop systems use readings from a CGM and deliver basal insulin using a proprietary algorithm, with or without the need to input basal insulin rates into the pump. Alternatively, the endocrinology team inputs basal rates and the pump uses an algorithm to deliver a fraction of a correction dose hourly to counteract hyperglycemia. Over time, in pumps such as the MiniMed 780G or Omnipod 5, the pump targets a glucose level rather than a preset basal rate. Insulin delivery is reduced or stopped when patients are predicted to develop hypoglycemia. Hybrid closed-loop systems require patients to push buttons to tell the pump to deliver mealtime insulin boluses.
Examples of hybrid closed-loop pumps include MiniMed 670G, 770G, and 780G, t:slim X2, Mobi with Control-IQ, and Omnipod 5. The MiniMed pumps work in closed loop only with the proprietary Guardian sensor. The t:slim X2 and Omnipod 5 are cleared by the FDA to work with the Dexcom G6 glucose monitor. The FDA has also cleared the Dexcom G7, Freestyle Libre 2 Plus, and Libre 3 CGMs, as well as interoperable CGMs (which can integrate with insulin pumps form various manufacturers), for integration with certain insulin pumps. These integrations are currently available with the t:slim X2 (with the Freestyle Libre 2 Plus and Dexcom G7 CGMs) and iLet ACE or Bionic Pancreas (with Dexcom G7) in the US market.9,26
The Mobi is smaller than other available pumps. It has a shorter (5-inch) tubing option and can be taped onto the skin and controlled with a compatible smartphone. Although FDA-cleared, the Mobi is not yet available in the consumer market. Because the Mobi uses the same “Control IQ” algorithm as the t:slim X2, it integrates with Dexcom G6 and it is anticipated that it will integrate with Dexcom G7. Integration of the Mobi pump with the Freestyle Libre 2 Plus CGM has not yet been announced.27
The iLet Bionic Pancreas, a hybrid closed-loop insulin pump, was cleared by the FDA in May 2023 for patients with type 1 diabetes.28 Its algorithm uses the patient’s body weight and glucose targets to determine basal insulin rates; instead of entering carbohydrate values, the patient indicates their meal size (usual, smaller or larger than usual). The device can be used with Dexcom G6 and G7.
Do-it-yourself hybrid closed-loop systems are open-source software systems that use a smartphone application and bridging devices to simulate hybrid closed-loop communication between a CGM and insulin pump. In 2023, the Tidepool application, which originated from a do-it-yourself program, received FDA clearance.29
Closed-loop systems, currently being researched and not yet available, would require no user input and ideally would calculate mealtime bolus dosing automatically. The highest-level closed loop system should counteract hypoglycemia by administering glucagon.
Patch pumpsMechanical disposable patches such as V-Go and Simplicity contain rapid-acting insulin like lispro or aspart. V-Go delivers insulin at a steady rate for 24 hours through a needle. When bolus insulin is needed at mealtime, the patient clicks a button on the pump to deliver 2 units of insulin. V-Go does not require a battery. It has different fixed-dose capacities consisting of 20, 30, and 40 units delivered at a uniform rate over 24 hours. It can deliver up to 36 units of bolus insulin in a 24-hour period. The patient needs to change the pump every day.30 The Simplicity patch is a bolus-only pump. It can be worn for 3 days and holds up to 200 units of rapid-acting insulin. Patients may use it to deliver a bolus before meals; each click delivers 2 units of insulin. These devices can be useful when patients do not want to use pens or needles and prefer discretion in public places.31
Basal-only patch pump. Apart from the Omnipod DASH and Omnipod 5 discussed above, the Omni-pod GO is a standalone tubeless pump that delivers rapid-acting insulin in 7 different preprogrammed rate options over 72 hours. It was cleared by the FDA in April 2023.32
Which patients are candidates for insulin pumps?Continuous subcutaneous insulin infusion demands more patient involvement than multiple injected doses. Factors to consider include the patient’s prior adherence to treatment, mental and psychological status, device preferences, and availability for follow-up visits, as well as device affordability for the patient.3,15,33 Automated insulin delivery systems are now the standard of care for patients living with type 1 diabetes.34 Automated insulin delivery- or sensor-augmented pumps may also be considered in other forms of insulin-deficient diabetes, including type 2 diabetes.
There are important considerations regarding insulin pumps. Patients must remove insulin pumps before undergoing computed tomography and radiography (none are radiation-safe), magnetic resonance imaging, and other imaging modalities. When using airport security, patients should avoid full-body scanners and request manual pat-downs and metal detectors. If insulin infusion is suspended for more than 1 to 2 hours, the patient needs to inject long-acting insulin to avoid complications such as diabetic ketoacidosis.
CLOSING THOUGHTSThe American Diabetes Association recommends that automated insulin delivery systems be offered to patients with type 1 and other forms of insulin-deficient diabetes, with the choice based on the patient’s circumstances, preferences, and needs.34 Clinical trials provide evidence of reduced A1c levels and improved time in goal range.3 Time below the desired glucose range is often reduced as well through features that can decrease or suspend insulin delivery based on predicted low glucose levels.35 Pivotal clinical trials for available systems show that all can achieve the recommended time-below-range targets of less than 4% of time spent below 70 mg/dL and less than 1% of time below 54 mg/dL. However, there are few head-to-head comparisons, and the patient populations and trial designs differed, so it is a challenge to make direct comparisons in terms of hypoglycemia.36
Several device options can improve outcomes or decrease the mental burden in people living with diabetes. Increased comfort level with this technology among clinicians will benefit patients. We encourage primary care physicians to work with endocrinology colleagues to identify patients who are candidates for these devices, ensure that patients who are candidates for these devices are offered the options to use them, and arrange timely access to them.
DISCLOSURESDr. Isaacs has disclosed teaching and speaking for Abbott Diabetes Care, Dexcom, Eli Lilly, and Mannkind Corporation, and consulting for Eli Lilly, Insulet Corporation, Medtronic, and Novo Nordisk. Dr. Lansang has disclosed receiving research funding support from Abbott, serving as a research principal investigator for Abbott and Dexcom, and conducting research for Xeris. Dr. Ambalavanan reports no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.
留言 (0)