Colorectal cancer is a common malignant tumor with a high incidence in the anorectal department. Its early symptoms are not obvious; most of them have developed to the middle and late stages when diagnosed.[1] The incidence of colorectal cancer ranks third among all malignant tumors in China. The incidence of colorectal cancer has increased year by year recently; the age of those affected is getting younger, and the number of male patients is significantly more than that of females.[2] The 5-year survival rate of colorectal cancer is only 30%. If colorectal cancer can be detected in time at an early stage and undergo surgical resection, patients can have a good prognosis, and the 5-year survival rate can be significantly improved.[3] Therefore, the key to improving the survival rate of cancer patients lies in early detection, diagnosis, and treatment, and effective early screening is an essential means for timely intervention and treatment.[4]
Studies have shown that improving early screening can significantly reduce the mortality rate of colorectal cancer.[5] The fecal occult blood test (FOBT), barium enema, and colonoscopy are all routine screening methods for colorectal cancer. The FOBT is convenient, but its false-positive and false-negative rates are high, and its sensitivity and specificity are poor.[6] Although barium enemas and colonoscopies have high sensitivity and specificity, they are difficult to promote as first-line screening due to the invasiveness of the procedures and high costs.[7] Stool testing is simple and specific, with high patient compliance, and can provide early screening and diagnosis of colorectal cancer.[8]
It has been found in clinical practice that the efficiency in the purification of exfoliated cells is low due to the large amount of impurities mixed in stool, which makes it challenging to popularize stool testing.[9] Nano-Fe3O4 folic acid magnetic beads are widely used in the cytological examination of pleural and ascites exfoliation. According to reports, nano-Fe3O4 folic acid magnetic bead technology can enrich cancer cells exfoliated in the sputum of lung cancer patients and tumor cells in the bladder cancer patients’’ urine while maintaining the isolated target cells’ complete morphology and function. Furthermore, it has the advantages of high sensitivity, high specificity, fast detection speed, and easy operation.[10] Antigen-antibody magnetic bead immune complexes are often used as a research tool to isolate specific cells when detecting circulating tumor cells. Based on previous studies, this study took 100 patients with colorectal cancer as the research object to analyze the enrichment efficiency of the improved fecal exfoliated cell enrichment method and its application value in colorectal cancer screening by providing reference materials for clinical screening of colorectal cancer.
MATERIAL AND METHODS Research subjectsOne hundred colorectal cancer patients (100 cases) admitted to the First Hospital of Hebei Medical University from January 2021 to June 2022 were divided equally (50/group) between the control (three-layer integrated sieve method) and experimental (nanofolate magnetic bead method) groups. The control group comprised 29 males and 21 females; ages ranged from 30 to 72 years, with an average of 53.50 ± 5.65 years; tumor distribution: 21 cases in the rectum, six cases in the descending colon, eight cases in the ascending colon, five cases in the transverse colon, and ten cases in the sigmoid colon; Tumor Dukes staging: 15 cases at stage A, 13 cases at stage B, and 22 cases at stage C; and degree of tumor differentiation: 24 cases were well differentiated, 16 cases were moderately differentiated, and ten cases were poorly differentiated. The experimental comprised 31 males and 19 females; ages ranged from 28 to 70 years, with an average of 54.25 ± 4.85 years; tumor distribution: 19 cases in the rectum, eight cases in the descending colon, nine cases in the ascending colon, five cases in the transverse colon, and nine cases in the sigmoid colon; Tumor Dukes staging: 16 cases at stage A, 14 cases at stage B, and 20 cases at stage C; and degree of tumor differentiation: 21 cases were well differentiated, 17 cases were moderately differentiated, and 12 cases were poorly differentiated.
There was no statistically significant difference in demographic data such as gender, age, tumor distribution, tumor Dukes stage, and tumor differentiation degree between the two groups.
Inclusion criteria are as follows: (1) Patients diagnosed with colorectal cancer by colonoscopy biopsy. (2) No operation, radiotherapy, or chemotherapy was performed before the feces collection. Exclusion criteria: (1) Patients with colorectal cancer and other malignant tumors. (2) Patients with various types of viral hepatitis. (3) Those with inflammatory bowel disease or other autoimmune diseases comorbidity. (4) Patients with severe cardiovascular disease. (5) Abnormal function of major organs (heart, spleen, and lung).
Instruments and reagentsImmune Fecal Occult Blood Kit (Wanhua Puman Bioengineering Co., Ltd., Cat. No.: PCS0703). Nano-Fe3O4 Folic Acid Magnetic Beads Detection Kit (Thermo Fisher Scientific, America, Cat. No.: A48310). Liquid-based Cell Preservation Solution (USA Thermo Fisher Scientific, Cat. No. 12648010). BCD826 bowel cleanser (Shanghai Tiancheng Biotechnology Co., Ltd.). Centrifuge (Beijing Liuyi Instrument Factory). SHZ-88A water bath constant temperature incubator (Taicang Experimental Equipment Factory, Jiangsu Province). WJ-T6400 automatic cell slicer (Zhejiang Ningbo Medical Equipment Co., Ltd.). ChemiDoc XRS Chemiluminescence imaging analysis system (Biorad, USA). Olympus BX-50 optical microscope (Olympus, Japan).
Sample collection Natural fecesFor both the control and experimental groups, 10 g of feces was collected from each subject and visually inspected. Normal appearance permitted suitability for sampling. A total of 5 g samples were collected from five different areas of the stool using a stool key.
Bowel cleansing liquidEach patient was given an oral laxative on the morning of a colonoscopy. Before colonoscopy, a cleansing enema was performed, and the fourth flushing was retained, filtered, added to the intestinal cell liquid-based preservation solution, and left at room temperature for 2 h.
Cell enrichment Three-layer integrated sieve methodNatural feces samples were combined with 30 mL of liquid-based preservation solution and mixed thoroughly with a pipette. The total volume, or 100 mL of preserved bowel cleanser sample, was filtered through a 30-mesh (600 μm) screen. The filtrate was transferred to a feces collection bottle with an integrated filter (40 mL) and centrifuged at 1200 rpm for 10 min at room temperature.
The filtrate was further passed through three layers of sieves (screen with 30, 100, and 200 mesh aperture), and the filter residue was discarded. The resulting cell pellet was suspended in 1 mL of liquid-based extraction solution, mixed using a pipette, transferred into the liquid-based thin-layer preparation layer of the automatic cell slicer, and centrifuged at 1000 rpm for 5 min at room temperature. The resulting cell smear was fixed in 95% ethanol for 10 min.
Nanofolate magnetic bead enrichment methodNatural feces samples were combined with 50 mL of cell preservation solution and mixed thoroughly with a pipette. The total volume, or 100 mL of preserved bowel cleanser sample, was filtered through a 30-mesh (600 μm) screen. The filtrate was transferred to a centrifuge tube, and the filter residue was discarded.
The filtrate was centrifuged at 1200 rpm for 10 min at room temperature. The supernatant was discarded, and the pellet was transferred to a conical centrifuge tube containing 10 mL of cell preservation solution. 40 μL of nanofolate magnetic bead suspension was added, mixed, and incubated in a 37°C water bath for 45 min.
The tube containing the nanofolate magnetic beads-cell suspension was subjected to a magnetic field for 15 min, following which the supernatant was transferred to another conical centrifuge tube.
The nanofolate magnetic bead-cell conjugate was washed down and suspended by mixing.
Both the cell suspension and the conjugation supernatant were centrifuged at 1000 rpm for 10 min. Supernatants were aspirated, leaving 1 mL in each sample. These were resuspended and transferred into the liquid-based thin-layer preparation layer of the automatic cell slicer, centrifuged at 1000 rpm for 5 min at room temperature. The resulting cell smear was fixed in 95% ethanol for 10 min.
Hematoxylin-eosin (HE) stainingThe fixed cell smears were dehydrated through an alcohol gradient of alcohol solutions. The alcohol was cleared by incubation in xylene before paraffin embedding. 4 μm slices were cut with a microtome, attached to a glass slide, and dried at 45°C. The samples were again dehydrated, washed with water, and stained with hematoxylin solution for 5 min. After washing, nuclear color depth was observed with an optical microscope. Differentiation was performed in a hydrochloric acid ethanol solution for a few seconds. Samples were counterstained with eosin, washed with water, and dehydrated again. Finally, neutral gum was used to seal the slide. The pathological changes of the cells were observed under an optical microscope, and the images were collected for sorting and analysis.
FOBTEither 10 mg of natural feces or a bowel cleansing liquid sample was combined with 0.5 mL of distilled water. These were then applied to the colloidal gold test strip, and the results were interpreted within 5 min following the manufacturer’s instructions.
Statistical analysisSPSS 23.0 software was used for statistical analysis of the obtained data. The measurement data were expressed as mean ± standard deviation. The independent sample t-test was used for comparison between groups, and the count data were expressed as a percentage. The χ2 test was used for comparison between groups. P < 0.05 indicates statistical significance.
RESULTS Comparison of FOBT-positive rates between two groups of patientsThe FOBT-positive rates of natural feces and intestinal cleansing liquid in the control group were 74.00% and 90.00%. The FOBT-positive rates of natural feces and intestinal cleansing liquid in the experimental group were 76.00% and 92.00%. The positive rate of FOBT in the two groups of bowel cleansing solution was higher than that of natural feces FOBT, and the difference was statistically significant (P < 0.05); [Table 1].
Table 1: Comparison of FOBT-positive rates between the two groups
Groups Sample Number of cases Positive cases (n) Negative cases (n) Positive rate (%) Control group Natural feces 50 37 13 74.00 Bowel cleanser 50 45 5 90.00 χ2 value 4.336 P-value 0.037 Experimental group Natural feces 50 38 12 76.00 Bowel cleanser 50 46 4 92.00 χ2 value 4.762 P-value 0.029 Comparison of cytology results of natural feces exfoliation between two groups of patientsThe sensitivities of natural fecal exfoliation cytology in the control and experimental groups were 82.00% and 92.00%, respectively. The sensitivity of the experimental group was higher than that of the control group, and the difference was not statistically significant (P = 0.137); [Table 2].
Table 2: Comparison of cytology results of natural feces exfoliation in two groups of patients.
Groups Number of cases Positive cases (n) Negative cases (n) Sensitivity (%) Control group 50 41 9 82.00 Experimental group 50 46 4 92.00 χ2 value 2.210 P-value 0.137 Comparison of exfoliated cytology results of intestinal cleansing fluid between two groups of patientsThe sensitivities of the exfoliated cytology examination of the intestinal cleansing fluid in the control and experimental groups were 88.00% and 98.00%, respectively. The sensitivity of the experimental group was significantly higher than that of the control group, and the difference was statistically significant (P = 0.050), as shown in Table 3.
Table 3: Comparison of exfoliated cytology results of intestinal cleansing fluid between two groups of patients.
Groups Number of cases Positive cases (n) Negative cases (n) Sensitivity (%) Control group 50 44 6 88.00 Experimental group 50 49 1 98.00 χ2 value 3.840 P value 0.050 Hematoxylin-eosin (HE) staining to observe the collection of exfoliated cells using the three-layer integrated mesh methodFigure 1a-f shows cancer cells of different shapes and sizes with different degrees of proliferation. Six cell smears were randomly selected to observe the exfoliated cells. Cell smear results show that the exfoliated cells collected by the three-layer integrated sieve method are unevenly distributed, with overlapping cells and a large number of impurities blurring the background, seriously affecting the observation of cell morphology. The cell structure is blurred, stained unevenly, and arranged in a disorderly manner [Figure 1].
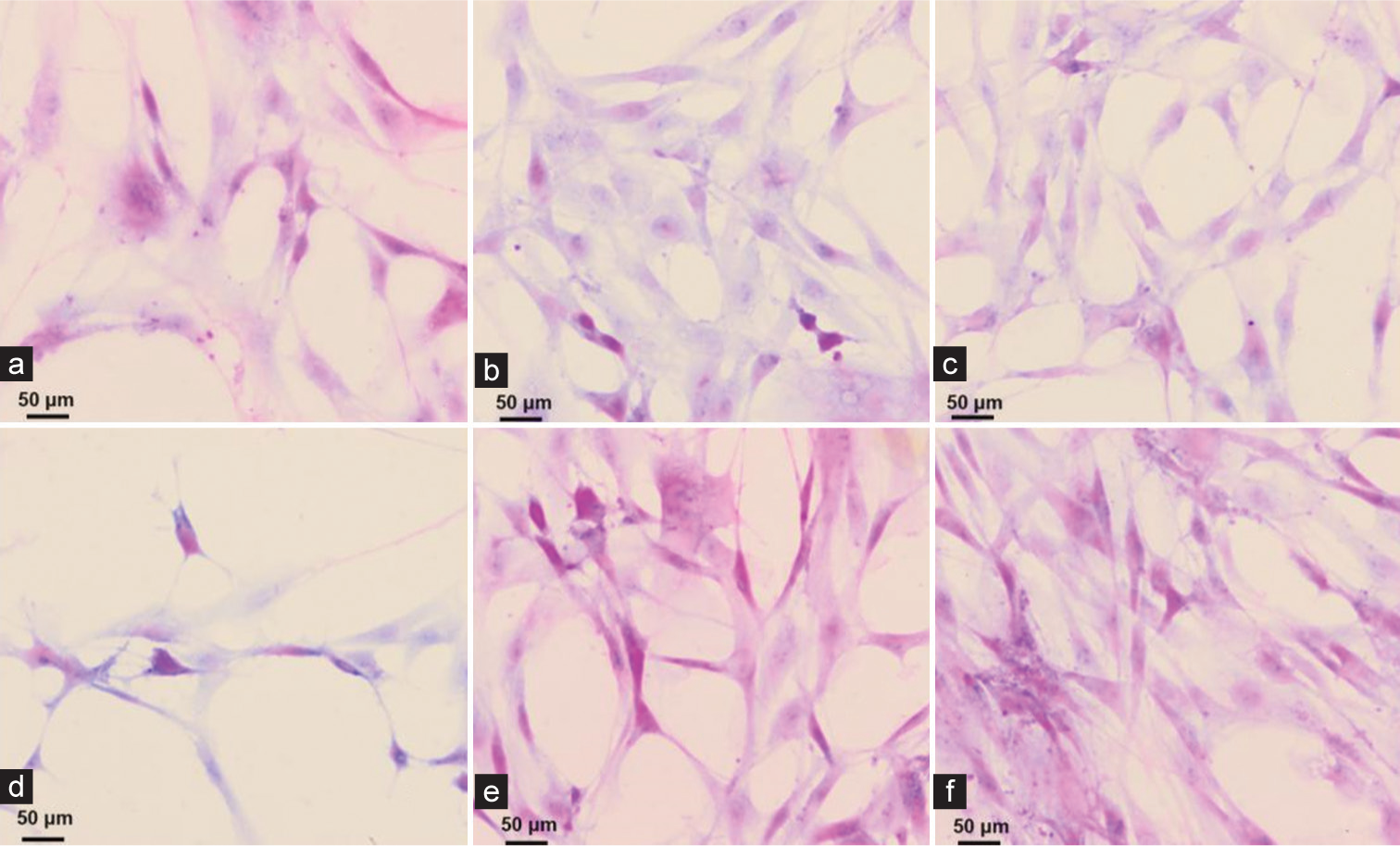
Export to PPT
HE staining to observe the collection of exfoliated cells using the nanofolate magnetic bead enrichment methodFigure 2a-f shows cancer cells of different shapes and sizes with different degrees of proliferation. Six cell smears were randomly selected to observe the exfoliated cells. The cell smear results showed that the exfoliated cells collected by the nanofolate magnetic bead enrichment method were relatively evenly distributed, with no overlapping of cells in patches. The background was clean, and the morphology of each cell could be clearly observed. The cell structure is relatively clear, stained evenly, and distributed evenly [Figure 2].
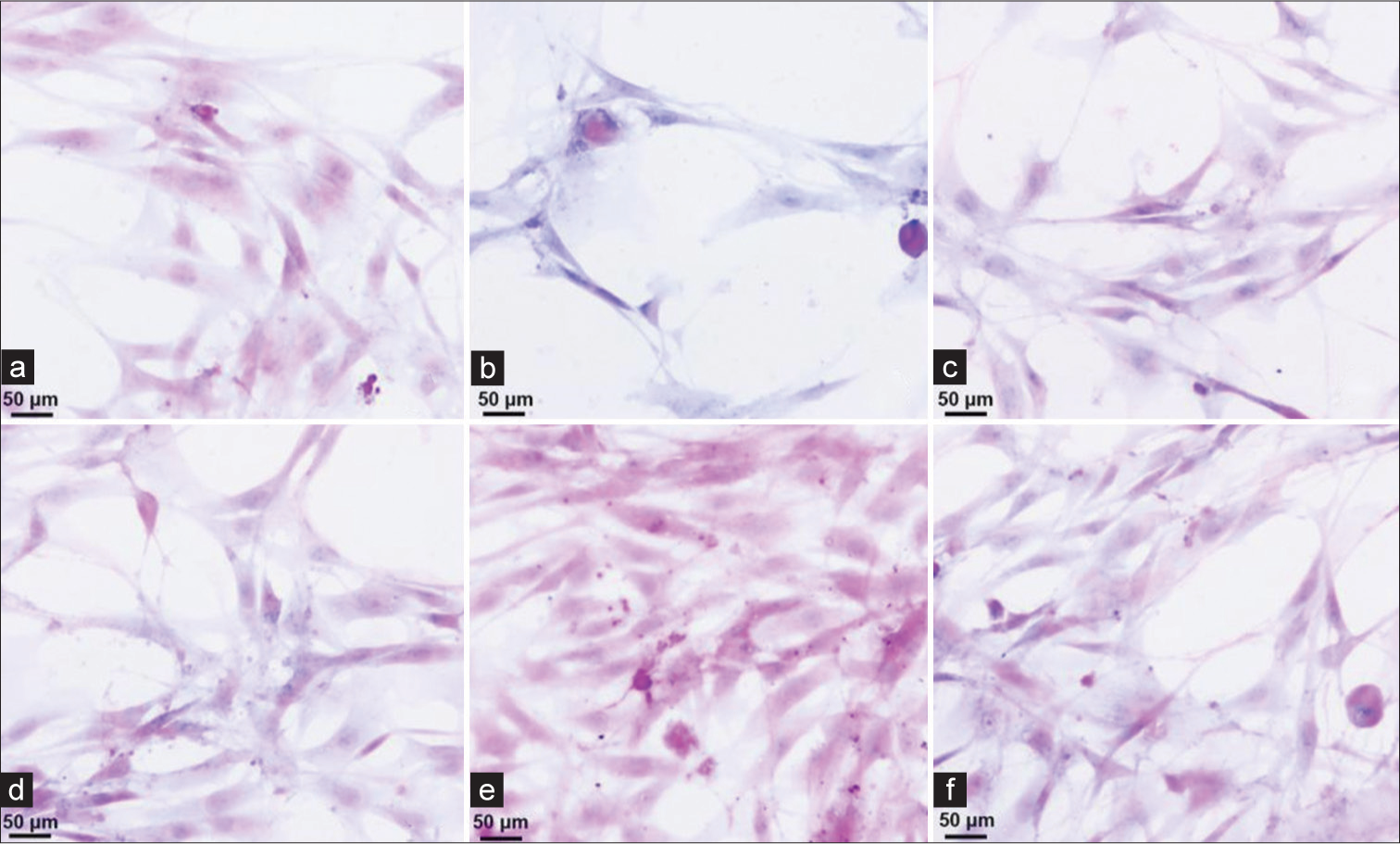
Export to PPT
DISCUSSIONEpidemiological surveys have revealed the association between colorectal cancer and both lifestyle and environmental factors; notably, a high-calorie, high-protein, and low-fiber diet accounting for the rapid increase in the incidence of colorectal cancer.[11] Most colorectal cancers develop from polypoid tumors, taking an average of 10 years to develop from adenoma to cancer.[12] Studies have found that improving early screening of colorectal cancer can significantly reduce the mortality rate of colorectal cancer.[13] If yearly stool screening is performed and further microscopic examination is conducted, informed by the results, the mortality rate can be reduced by 33% within 10 years.[14] There are currently many screening methods for colorectal cancer, among which stool testing has been proven to help detect early-stage cancer and adenoma to improve the prognosis of the disease, in conjunction with removing polyps, which can prevent the occurrence of cancer.[15] The FOBT is easily affected by diet and has low sensitivity to early cancer and adenoma.[16] There are some limitations with the magnetic bead technology, including the inability to discriminate immunological marker expression by tumors and normal tissues, resulting in false-positive or false-negative results, which will affect the development of clinical applications.[17] In addition, when preparing magnetic beads, the antigen coating concentration and coating efficiency can vary, and the prepared magnetic beads have different effects in immunoassay, hampering the unification of detection results.[18] Therefore, it is essential to find safe and effective screening methods to prevent the occurrence of colorectal cancer that endangers the lives and health of patients. In recent years, nanomagnetic beads have attracted much attention in clinical applications due to their advantages of high separation rate, less impact on active cells, and high specificity.[19] Folic acid magnetic bead technology provides reference materials for screening, diagnosing, and treating colorectal cancer in the clinic.
In this study, the group was coated on the surface of nanoFe3O4 folic acid magnetic beads to make nanofolate magnetic beads with positive/negative charges. Combined with the principle of tumor cell surface specific screening of cancer cells. It is suggested that the bowel cleansing solution in FOBT can improve the sensitivity to a certain extent. In this study, we investigated the results of exfoliated cytology in natural feces and bowel cleansing fluid of patients with colorectal cancer and found that the positive rate of FOBT in bowel cleansing fluid in both groups was higher than that in natural feces, but the difference was not significant. In the work presented here, the number of cells isolated through the nanofolate magnetic bead method was 0.91 × 106, confirming the ability of this protocol to enrich colorectal cancer cells, with good specificity shown by the negligible cell density found in the conjugation supernatant. In the exfoliation cytology test, the sensitivity of the experimental group was higher than that of the control group, irrespective of natural feces samples or intestinal cleansing liquid samples, indicating that the enrichment method of nano folic acid magnetic beads is more effective than the traditional three-layer integrated sieve method.
Enriching fecal exfoliated cells has always been the main factor determining whether the exfoliated cytology method can be promoted in clinical practice.[20] As early as 1959, the enema method was used to collect exfoliated cells in the intestinal tract to diagnose intestinal tumors. However, its cumbersome operation and low cell enrichment rate make it difficult to popularize.[21] Studies have used elutriation and silica gel density suspension density gradient centrifugation to extract exfoliated cells from feces. However, due to the anaerobic environment of feces, it is difficult to extract active cells with good morphology.[22] These limitations have been overcome by a methodology combining whole-feces elution and immunomagnetic beads to extract fecal exfoliated cells, allowing observation of the exfoliated intact cells from feces, and has been applied to the research of other colorectal cancer-related analytical markers.[23] However, the cell enrichment rate was still low, and a large number of enriched cells were squamous epithelial cells exfoliated from the anal canal.[24] Nanomagnetic materials are nanoscale particles with a diameter of about 1–100 nm possessing unique magnetic and quantum size effects.[25] The magnetic nanomaterials can freely penetrate blood vessels and gather under the action of an external magnetic field.[26] In this study, cell smears were prepared from the enriched cancer cells and atypical cells, allowing specific morphology and structure of exfoliated cells to be observed after fixation and staining. This study found that the nanofolate magnetic bead enrichment method has a higher detection rate of heterogeneous cells than the traditional three-layer integrated screen method while maintaining the complete morphology and function of the isolated target cells. The quality of the cell smears was higher, with low background, intact cell outline, and even cell distribution, which can aid in better observation of the cell morphology. However, this study has limitations that will be addressed in future work, including a limited sample size, testing a single type of magnetic beads, and using a predetermined concentration of nanofolic acid magnetic beads. At present, there are a variety of early detection methods for colorectal cancer. This study does not claim that one screening method is superior to others; new screening methods are still being explored. It does provide a reference for colorectal cancer screening methods.
SUMMARYColorectal cancer is a common anorectal malignancy, and physicians and patients should give early screening sufficient attention. In the cytological examination of fecal exfoliation of colorectal cancer, the nano-Fe3O4 folic acid magnetic bead enrichment method can enrich more target cells compared with the traditional three-layer integrated screen method, improve the detection rate of colorectal cancer by maintaining cell quality and morphology which can help doctors better observe the shape of the exfoliated cells. Nano-Fe3O4 folic acid magnetic beads enrichment method can become a simple, efficient, and relatively safe screening method for colorectal cancer, which positively affects the development of diagnosis and early screening.
留言 (0)