A 40-year-old white woman with systemic lupus erythematosus (SLE), reported positive lupus anticoagulant, and avascular necrosis of the left hip underwent elective left-hip hemiarthroplasty at an outside facility. No complications were reported in the intraoperative period. The postoperative period was complicated by a left-hip surgical-bed hematoma measuring 6.9 by 4.3 cm, with evidence of soft-tissue-hematoma formation throughout the pelvis. The hematoma was greatest in the left gluteal region, hip, and thigh, causing peroneal nerve palsy. The patient had a significant drop in hemoglobin requiring 5 units of red blood cells and 2 units of fresh frozen plasma. Her activated partial thromboplastin time (aPTT) was 150 seconds, prompting transfer to the intensive care unit.
On physical examination, the patient was tachycardic and pale. Active bleeding from the surgical site was noted. She had a left foot drop and no sensation to light touch over the left foot.
The patient’s complete blood cell count and laboratory results from the period after hip surgery through hospital discharge are shown in Table 1. Her coagulation profile results after hip surgery though hosptial discharge are shown in Table 2.
TABLE 1Patient’s complete blood cell count and laboratory results
TABLE 2Patient’s coagulation test results
On postoperative day 3, computed tomography of the abdomen and pelvis showed a large area of soft-tissue thickening and inflammation throughout the pelvis that was greatest in the left gluteal region, hip, and thigh. Computed tomography angiography did not show any active arterial bleeding.
1. What is the most likely etiology of bleeding in this patient?
Arterial injury during surgery
Disseminated intravascular coagulation
Lupus anticoagulant
Deficiency of intrinsic pathway factors or presence of an acquired inhibitor
When a bleeding disorder is suspected, a thorough bleeding assessment and family history of bleeding diathesis or known bleeding disorders should be obtained.1,2 A detailed history on current medications is also warranted, as certain prescription drugs, dietary supplements, and herbal preparations may interfere with platelet function and coagulation proteins. A complete blood cell count, coagulation profile including prothrombin time, aPTT, and fibrinogen, and a peripheral blood smear should be completed.2
The computed tomography angiogram in the patient did not show active arterial bleeding, ruling out an arterial injury. The complete blood cell count and coagulation profile were not consistent with disseminated intravascular coagulation. Disseminated intravascular coagulation can be diagnosed using a simple scoring system proposed by the International Society on Thrombosis and Haemostasis,3 which has a sensitivity of 91% and specificity of 97%.4 In patients with disseminated intravascular coagulation, both prothrombin time and aPTT are prolonged. The serum fibrinogen is low, D-dimer is markedly elevated, and platelets are low owing to consumptive coagulopathy. Prothrombin time is most sensitive to disseminated intravascular coagulation. Because factor VII has the shortest half-life of all clotting factors, an isolated prolonged prothrombin time can be the earliest manifestation of disseminated intravascular coagulation.5
The differential diagnosis of a patient with bleeding and an isolated prolonged aPTT includes deficiency of factors VIII, IX, or XI, deficiency of von Willebrand factor, and the presence of an acquired inhibitor. In mild von Willebrand disease, factor VIII activity is usually in the low normal range and the aPTT is normal. In severe von Willebrand disease, however, a marked reduction in von Willebrand factor can lead to a decrease in factor VIII sufficient to prolong aPTT. Also, antibodies that inhibit factors VIII, IX, and XI and von Willebrand factor and anticoagulants like heparin and direct thrombin inhibitors can prolong aPTT.6 Lupus anticoagulant can prolong aPTT, but patients with lupus anticoagulant often present with thrombotic events and only rarely experience bleeding diathesis, a disorder known as lupus anticoagulant-hypoprothrombinemia syndrome.1
After finding a prolonged aPTT, the presence of heparin and other coagulation inhibitors must be ruled out. The anti-factor Xa assay can exclude the presence of heparin. A mixing study differentiates factor deficiency from the presence of an inhibitor.2 Prolonged aPTT that corrects into the normal range on immediate repeat testing after patient plasma is mixed with normal plasma in a 1:1 ratio implies factor deficiency in the intrinsic pathway, whereas persistent prolongation of aPTT implies the presence of an inhibitor or antibody.2 Factor VIII inhibitors can be time- and temperature-dependent; hence, prolonged aPTT may partially correct on testing done immediately after mixing but not correct on testing done after 1 to 2 hours of incubation at 37°C.2 In patients who have prolonged aPTT and lupus anticoagulant, aPTT will not fully correct with normal plasma, but partial correction may be seen after an hour of incubation at 37°C.
Figure 1 summarizes our approach to a patient presenting with bleeding diathesis and prolonged aPTT.2
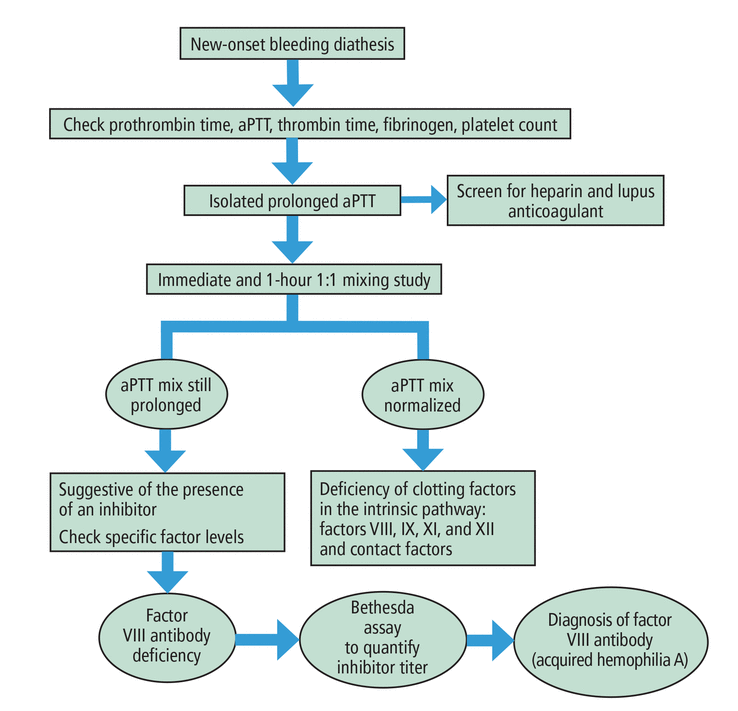
 Figure 1
Figure 1 Approach to a patient with bleeding diathesis and prolonged activated partial thromboplastin time (aPTT).
Based on information from reference 2.
PATIENT HISTORY AND EVALUATIONThe patient had been diagnosed with SLE in her twenties when she presented with fever, malar rash, pleurisy, anemia, synovitis, and photosensitivity. Serologies were positive for antinuclear antibody and double-stranded DNA (titer of 1:160). Her medications for SLE included hydroxychloroquine 300 mg daily, mycophenolate mofetil 500 mg twice daily, and subcutaneous belimumab 200 mg weekly. She denied taking herbal remedies or over-the-counter medications. Personal and family history were negative for thrombotic events or bleeding diathesis, except for the event after her hip surgery. However, the patient reported first having significant bruising almost 2 years before her surgery. The surgery initially had been canceled during preoperative evaluation owing to a significantly elevated aPTT. Despite that, surgical clearance was provided by an outside hematologist, who attributed the prolonged aPTT to the patient’s autoimmune disorder.
Laboratory test results after hip surgeryOn testing done in the postoperative period after her hip surgery, peripheral blood smear showed normal platelet morphology. Her coagulation profile at that time showed a markedly elevated aPTT, with a chromogenic factor VIII level less than 1% and a 1-stage factor VIII level less than 0.7% (reference range 50%–242%). Results of the mixing study showed that her prolonged baseline aPTT of 92.4 seconds (27.5–35.5 seconds) partially corrected (43.4 seconds) immediately after mixing and did not correct after 1 hour of incubation at 37°C. Her diluted thrombin time was normal at 5.4 seconds. The factor VIII inhibitor human assay titer was positive at 80 Bethesda units/mL (Table 2).
Results of other laboratory tests done after the patient’s hip surgery are shown in Table 3.
TABLE 3Results of other laboratory tests done after hip surgery
2. What is the diagnosis?
An isolated prolonged aPTT should always be investigated further even if the patient is asymptomatic. Hemophilia A and B are X-linked disorders characterized by inherited deficiency of factors VIII and IX, respectively. The aPTT fully corrects during the mixing study in patients with hemophilia A and B and inherited deficiencies of factor XI, factor XII, and contact factors.7 Hemophilia C, or factor XI deficiency, is an autosomal dominant or recessive trauma-associated bleeding disorder caused by factor XI deficiency and is commonly seen in Ashkenazi and Iraqi Jewish populations.8 Although factor XII deficiency and other contact factors (high-molecular-weight kininogen and plasma prekallikrein) cause markedly prolonged aPTT, factor XII deficiency does not cause any bleeding diathesis.9
New-onset bleeding diathesis in a patient with no family history of bleeding, as seen in our patient, is highly suggestive of AHA. The constellation of clinical and laboratory features, including a prolonged aPTT, failure of the aPTT to correct during the mixing study, and presence of a high-titer inhibitor, also pointed to a diagnosis of AHA.
The patient reported a history of new-onset easy bruising that began almost 2 years before diagnosis. Her abnormal preoperative aPTT with a history of significant bruising should have been thoroughly investigated, avoiding this life-threatening situation.
3. What is the most likely etiology of AHA in this patient?
Surgery
SLE
Malignancy
Drug-induced
AHA is a rare and potentially fatal autoimmune disease characterized by antibodies against factor VIII. It has a reported incidence of 1.3 to 1.5 per million per year.10–12 AHA typically manifests with bleeding diathesis, which can occur spontaneously, post partum, or after surgery.12 Bleeding in AHA may include extensive subcutaneous ecchymoses, large hematomas, and gastrointestinal, genitourinary, or retroperitoneal bleeding. Spontaneous hemarthroses, a hallmark of congenital hemophilia, are rare in AHA. Bleeding associated with AHA is a medical emergency and tends to be more severe than bleeding in congenital hemophilia even with the same factor VIII levels.12 The mortality rate in AHA ranges from 7.9% to 22%.11,12
The most common predisposing factors for AHA include autoimmune diseases, pregnancy or the postpartum period, and malignancy.12–14 The patient had a known history of SLE, and this was the likely trigger for AHA. Among autoimmune disorders, AHA is often seen in patients with SLE and rheumatoid arthritis.15 Very rarely, drugs such as clopidogrel, alemtuzumab, and omalizumab have been associated with AHA.16 The patient did not have exposure to these drugs. Extensive imaging did not reveal an underlying malignancy.
CASE CONTINUEDAutoimmune serologies revealed a positive antinuclear antibody with a titer of 1:80. Negative results were reported for the following tests: anti-double–stranded DNA, anti-Ro (Sjögren syndrome [SS]-A) and anti-La (SS-B) antibodies, rheumatoid factor, cytoplasmic and perinuclear antineutrophil cytoplasmic antibody, antiribonucleoproteins and liver kidney microsomal antibody, Jo-1 (histidyl-tRNA synthetase) antibody, and antimitochondrial antibody serologies.
The patient was started on oral prednisone 1 mg/kg daily and weekly rituximab 375 mg/m2. For AHA, she received 2 doses of activated prothrombin complex concentrate (aPCC), known as FEIBA (factor eight inhibitor bypass activity). Hydroxychloroquine and mycophenolate mofetil for SLE were continued.
Two days later, after the infusion of the third dose of aPCC, she developed severe abdominal pain, nausea, and vomiting. Computed tomography revealed a retroperitoneal bleed, raising concern for ischemic colitis. The patient was taken for exploratory laparotomy and found to have colon necrosis. On day 13 after her hip surgery, she underwent subtotal colectomy with colostomy placement at the outside facility.
Ischemic colitis is a rare and challenging clinical situation in a patient with AHA. The patient likely developed spontaneous retroperitoneal hematoma in the setting of AHA, and the necrotic bowel was probably secondary to ischemia caused by the pressure effect from the hematoma. A vascular event such as superior mesenteric artery or vein thrombosis in the setting of aPCC cannot be completely ruled out. Suzuki et al17 reported a case of a 66-year-old man with AHA who developed ischemic colitis and was managed conservatively, with a favorable outcome.
Transfer to our facilityThe day after undergoing colectomy, the patient was critically ill and transferred to our hospital for a higher level of care. She had life-threatening hemorrhage requiring massive transfusion support and admission to the intensive care unit. At the time of transfer, she was alert, awake, and oriented to person, place, time, and situation, and did not require vasopressors. Cardiovascular examination showed tachycardia, and abdominal examination revealed active bleeding from the laparotomy wound vacuum device and drains. Peripheral smear examination did not show any schistocytes or evidence of hemolysis.
The patient was started on therapeutic daily plasma exchange to decrease factor VIII antibodies, and recombinant activated factor VII (90 μg/kg) was given every 4 to 6 hours to stop the bleeding. She also received 2 doses of recombinant porcine factor VIII. Immunosuppressive therapy was continued.
Three days after laparotomy, the patient developed extensive bilateral venous thrombosis of the lower extremities.
4. What is the most likely etiology of thrombosis in this patient?
AHA
Immunosuppressive therapy
Plasma exchange
Multifactorial from recent surgery, massive transfusion, history of SLE, use of bypassing agents
Venous thrombosis is a rare event in patients with AHA. Cases of patients with AHA and simultaneous thrombosis are summarized in Table 4 (expanded version of table available online).18–25 Thrombotic events in the patients in studies 3, 4, 5, 7, and 8 were associated with use of bypassing agents.20–22,24,25 Hemorrhage and thrombosis represent opposite ends of the coagulation cascade, but each can be independently fatal. Deep vein thrombosis can be triggered by recent surgery, malignancy, autoimmunity, or inherited or acquired thrombophilia. Massive transfusion protocols in trauma patients can increase the risk of venous thromboembolism, presumably from overcorrection of the coagulation factor deficit.26 Plasma exchange with immunosuppressive therapy alone does not significantly increase the risk of venous thrombosis.27
TABLE 4Summary of patients with acquired hemophilia A and thrombosis
Although thrombosis associated with aPCC therapy has been described, it is a rare event. Clinical trial data have shown that thrombosis is rare in patients with congenital hemophilia and inhibitors who are treated with bypassing agents.28,29 Similarly, a thrombosis risk of 1% was reported in patients with congenital hemophilia and inhibitors treated with aPCC.30 However, risk of thrombosis is higher in patients with AHA treated with bypassing agents. Data from the European AHA (EACH2) registry showed a 2.9% thrombosis risk with recombinant activated factor VII and a 4.8% risk with aPCC.31 Aslam et al25 reported on a 64-year-old woman with AHA who developed fatal pulmonary embolism with aPCC therapy.
Administration of recombinant activated factor VII seemed most temporally related to our patient’s deep vein thrombosis. However, it is possible that she already had deep vein thrombosis when she was transferred from the outside hospital, as we did not perform Doppler ultrasonography until day 3 of admission. Both bypassing agents may have contributed to the deep vein thrombosis. It is difficult to establish a causal relationship between bypassing agents and thrombotic risk in AHA, but older age, medical comorbidities, and presence of other thrombotic risk factors also play a role. Our patient was an active smoker, and smoking is a well-established risk factor for venous thrombosis. Although debatable, it is believed that the risk of venous thrombosis is significantly higher in patients with SLE than in the general population.32 Our patient’s postoperative state and immobility also contributed to her risk for venous thrombosis.
CASE CONCLUSIONSimultaneous thrombosis and life-threatening hemorrhage can be extremely challenging to manage, and the treatment priority should be to control bleeding. Given the patient’s contraindication to anticoagulation, an inferior vena cava filter was placed to minimize the risk of pulmonary embolism. Glucocorticoids were weaned over a period of 4 weeks, and a maintenance dose of prednisone 10 mg daily was continued. Mycophenolate mofetil 1 g twice daily was continued, and the patient completed 4 doses of rituximab and 10 sessions of therapeutic plasma exchange. During hospitalization, she required 30 units of packed red blood cells, 17 units of fresh frozen plasma, 8 units of platelets, and 3 units of cryoprecipitate. Her hospital course was complicated by acute kidney and liver injury; both improved with conservative measures. At the time of discharge, she was hemodynamically stable, and there was no evidence of active bleeding.
DISCUSSIONAs noted above, AHA is a life-threatening hematologic emergency associated with high mortality. The most important steps in the management of a bleeding patient with AHA are to achieve hemostasis and eradicate the antibody.
Controlling bleedingThere is poor correlation between factor VIII levels, inhibitor titer, and bleeding, and these laboratory results should be interpreted with caution.33 Three drugs are currently approved to treat bleeding in patients with AHA: recombinant activated factor VII, aPCC (FEIBA), and recombinant porcine factor VIII. High doses of factor VIII concentrate can be used in patients with low-titer inhibitors (eg, < 5 Bethesda units/mL), but high-dose factor VIII is generally not recommended in patients with high-titer inhibitors (≥ 5 Bethesda units/mL) given the superior efficacy of recombinant porcine factor VIII and the factor-bypassing agents aPCC and recombinant activated factor VII.31,34,35
Recombinant porcine factor VIII theoretically is less likely to be inactivated by the factor VIII inhibitor because its protein sequence is different from that of human factor VIII.36,37 Autoantibodies to human factor VIII may cross-react with recombinant porcine factor VIII in up to 49% of patients with AHA and high-titer inhibitors.38 In a study of 28 patients with AHA and severe bleeding, recombinant porcine factor VIII controlled the bleeding in 24 patients (86%).39
It is reasonable to use recombinant porcine factor VIII as the initial hemostatic therapy in AHA. The US Food and Drug Administration–approved starting dose of recombinant porcine factor VIII is 200 U/kg.40 A potential advantage of recombinant porcine factor VIII is that the standard 1-stage clotting FVIII assay can be used to monitor factor VIII levels and help guide dosing. Using bypassing agents as first-line therapy is another option in AHA, reserving recombinant porcine factor VIII for patients with life-threatening hemorrhage that is unresponsive to a bypassing agent.
aPCC or recombinant activated factor VII is highly recommended in patients with higher-titer factor VIII inhibitors (ie, ≥ 5 Bethesda units/mL) and life-threatening hemorrhage. These agents are believed to have similar efficacy, with preference typically based on cost and the experience of the treating physician.41,42 The aPCC FEIBA contains mainly nonactivated therapeutic levels of factors II, IX, and X and activated factor VII; this agent facilitates thrombin generation.43 For life-threatening hemorrhage, aPCC is dosed at 100 U/kg, with repeat doses every 4 to 12 hours as needed; recombinant activated factor VII is dosed at 90 μg/kg and repeated every 2 to 3 hours as needed.40,44–46 Standard laboratory assays cannot be used to monitor the efficacy of aPCC or recombinant activated factor VII. Accordingly, dosing frequency depends on improvement in bleeding symptoms. In refractory bleeding events, sequential administration of aPCC and recombinant activated factor VII has been used successfully.45
An important consideration when using a bypassing agent is the risk of thrombosis, which appears to be similar with aPCC and recombinant activated factor VII. However, there are conflicting reports on thrombosis risk with these agents. In postmarketing surveillance studies of aPCC, thromboembolic events were reported with doses above 200 units/kg/day.46 A pharmacovigilance study found a higher thrombotic risk with recombinant activated factor VII compared with aPCC.47 A recent French multicenter study of patients with AHA showed that recombinant activated factor VII was safe, with no thromboembolic events reported.48
Concomitant use of aPCC and antifibrinolytics is another therapy option, although theoretically this combination might increase the risk of thrombosis. A retrospective study from the FEIBA on Acquired Hemophilia A Italian Registry (FAIR) showed that combination aPCC and antifibrinolytics was highly effective in achieving hemostasis in AHA without increasing thrombosis risk.49
Emicizumab is a factor VIII-mimetic therapeutic bispecific antibody that bridges enzyme factor IXa and the substrate factor X. A study with 12 patients showed that emicizumab was safe and highly effective in achieving hemostasis in patients with AHA.50 There are reports of patients with acquired hemophilia developing thromboembolic events after receiving emicizumab. For this reason, our patient was not given emicizumab, especially after she developed an ischemic bowel. Larger studies are currently ongoing (NCT05345197) to investigate the role of emicizumab in AHA.
Suppressing the antibodyAlthough some coagulation inhibitors may regress spontaneously, immunosuppressive therapy remains an important pillar in the management of AHA. The optimal immunosuppressive therapy paradigm is unclear. The EACH2 study (N = 31) showed that steroids in combination with cyclophosphamide achieved a higher rate of complete remission (70%) compared with glucocorticoids alone (48%) or rituximab-based regimens (59%).51 The median time to achieve complete remission in the cyclophosphamide group was 5 weeks, and was longer with rituximab-based therapy. The choice of first-line therapy did not determine the clinical outcome, and the likelihood of achieving stable remission was predicted by the factor VIII level and inhibitor titer, not by the underlying etiology of AHA.51
Prolonged immunosuppressive therapy in AHA is associated with significant illness and infection-related deaths. Green et al52 evaluated prednisone, cyclophosphamide, and these agents in combination. The complete remission rate for single-agent prednisone was 32%, and this is a good option for patients with high factor VIII levels (≥ 1 IU/dL) and antibody titers of 20 Bethesda units/mL or less.44,52,53 Tiede et al53 analyzed the prognostic factors in AHA and observed that patients with low factor VIII levels (< 1 IU/dL) and inhibitor concentrations greater than 20 Bethesda units/mL had a lower remission rate and decreased survival. Hence, most experts agree on a risk-adapted immunosuppressive therapy regimen involving more intense therapy with glucocorticoids and cyclophosphamide or rituximab in this high-risk population.44
Infection risk seems to be lower with rituximab-based regimens (12%) than with the cyclophosphamide-based regimen (27%).51 Given our patient’s postoperative state and higher risk of infection, we opted for a rituximab-based regimen in addition to mycophenolate mofetil. Alternatively, recent studies have shown that upfront triplet immunosuppressive therapy consisting of cyclophosphamide, dexamethasone, and rituximab is highly effective and can achieve a durable complete remission rate of 96.8%.54 In patients with inhibitor titers exceeding 100 Bethesda units/mL, it is reasonable to consider triplet immunosuppressive therapy after carefully considering the infection risk.55,56
When first-line therapy fails, approximately 60% of patients can achieve a stable complete remission with second-line therapy. The choice of second-line therapy, rituximab vs cyclophosphamide, is primarily dictated by the initial regimen.
Therapeutic plasma exchange can also be used as an adjuvant to immunosuppressive therapy in patients with AHA.57
TAKE-HOME POINTSThis rare case of a patient with AHA was secondary to SLE, and the diagnosis was missed preoperatively despite a positive clinical history of bruising and prolonged aPTT.
Venous thromboembolism is a rare but potentially fatal complication associated with AHA.
Hemostatic therapy with agents such as recombinant activated factor VII and aPCC is an important pillar in the management of AHA, although these agents can increase thrombotic risk.
Simultaneous life-threatening hemorrhage and venous thrombosis in AHA is rare and poses major therapeutic challenges.
A complex clinical case like the one described here should be managed by an expert hematologist and a multidisciplinary team of specialists.
DISCLOSURESDr. Escobar disclosed consulting for Bayer, Biomarin, Genentech/Roche, Hemabiologics, LFB, Magellan, NovoNordisk, Regeneron, Sanofi, Takeda; participating in a clinical trial sponsored by Bayer, Genentech/Roche, Regeneron, Sanofi, Takeda; being principal investigator for LFB research. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
AcknowledgmentThe authors thank Virginia Mohlere for editorial assistance.
Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.
留言 (0)