Considerable advancements have been made in the theoretical foundations, design, methodologies, and ethical considerations within palliative care research, highlighting the need for widespread dissemination of findings. Palliative care research is multifaceted, covering investigations into comprehending patients’ values, preferences, and care objectives, which entail addressing biopsychosocial symptoms and spiritual needs. In addition, it aims to improve overall quality of life, refine communication and decision-making processes, and foster effective teamwork among healthcare providers. These aspects are explored within the context of serious, life-threatening, or life-limiting illnesses.[1] The disciplines of this field include bioethics, biostatistics, epidemiology, humanities, education, economics, e-health, health services research, and implementation science, underscoring the interdisciplinary nature of high-quality, ethically sound research.[2]
Despite advancements, challenges persist in the form of poor reporting practices throughout the research process. For instance, under-reporting methodological aspects: Researchers might neglect to adequately report crucial methodological details in their observational studies within palliative care, for example, failing to describe the process of participant selection, data collection methods, or data analysis techniques. This under-reporting can compromise the transparency and reproducibility of the study findings, hindering the advancement of palliative care knowledge. Inaccuracies or discrepancies between abstracts and main texts of published articles can mislead readers and affect the interpretation of study findings. For instance, if the abstract of a palliative care study strongly supports the effectiveness of an experimental intervention, but the main text does not provide substantial evidence to support this claim, it can create confusion and potentially lead to inappropriate clinical decisions or resource allocations. Moreover, insufficient and deficient reporting of research in medical literature, such as failing to provide sufficient information about the rationale behind choosing a particular study design or not calculating the sample size needed to detect meaningful effects. As a result, the study may lack statistical power to draw valid conclusions, reducing its impact on clinical practice which may impede the comprehensive evaluation of the merits and limitations of the studies. Understanding the planned methodology, executed procedures, findings and the implications of results is imperative for readers. However, addressing the global issue of inadequate reporting requires a multifaceted approach, as challenges emerge at each stage of the research process.[3] Factors contributing to poor reporting include poorly formulated research questions, lack of awareness about this guideline, and disparities among researchers from resource-poor and resource-rich settings. Limited resources further constrain research activities in resource-poor settings, hindering the overall improvement of reporting quality. [4] A commitment to transparent and complete reporting is imperative to address these challenges. Structured approaches guided by established reporting guidelines are pivotal in achieving comprehensive and transparent research reporting. These guidelines ensure transparency by detailing key elements: The research process, outcomes, benefits, potential biases, and harms.[5] Academic research should focus on accurately relating research to existing knowledge, considering design and innovations to maximise impact.[6] Reporting guidelines required for Palliative Medicine submissions can help prepare study protocols and ensure ethical approvals, transparency, and rigour.[7] By following guidelines, researchers in palliative care can ensure comprehensive and transparent reporting of their studies, thereby enhancing the trustworthiness and reliability of their findings and facilitating the translation of research evidence into meaningful improvements in palliative care practice and policy. Thus, the imperative for producing and publishing high-impact health research underscores the importance of journals in promoting transparent reporting. Journals should encourage adherence to structured and standard reporting guidelines, fostering a culture of rigour and transparency in palliative care research.[8]
The endorsement of reporting guidelines by esteemed medical journals serves as a constructive measure to enhance the reporting quality of studies. Implementing structured reporting in research not only elevates overall quality but also effectively mitigates reporting deficiencies. Journals can foster transparency and completeness in reporting by adhering to and advocating for the utilisation of structured guidelines. Therefore, this article aims to familiarise potential authors with the reporting guidelines used in the Indian Journal of Palliative Care (IJPC).
REPORTING GUIDELINESReporting guidelines are essential for health researchers when writing manuscripts, offering a structured framework to ensure clarity and transparency. They provide a minimum set of information necessary for readers to understand, researchers to replicate, clinicians to make informed decisions, and systematic reviews to include the study. These guidelines are crucial for specific study designs, presenting checklists, flow diagrams or structured text to guide authors in effectively communicating their research details. Following these guidelines enhances comprehension of research design, conduct, and analysis, enabling critical appraisal and appropriate interpretation of conclusions. Developed through explicit methodology, reporting guidelines are not mere suggestions but a structured approach to improve the reliability and value of health research literature. They aim to promote transparent and accurate reporting, encouraging widespread use for robust research communication.
In this article, the authors are trying to give details about the reporting guidelines followed for IJPC. Reporting Guidelines for Specific manuscript types are given in Figure 1.
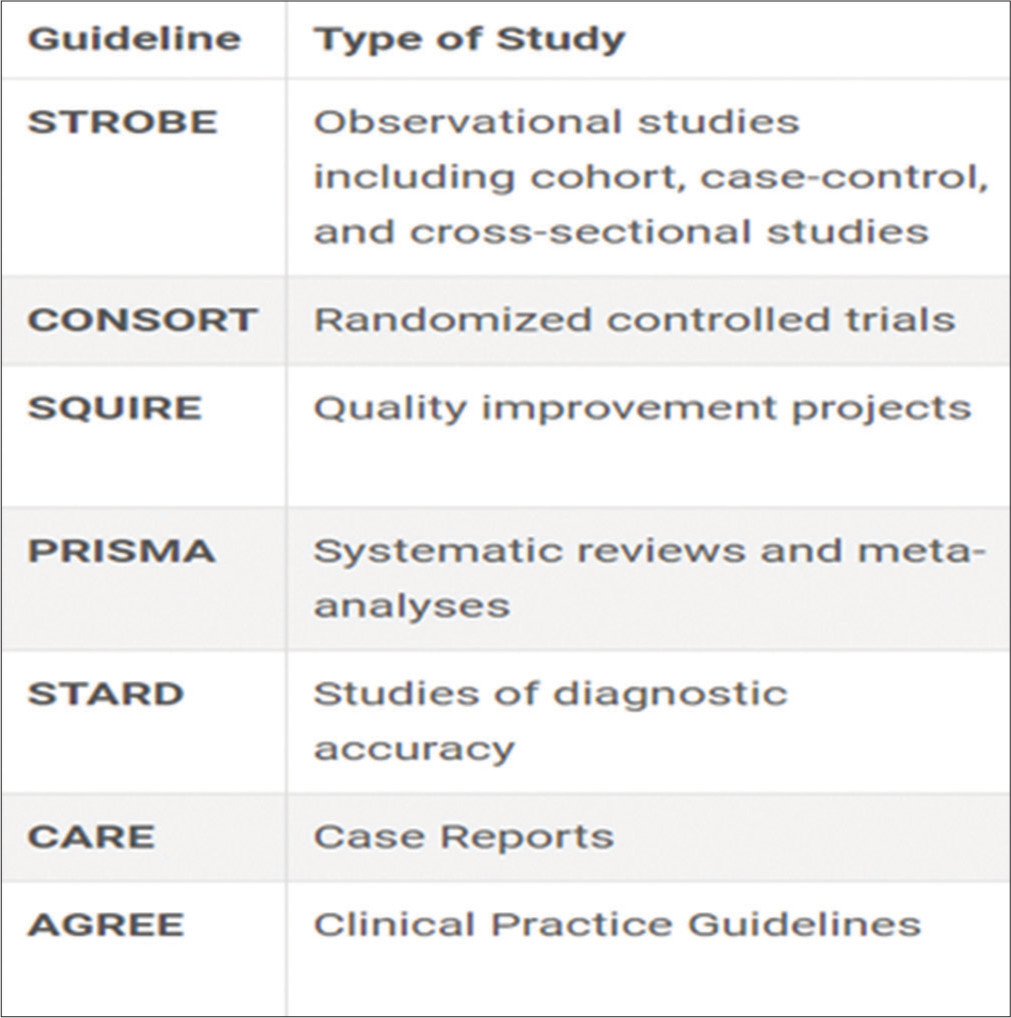
Export to PPT
These guidelines extend beyond mere reflections on academic paper content. They can be defined as structured tools, such as checklists, flow diagrams, or detailed text, offering guidance to authors in reporting specific research types. These guidelines are crafted through explicit methodologies, presenting a clear and systematic list of items that should be included in a paper and providing insights into the development process. In palliative care, evidence-based decision-making is paramount. Therefore, we need to adhere to the Enhancing the Quality and Transparency of Health Research (EQUATOR) guidelines. It offers a structured framework for transparently reporting various types of health research, ensuring that essential information is provided for critical appraisal and interpretation and it also enhances the quality and reliability of their research reporting. As an international initiative, the EQUATOR network[8] strives to enhance the reliability and value of published health research literature. Its focus lies in promoting transparent and accurate reporting and fostering the widespread adoption of robust reporting guidelines. All available guidelines can be found on this website (https://www.equator-network.org/).
The list of most frequently used reporting guidelines in IJPC is detailed below:
Strengthening the reporting of observational studies in epidemiology (STROBE)These guidelines were developed to provide a checklist for authors reporting observational research, focusing on cohort, case-control, and cross-sectional studies. STROBE, comprising 22 items, offers guidance on clear and comprehensive reporting. Authors following these guidelines increase their chances of successfully publishing observational studies in journals, contributing to robust and reliable research reporting.[9] Adhering to guidelines in palliative care research is essential for ensuring that studies are well-designed, conducted, and reported, advancing knowledge in this critical and sensitive area of healthcare, enhancing the transparency and completeness of study reports, and facilitating critical appraisal and evidence synthesis. The weakness of this is that it may not address all potential sources of bias inherent in observational studies, which could affect the reliability of findings in palliative care research, and subjectivity in the interpretation of guidelines may lead to variability in reporting quality, potentially impacting the comparability of studies.
Consolidated standards of reporting trials (CONSORT)The CONSORT statement is a critical tool in clinical research to ensure transparent reporting of randomised controlled trials (RCTs). RCTs are considered the gold standard in evaluating and translating research data into clinical practice and the CONSORT statement plays a pivotal role in maintaining standards for their reporting. Clinicians, patients, and policymakers rely on published trial results for evidence-informed decision-making. This guideline comprises a 25-item checklist that guides authors in presenting RCTs with clarity and completeness, focusing on trial design, analysis, and interpretation. It emphasises the importance of providing complete information on planned, conducted, and found aspects of the trial. It serves as a framework designed to improve the standardisation and reproducibility of (RCTs).[10] Moreover, the CONSORT-Outcomes 2022 extension, building on the CONSORT 2010 statement, introduces 17 outcome-specific items that should be addressed in all published clinical trial reports. This extension aims to enhance trial utility, replicability, and transparency while minimising the risk of selective non-reporting of trial results.[11] CONSORT compliance in palliative care trials is crucial for enhancing evidence-based decision-making among patients, caregivers, and clinicians. Given the sensitive nature of palliative care interventions, clear and comprehensive reporting of trial results enables informed decisions regarding symptom management, endof-life care (EoLC), and treatment preferences. Compliance with the statement is crucial for authors seeking publication, ensuring that manuscripts adhere to established reporting standards. On the other hand, there is limited applicability to other study designs commonly used in palliative care, such as observational studies or qualitative research, and it emphasises RCTs, which may not always be feasible or ethical in palliative care research due to patient preferences and disease trajectory.
Standards for quality improvement reporting excellence (SQUIRE)SQUIRE guidelines are designed for reports that detail system-level efforts to enhance the quality, safety, and value of healthcare, emphasising the use of methods to attribute observed outcomes to specific interventions. The SQUIRE framework aids researchers in systematically documenting and reporting the implementation and outcomes of complex interventions. It underscores the necessity of attributing observed outcomes to specific interventions, particularly crucial in palliative care trials featuring multifaceted interventions such as interdisciplinary team-based care, psychosocial support, patient preferences, caregiver involvement, communication strategies and spiritual care. This approach offers several benefits, given the unique characteristics of the patient population and the nature of palliative care interventions, enhancing the understanding of effective strategies in palliative care by facilitating reporting novel insights into healthcare improvement initiatives.[12] SQUIRE 2.0 builds on the SQUIRE and provides a structured framework for reporting insights into system-level healthcare improvement initiatives.[13] SQUIRE also promotes reporting novel insights vital in palliative care, where innovation is key for addressing diverse patient and family needs, thus advancing care delivery.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)The PRISMA statement, initially published in 2009 and updated in 2020, provides evidence-based guidelines for transparent reporting in systematic reviews and meta-analyses. The PRISMA 2020 statement provides updated reporting guidance for systematic reviews that reflect advances in methods to identify, select, appraise, and synthesise studies. It consists of a 27-item checklist that details reporting recommendations for each item. It focuses on improving the reporting of reviews assessing interventions and can be adapted for other review objectives. Thus, the PRISMA 2020 statement aims to ensure clear, comprehensive, and accurate reporting.[14] The aim is to benefit authors, editors, peer reviewers, guideline developers, policymakers, healthcare providers, patients, and other stakeholders by promoting more transparent, complete, and accurate reporting of systematic reviews, ultimately facilitating evidence-based decision-making.[14] Authors should prepare a transparent, complete, and accurate account of why the review was done, what they did, and what they found. However, the limited availability of high-quality evidence for systematic reviews in palliative care may restrict its applicability in this field. Its overemphasis on quantitative data synthesis may overlook the inclusion of qualitative evidence, which can provide valuable insights in palliative care research.
Standards for reporting diagnostic accuracy (STARD)STARD statement addresses incomplete reporting in biomedical research, specifically in diagnostic accuracy studies. The 2015 update introduces a checklist of 30 essential items for comprehensive reporting.[15] Diagnostic accuracy studies face bias risks, and STARD assists in mitigating them by promoting transparent reporting.[16] Given the inherent challenges and sensitivities in palliative care research, STARD 2015 is a valuable tool to mitigate bias risks by promoting clear and thorough reporting. It increases the transparency and reliability of reporting diagnostic and prognostic studies, which are crucial for decision-making in palliative care, and it provides structured frameworks for transparent reporting of methodology and results, aiding critical appraisal in this field. However, diagnostic and prognostic accuracy may vary in palliative care populations due to advanced disease and symptom complexity, impacting the generalizability of guidelines and limited guidance for addressing complexities of palliative care settings in study design and analysis, such as accounting for palliative care interventions or patient preferences.
CAse REporting (CARE)CAREs are crucial narratives detailing medical issues in patients that hold significant importance in the realm of palliative care for medical, scientific, and educational purposes. The CARE guidelines have been established to improve the precision and transparency of CAREs. The CARE guidelines, featuring a 13-item checklist and narrative for CAREs, serve as a valuable framework for systematically collecting and reporting data in healthcare. Following these guidelines enhances the completeness and transparency of published CAREs. Such reports, aligned with CARE, provide practice-based data on interventions and clinical outcomes, facilitating comparisons with other interventions. The systematic aggregation of information from these reports informs clinical study design, offers early signals of effectiveness and potential harms, and contributes to improved healthcare delivery.[17] However, the limited generalizability of CAREs, which may restrict their impact on evidence-based practice in palliative care, and subjectivity in interpreting CARE guidelines may affect consistency in reporting quality, potentially limiting comparability across studies.
Appraisal of guidelines, research, and evaluation (AGREE)The AGREE Reporting Checklist serves as a tool for practice guideline developers to enhance the completeness and transparency of their reporting. The international AGREE research team developed a tool to assess the methodological quality of practice guidelines – the original was released in 2003 (AGREE I), and the revised and updated version in 2009 (AGREE II). The checklist has six quality domains and 23 key items, offering a systematic and logical approach to reporting essential information. Each of the 23 items includes a summary statement and specific reporting criteria in a bulleted list.[18]
Consolidated criteria for reporting qualitative research (COREQ)COREQ is a comprehensive checklist designed to enhance the transparency and completeness of qualitative research reporting, particularly in palliative care. It comprises 32 items categorised into three main domains: research team and reflexivity, study design, and data analysis and reporting. By ensuring that researchers provide detailed information about their methods, analysis processes, and findings, COREQ enhances the trustworthiness and replicability of qualitative studies. This checklist aids researchers in reporting essential aspects such as the research team’s background, study context, methodology, analysis techniques, and interpretations, thereby facilitating a deeper understanding of complex interactions in palliative care.[19]
Standards for reporting qualitative research (SRQR)SRQR offers a comprehensive set of 21 items to ensure rigour, transparency, and ethical conduct in qualitative research, particularly helpful in the sensitive domain of palliative care. These standards cover critical aspects, including the study rationale, design, data collection, analysis, and reporting. By adhering to SRQR, researchers uphold integrity, respect, and sensitivity toward participants, thereby safeguarding their dignity and well-being. The ultimate goal of SRQR is to enhance the transparency of qualitative research, aid authors in manuscript preparation, assist editors and reviewers in evaluating submissions, and empower readers to critically appraise and apply study findings.[20]
Methods of researching end-of-life care (MORECare)The MORECare statement offers 36 best practice solutions to elevate the quality of research in EoLC, aiming to set a standard for future studies in this crucial healthcare field. It provides clear and standardised recommendations, serving as a valuable resource for researchers, trainees, funders, ethics committees, and editors engaged in EoLC research.[3,21]
CONCLUSIONAdhering to ethical standards is crucial in palliative care research, given its focus on vulnerable populations and sensitive topics. Reporting guidelines establish standards for conducting and evaluating qualitative research, covering key aspects such as study rationale, design, data collection, analysis, and reporting. These guidelines enhance research transparency and support stakeholders throughout publication by emphasising rigour, transparency, and ethical considerations. Compliance with such guidelines enhances manuscript quality, mitigates reporting bias, ensures ethical conduct, and facilitates critical appraisal. Ultimately, adherence to reporting guidelines fosters the generation of high-quality evidence in palliative care, improving patient and family outcomes while minimising wasteful research practices. This leads to the generation of high-quality evidence that can inform and improve the delivery of palliative care services, ultimately enhancing the quality of life for patients and their families. In essence, these guidelines promote accountability, enrich reader comprehension, and uphold the integrity of scientific literature in palliative care research.
留言 (0)