Palliative care (PC) is a key component of the comprehensive treatment of children with cancer and their families who have to cope with physical, psychological, social and spiritual suffering because of the disease and treatment. Paediatric PC has emerged as a speciality to address these problems scientifically and methodically and to help children and families improve their quality of life through the disease and grief of the child.[1] Among paediatric cancers, metastatic neuroblastoma (NB) is associated with suboptimal cure rates and limited lifespan despite all aggressive oncological management.[2] Affected children have high symptom load due to primary and metastatic disease, toxicities related to the treatment and early relapses.[3] Their families need psychosocial support to cope with the illness, grave prognosis, disease progression and imminent death of their child. It represents a prototype in childhood cancer where early integration of PC and sharing the care along with the paediatrician can result in a significant impact on the quality of care provided to the patient and family. We studied the children with metastatic NB treated in our hospital to define the role of PC in their illness trajectory and the effectiveness of hospital-based PC services in catering to these patients.
MATERIALS AND METHODSThis is a retrospective study of case records of all children 1–14 years of age with Stage 4 NB treated at our centre over 10 years (1 January 2008 to 31 December 2017). The diagnosis of metastatic NB was established by histopathology of the primary tumour or bone marrow biopsy showing tumour cells and skeletal X-rays showing lytic bone lesions.
Inclusion and exclusion criteriaAll patients over 1 year of age diagnosed with metastatic NB and received treatment at our centre were included in the study. Patients who received treatment elsewhere and those who expired before starting treatment were excluded from the study.
TreatmentThe patients with resectable primary disease, a limited number of metastases and good general condition received 8–12 cycles of moderately aggressive chemotherapy comprising of vincristine, doxorubicin, cyclophosphamide, cisplatin and etoposide, with response assessment after four cycles followed by local treatment (surgery/radiation). If residual disease was unresectable, they received metronomic maintenance chemotherapy with oral cyclophosphamide and etoposide for 6–8 courses. Patients who presented with multiple metastases or poor clinical condition received palliative chemotherapy with 3-weekly vincristine, doxorubicin and cyclophosphamide for 6 cycles. Patients with severe pain (7–10 on faces/numerical rating scale as age-appropriate) were started on opioids after consultation with the PC Department, and their chemotherapy was continued. Patients with progressive disease/relapse were referred to the PC department for best supportive care. The treating team consisted of the paediatric oncologists, PC consultants, nurses and social workers.
Statistical methodsThe descriptive analysis included the absolute and relative frequency for categorical variables. Variables studied were age, primary site, metastatic site, symptom burden, treatment received, relapses, time to death, time to palliative referral, time from palliative referral to death and palliative interventions.
RESULTSThere were 119 patients with metastatic NB, with a mean age of 3.5 years (range 1–14 years) and male: female ratio of 1.1:1 most common site of primary tumour was suprarenal (n = 66, 55.5%), and the most common site of metastasis was bone marrow (n = 67, 76.3%), and bone (n = 13, 15.9%). Thirty-three patients (27.7%) had multiple metastases. The commonest clinical presentations were fever, malaise and pallor (n = 53, 44.5%), followed by abdominal pain and distension (n = 41, 34.5%). Pain was the most common symptom (n = 87, 73%), and 37 patients (31%) presented with severe pain. Nausea and vomiting (n = 13), constipation (n = 8), abdominal distension (n = 33), bony swellings (n = 35), proptosis/periorbital ecchymosis (n = 12), oedema (n = 2) and seizures (n = 6) were noted as the major symptoms at presentation.
Details of symptom burden are depicted in Table 1.
Table 1: Symptom burden at presentation and at PC referral.
Symptom AtEighty-nine patients received cancer-directed treatment, while 30 patients (24.4%) did not receive cancer treatment due to parental refusal. Thirty-eight patients (31.9%) received aggressive treatment with intensive chemotherapy, surgery, radiotherapy and metronomic maintenance chemotherapy. Fifty-one patients (42.8%) presented with advanced disease or poor clinical condition and received palliative chemotherapy. Seventy-four patients (86%) developed disease progression/recurrence within a median time of 10 months (range 1–123 months), and 71 patients (85%) died of the disease. The median time to death was 9 months (range 0–120 months) from diagnosis and 4 months (range 0–12 months) from relapse.
Eighty-seven patients received paediatric palliative care (PPC) consultation at different time points during treatment. Thirteen patients (14.9%) received PC at presentation/early in the course of the disease, whereas 58 patients (66.6) were referred to PC at disease relapse, and 16 (18.3%) received PC intervention at end-of-life only. The patients were directly taken care of by the hospital PC team or linked to local PC services while maintaining telephonic communication with the oncology team. The pain was the prominent reason for referral to PC, and pain management, including opioids and adjuvant pain medications, was provided in all patients. Nausea/vomiting (n = 69), constipation (n = 68) and abdominal distension (n = 67) were the most frequently reported symptoms toward end-of-life, followed by bony swellings (n = 43), proptosis (n = 17), oedema (n = 6) and seizures (n = 3). Palliative radiation was given to six patients, and three patients required drainage of pleural/ascitic fluid. Psychosocial support was provided to families of 53 children by the treating oncologist and PC team whenever required. The mean time from initiation of PC to death was 4.2 months.
Details of palliative intervention are provided in Table 2.
Table 2: Type of PC intervention.
Intervention Percentage (n=87) (%) Pain medication (opioids) 87 (100) Palliative chemotherapy 58 (66.6) Palliative radiotherapy 6 (6.9) Palliative procedures (pleural/ascitic tapping) 3 (3.4) Psychosocial support 53 (61) DISCUSSIONPaediatric PC needs skill, expertise and organisation different from adult PC because the needs of children are unique and developmentally defined and require close integration with the family as a whole.[4] The main challenges to the provision of PPC in low- and middle-income countries (LMICs) are lack of awareness of its role, high burden of disease, lack of access to PPC and financial and workforce constraints.[5] Although paediatric cancers largely have favourable survivals, metastatic NB is one of the life-limiting cancers in children with <20% 5-year survival rates in the LMICs[2] and is mostly treated with palliative intent in resource-limited settings.
Children with metastatic NB have a higher anticipated symptom burden than other PC patients,[6] but not many studies have focused on the PC aspect of this population. Our study revealed barriers to providing optimal PC to metastatic NB patients at multiple levels. Upfront PC was not offered to many families, so they opted for chemotherapy in the hope of a cure and had to stay near the oncology tertiary hospital for supportive care needs for a long duration, causing a considerable financial burden. These patients were initially taken care of by the oncology team alongside patients on curative treatments, and the sudden transfer of care to the PC department at disease progression created distress for most of the patient’s families, who expressed unfamiliarity and lack of trust toward the new team. Due to workforce constraints, PC intervention was limited to initiation of opioids, symptom management and linking to local PC services, and we could not ensure continued psychosocial support, follow-up or documentation of complications, endof-life-care issues or place of death (home/hospital) in most of these patients. The lack of PC-trained paediatricians in the team was also felt as a major disadvantage.
Despite increasing evidence regarding the benefits and recommendations of early PC in paediatric patients, in practice, the referrals to PC are inconsistent and often late in the disease trajectory. Balkin et al. in a questionnaire-based study involving paediatric providers, reported uncertainty in prognosis, non-acknowledgement of poor outcomes by family and time constraints as major barriers in initiating early PC in children.[6] Similar to our findings, Bhat et al., in a retrospective study, have also found a low incidence of PC referral in paediatric cancer patients, mainly for symptom management and psychosocial problems.[7] Another interview-based study in paediatric oncology practice by Cuviello et al. has identified triggers for early PC consultation to be poor prognostic disease itself, high symptom burden, comorbidities and psychosocial issues.[8] Pediatric oncologists are increasingly aware of improving the integration of PC for their patients and the need of standardisation of referral practices.
The current preferred approach to the treatment of metastatic and incurable cancers of childhood is an integrated approach combining PC concurrently and flexibly with cancer-directed treatment for whatever duration it is planned. A study from the Royal Brisbane Hospital has concluded that a palliative approach to the management of metastatic NB patients should be initiated by the oncology team with early intervention by the PC team, and a formal transition to PC occurring later in the illness trajectory.[9] Salins et al. have proposed a three-tier model to provide PC in paediatric oncology, with coordinated and problem-based involvement of paediatric oncologists and paediatric PC specialists.[10] We believe strongly that in LMIC, where resources are limited, the integration of paediatricians practising in peripheral hospitals, easily accessible to patients, will ensure a continuum of evidence based care from the tertiary hospital to care near home. To provide comprehensive care at all levels to children as well as families needing PC, we recommend a shared care model that includes – the tertiary-hospital-based PC team, local paediatrician-led PC team and the primary oncology team [Figure 1]. The role of each team in patient management is clear and the teams remain connected with each other and with the family throughout the child’s illness, end-of-life and bereavement to ensure uninterrupted care. The oncology team – takes the decision regarding the palliative intent of treatment based on multidisciplinary tumour board discussion, has the initial communication to the family explaining the poor prognosis and limited chance of cure, takes a decision regarding antineoplastic therapy and initiates early referral to PC.
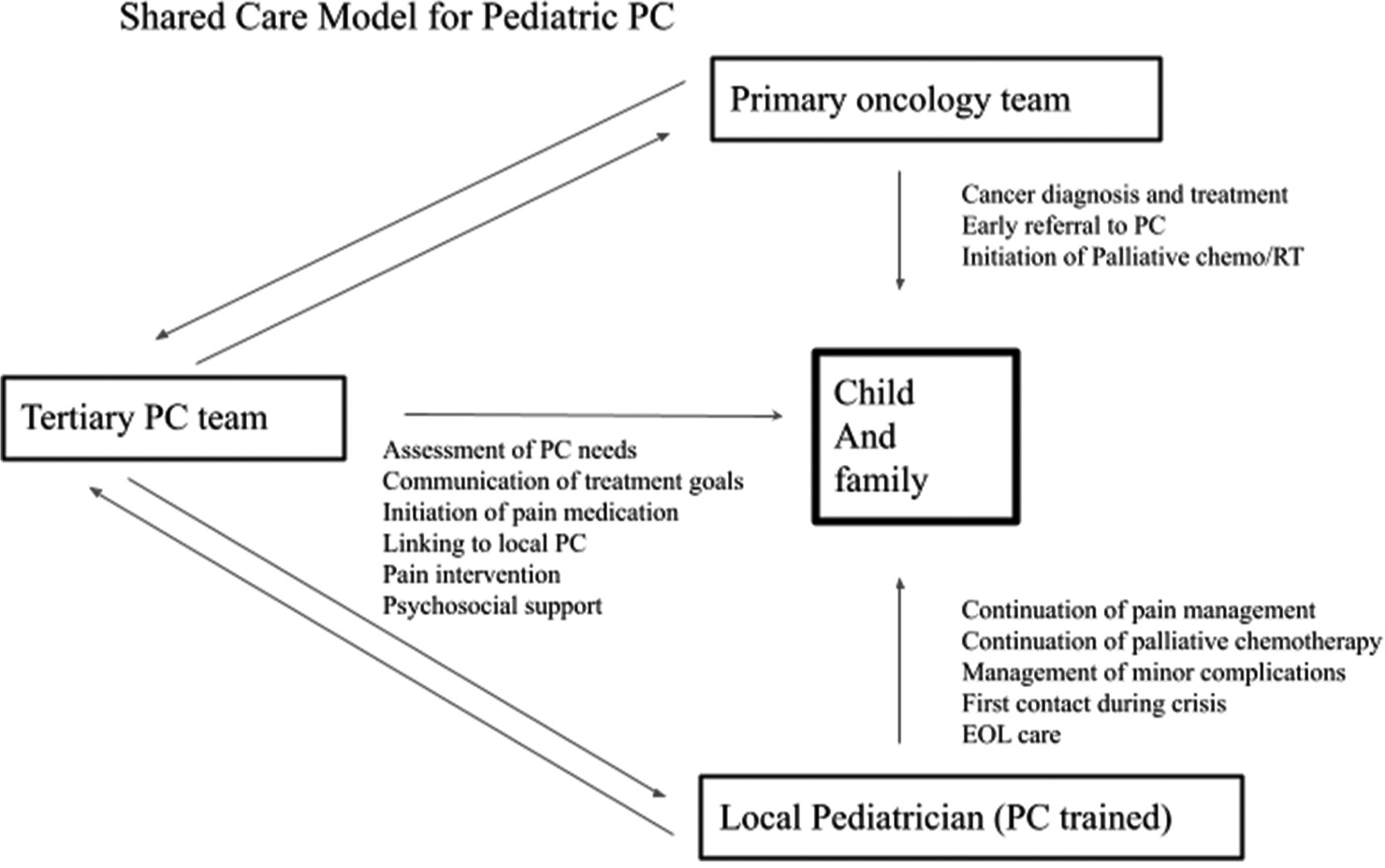
Export to PPT
The tertiary hospital-based PC team – initiates pain and symptom management, has multiple counselling sessions with the family to communicate clearly and sensitively regarding treatment goals, provides guidance regarding advanced symptom management and manages psychosocial issues such as distress, emotional burden and communication issues. The team facilitates the transfer of care from tertiary hospital to local PC services gradually as the family comes into acceptance of the child’s poor prognosis, prepares them for the end-of-life of the child and provides training in PC skills to practising paediatricians from the area of child’s residence, who can be integrated into the PC network to lead local PC teams to provide care for the child.
The PC-trained paediatrician – is the crucial link in providing PCs at tier-3 level near the child’s home. The role includes providing home-based medical care, frequent reassessment of symptoms, identifying and initiating referral to specialised PC services when required, involvement in family communications and critical decision-making, and providing end-of-life care. Minor procedures such as pleural/ascitic fluid drainage, blood transfusions, management of infections and acute emergencies such as seizures, dyspnoea and bleeding can be done locally by PC-trained paediatricians, avoiding referrals to tertiary hospitals. They can train and empower the caretakers by teaching them skills such as pharmacological management of constipation, breathlessness, dry mouth, and pain, care for bed-bound children, management of minor bleeds and breathlessness, to improve their confidence, reduce anxiety and increase their feeling of being in control.
The teams will remain connected to each other and to the family through telephone calls, video calls, teleconferencing or social media platforms for regular updating and sharing information. Referrals can be initiated during crises, when the oncology team responds for any palliative intent of treatment for symptom control and the PC team responds to manage difficult symptom burden. This model is possible and sustainable even in LMICs with limited resources, where the optimum utilisation and streamlining of the available facilities can result in quality improvement of the services provided. Further ahead, this care model can be extended to all children with cancer, irrespective of whether the treatment is curative or palliative intent.
The major limitation of our study is that it is a retrospective analysis of heterogeneously treated patients for whom the PC consultations and interventions were not uniform or consistent and inadequately followed up. A prospective study on the quality of life of children with cancer and their families after the establishment of a streamlined PC program will help us to know the impact of such a service.
CONCLUSIONChildren with metastatic NB form a unique set of patients with limited lifespans having diverse PC needs throughout their illness trajectory. PC-trained paediatricians can play a major role in the shared care of these patients. Coordination between the primary oncology team, hospital PC team and paediatrician-led local PC services can be instrumental in optimising the continued care for children with incurable cancer in the LMIC setting.
留言 (0)