Atypical squamous cells of undetermined significance cytology (ASC-US) is a challenging and equivocal diagnosis for pathologists in cervical Pap smears. According to the 2014 Bethesda system for reporting cervical cytology, cervical epithelial abnormalities are categorized into squamous cell and glandular cell abnormalities each of which has several types.[1] The squamous cell abnormalities are subcategorized into atypical squamous cells (of undetermined significance [ASC-US] and cannot exclude high-grade squamous intraepithelial lesions [ASC-H]), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and squamous cell carcinoma.[1] The atypical squamous cell subtype is the most prevalent of all abnormal cervical cytology interpretations, and ASC-US specifically compromises more than 90% of all squamous epithelial cell cervical smear cytology results.[1] ASC-US refers to “changes that are suggestive of LSIL, but which are insufficient for a definitive interpretation as such.”[1] The criteria for the diagnosis of ASC-US are the presence of mature squamous and squamous metaplastic cells with a high nuclear-to-cytoplasmic ratio and nuclear changes including hyperchromasia, chromatin clumping, irregularity, smudging, and/or multinucleation.[1] The reporting rates of ASC-US among all cervical cytology results range between 2% and 10.9% in different regions of the world.[2-5]
Human papillomavirus (HPV) is a well-established risk factor for cervical cancer. Twelve oncogenic types of HPV are classified as high-risk HPV (hrHPV), including types 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, and 59.[6] Cervical cancer is the eighth most common type of cancer among women in Bahrain, with an age-standardized incidence rate of 3.92/100,000 women/year.[7] A relatively high proportion of women diagnosed with ASC-US are positive for hrHPV, which is reported to be 19–48.7%.[3,4,8,9] ASC-US cytologic changes may increase the risk of developing high-grade cervical lesions, including cervical intraepithelial neoplasia-2 (CIN-2) and carcinomas, particularly in patients who tested positive for hrHPV.[2,3,5,8] The current practice of cervical cancer screening in the primary health care and the gynecology departments of the secondary care hospitals in Bahrain is following the guidelines of the cervical cancer screening of the American Cancer Society.[10]
The data on ASC-US cervical cytology in Bahrain are deficient. This study reviewed and identified the reporting rates, hrHPV testing results, the histopathologic follow-up findings, along with the cytologic progression of patients diagnosed with ASC-US in the main referral tertiary hospital in Bahrain, and the only center for the analysis of Pap smear samples received from all primary care health centers and the gynecology department, Salmaniya Medical Complex. The findings of this study have major implications for defining clinical and prognostic metrics of cervical cancer screening and therapeutic strategies in Bahrain.
MATERIAL AND METHODS Study designA retrospective chart review was conducted on the medical records of patients at Salmaniya Medical Complex, available at the National Health Information System (I-SEHA). The cytopathology reports for all Pap smears performed between January 2019 and March 2022 were reviewed to identify cases diagnosed with ASC-US. All women diagnosed with ASC-US cervical cytology in the study period were included in the study and no exclusion criteria were applied. Data on the demographic characteristics and the types of hrHPV identified were recorded. Histopathological results of cervical biopsy, endocervical curettage, or loop electrosurgical excision procedure/cone biopsy performed within 6 months of the Pap test were included in the study and were correlated with the cytology findings. Biopsy specimens were stained with hematoxylin and eosin. In addition, the specimens were stained with Ki-67 and P16 immunohistochemical stains. Biopsy results were categorized into three groups: (1) negative, (2) low-grade cervical lesion, cervical intraepithelial neoplasia-1 (CIN-1), and (3) high-grade cervical lesion (CIN-2 and higher). Follow-up Pap smear reports within 1 year of diagnosis were tracked to monitor the progression or regression of the cervical cytology changes.
Cytologic examination of the Pap smearsCervical Pap smears were collected in liquid fixative vials (BD SurePath™ liquid-based Pap test, Becton Dickinson, Franklin Lakes, New Jersey, USA). The slide preparation and staining were done using an automated system (BD PrepStain™ System, Becton Dickinson, Franklin Lakes, New Jersey, USA). Microscopic examinations and reporting were performed by qualified pathologists according to the 2014 Bethesda System.[1]
High-risk HPV detectionThe detection of hrHPV was performed using the Xpert™ HPV assay (Cepheid, Sunnyvale, California, USA), and the real-time polymerase chain reaction (PCR) was run in the GeneXpert system (Cepheid, Sunnyvale, California, USA). 14 hrHPV types were tested, and the results were reported as negative or positive for HPV16, HPV18/45, or other hrHPV (31,33,35,52,58,51,59,39,56,66,68).
StatisticsThe age was reported as mean age ± standard deviation and median age. The ASC-US reporting rates were calculated from the total Pap smear tests conducted in the study period. To compare the histopathologic findings or the cytologic follow-up findings with the hrHPV test results, a Chi-square test of independence was conducted. P ≤ 0.05 was considered statistically significant. The strength of the relationship between those categorical variables was tested using Cramer’s V.[11] The statistical analysis was done using IBM Statistical Package for the Social Sciences Statistics version 29.0.1.0.
RESULTS ASC-US reporting ratesDuring the 39-month retrospective study period, a total of 23,888 Pap smear tests were analyzed. From which 259 women were diagnosed with ASC-US, with an overall reporting rate of 1.1%. The reporting rate of ASC-US showed an increasing trend over the years where it doubled from 0.8% in 2019 to 1.6% in 2021. While in the first 3 months of 2022, only the reporting rate was 1% [Table 1].
Table 1: ASC-US reporting rates per year (2019–2022).
Year Number of total Pap smears Number of ASC-US Reporting rate of ASC-US (%) 2019 8581 67 0.8 2020 3674 49 1.3 2021 4485 74 1.6 2022 (Jan-March) 7148 69 1.0 Total 23,888 259 1.1 Demographic features of the study populationThe mean age of the 259 women residing in Bahrain who were diagnosed with ASC-US is 43 ± 11.6. Based on the age group, 55.6% of the participants were between 30 and 49 years old, 31.7% were 50 years old and above, and 12.7% were <30 years old. Most of the ASC-US cases were Bahrainis (81.5%) [Table 2].
Table 2: Nationality and age of women diagnosed with ASC-US in the study period.
Variables ASC-US cases N(%) 259 (100) Nationality Bahraini 211 (81.5) Non-Bahraini 48 (18.5) Age Mean±SD 43±11.6 Median 42 Age group <30 33 (12.7) 30–49 144 (55.6) ≥50 82 (31.7) The hrHPV rates among ASC-USAmong the ASC-US cases, 160 (61.8%) had hrHPV genotyping test results. The overall hrHPV positivity rate among them was 30%. Women aged 30-49 had the highest hrHPV rate (62.5%), and those aged <30-years-old had the lowest rate (16.7%) [Table 3]. Most of the ASC-US cases tested positive for the other hrHPV (75%). This was followed by HPV 16 (12.5%) and HPV 18/45 (4.2%). The remaining 8.4% of the hrHPV-positive ASC-US cases had coinfection with at least two types of the virus (HPV 16 and 18/45 [2.1%], HPV 16, and other hrHPV [4.2%], and HPV 16, HPV 18/45, and other hrHPV [2.1%]) [Table 3].
Table 3: HrHPV among ASC-US.
Variables n(%) hrHPV test status High risk-HPV test done 160 (61.8) High risk-HPV not done 99 (38.2) hrHPV test result Negative 112 (70) Positive 48 (30) hrHPV-positive test result based on the age group <30 8 (16.7) 30–49 30 (62.5) ≥50 10 (20.8) hrHPV type HPV 16 6 (12.5) HPV 18/45 2 (4.2) Other hrHPV 36 (75) HPV 16 and 18/45 1 (2.1) HPV 16 and other hrHPV 2 (4.2) HPV 16 and HPV 18/45 and other hrHPV 1 (2.1) Histopathologic findings of women diagnosed with ASC-USOf 259 women with ASC-US cytology, only 28 (10.8%) underwent histopathologic examination within 6 months of diagnosis. Among them, 82.1% had normal histopathologic findings. However, high-grade cervical lesions (CIN-2 and higher) were reported in three cases (10.7%), and CIN-1 was reported in two cases (7.1%) [Table 4]. Two out of three cases diagnosed with CIN-2 and higher were positive for the other hrHPV, and the third case was not tested for hrHPV. Regarding the two cases diagnosed with CIN-1, one of them tested positive for hrHPV (HPV 18/45 genotype) and the other one tested negative [Table 4]. A Chi-square test of independence was conducted between the histopathologic findings of the ASC-US cases and the hrHPV test results. There was not a statistically significant association between the histopathologic findings of the ASC-US cases and the hrHPV test results, χ2(1) = 0.787, P < 0.375 [Table 4]. A representative example of a cervical biopsy specimen diagnosed with cervical intraepithelial neoplasia-3 is shown in Figure 1.
Table 4: Histopathologic follow-up findings and hrHPV of women diagnosed with ASC-US.
Histopathologic findings n=28 hrHPV status hrHPV type Diagnosis n(%) HPV negative HPV positive HPV test not done HPV 16 HPV 18/45 Other hrHPV HPV 16/18/45 Negative 23 (82.1) 7 7 9 1 0 5 1 Low-grade cervical lesion (CIN-1) 2 (7.1) 1 1 0 0 1 0 0 High-grade cervical lesion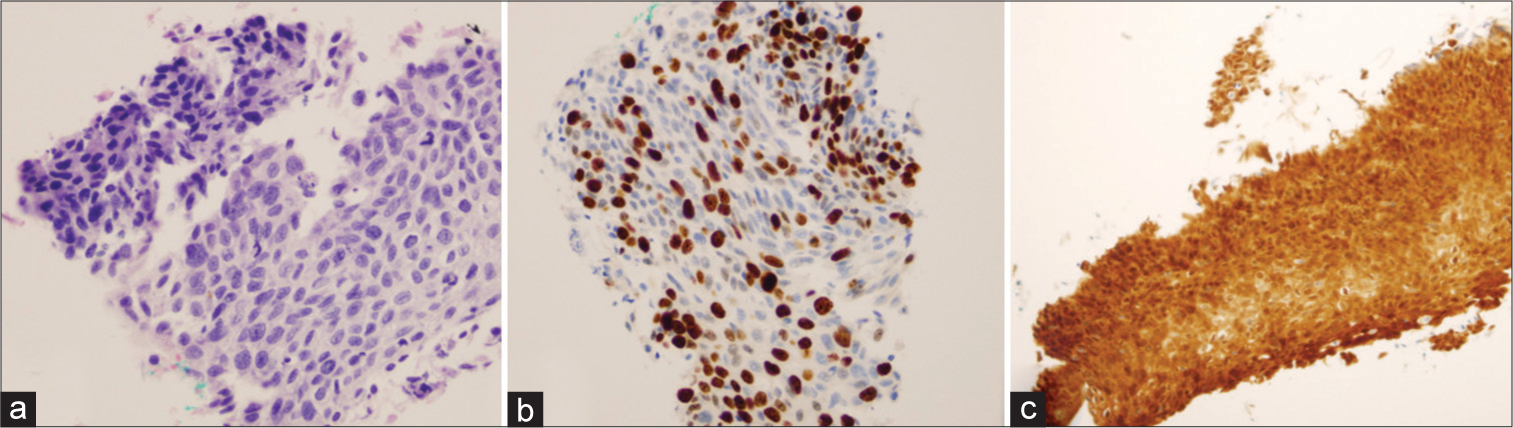
Export to PPT
Cytology follow-up findings of women diagnosed with ASC-USOverall, 32.4% of patients had a repeated Pap smear test within 1 year of ASC-US diagnosis. The majority of the ASC-US cases were negative for intraepithelial lesion or malignancy (NILM) (73.8%) or had persistent ASC-US (17.9%). The remaining minority of cases progressed into either LSIL (6%) or HSIL (2.4%) [Table 5]. Representative examples of ASC-US, LSIL, and HSIL cervical cytology are shown in Figure 2. It was observed that 25 out of 34 (73.5%) cases that regressed into NILM and seven out of 13 (53.8%) cases that had persistent ASC-US were hrHPV negative. All three cases that progressed into LSIL and the one case that progressed into HSIL (with an HPV test done) were positive for hrHPV [Table 5]. A Chi-square test of independence was conducted between the cytology follow-up findings of ASC-US cases and the hrHPV test results. There was a statistically significant association between the cytology follow-up findings and the hrHPV test results, χ2(2) = 8.869, P < 0.012 [Table 5]. The association was relatively strong (Cramer’s V = 0.417).[11]
Table 5: Cytology follow-up findings and hrHPV of women diagnosed with ASC-US.
Cytology findings n=84 HrHPV status HrHPV type Diagnosis n(%) HPV negative HPV positive HPV test not done HPV 16 HPV 18/45 Other hrHPV HPV 16/18/45 HPV 16 and other hrHPV NILM 62 (73.8) 25 9 28 0 2 6 0 1 ASC-US 15 (17.9) 7 6 2 1 0 5 0 0 LSIL 5 (6) 0 3 2 2 0 1 0 0 HSIL 2 (2.4) 0 1 1 0 0 0 1 0 χ2(2) = 8.869, P-value < 0.012, cramer’s V=0.417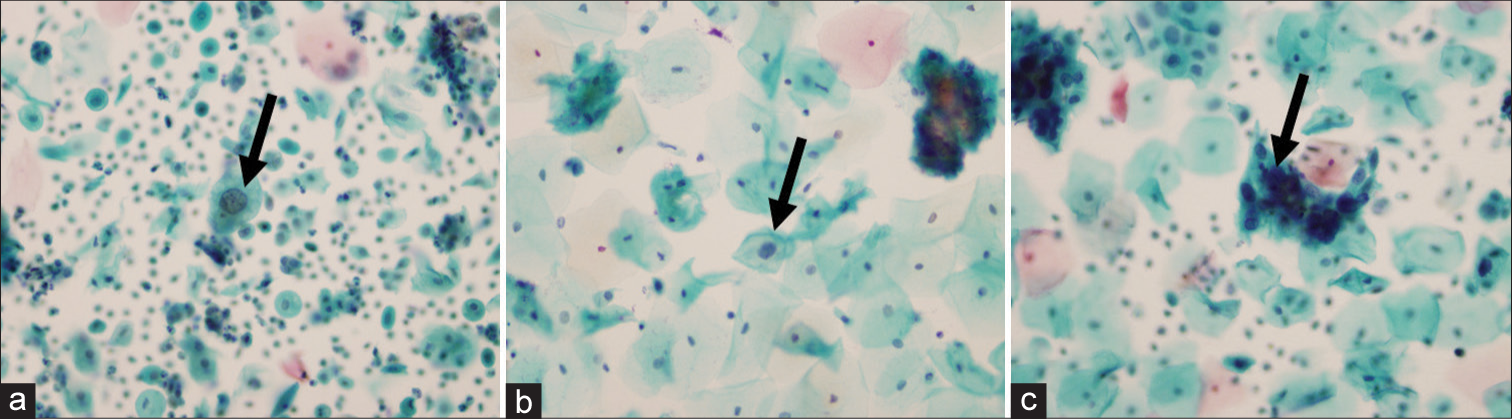
Export to PPT
To elaborate further, the two ASC-US cases that progressed into HSIL were for 35-years-old and 50-years-old patients. The Pap smear was repeated for them at 8 months and 3 months, respectively, after the first ASC-US diagnosis where HSIL cytologic changes were observed. Both cases regressed into NILM on further follow-up within 1–2.5 years.
DISCUSSIONIn this study, a total of 23,888 Pap smear tests were interpreted, of which 1.1% were diagnosed with ASC-US cervical cytology. Although there was an increase in the reporting rate over the years in the study period, it is still falling within the benchmark range reported by the CAP survey.[12] Higher rates were reported in the Gulf Cooperation Council countries (GCC) overall (10.9%) and in the United Arab Emirates (5.3%).[4,5] In China, the reporting rates were 3.8% and 4.2% in two different studies.[2,3]
HrHPV positivity among women with ASC-US varies among different populations and is based on the technique used to detect HPV. The proportion of ASC-US patients who tested positive for hrHPV is 34.89–48.7% in the Chinese population, 30.3–40.1% in the United States, 21% in the GCC countries, and 19% in Kuwait.[3,4,8,9] In a large-scale study, the mean percentage of hrHPV positivity for ASC-US was 43.74% among 69 laboratories in the United States.[13] Those figures are comparable to the findings of this study, where the hrHPV positivity among ASC-US is 30%. In line with the results of two studies conducted in the GCC, the most identified genotype of hrHPV in ASC-US cases was the other hrHPV.[4,9] This can be partially explained by the fact that the most prevalent hrHPV type reported in Bahrain is the other hrHPV.[14] On the contrary, hrHPV types 16 and/or 18 were commonly identified genotypes in ASC-US patients in China.[2,3]
In our study, three out of 28 women diagnosed with ASCUS (10.7%) developed high-grade cervical lesions, and two of them tested positive for other hrHPV. The association between ASC-US and the presence of high-grade cervical lesions have been studied previously. In one study, the histopathologic follow-up findings of ASC-US cases revealed that 7.87% of ASC-US cases had high-grade cervical lesions.[2] Those high-grade cervical lesions were reported in 13.98% of women with hrHPV-positive ASC-US and only in 2.84% of hrHPV-negative ASC-US cases.[2] In another study, the immediate histopathologic follow-up revealed that 7.1% of cases who were diagnosed with ASC-US had high-grade cervical lesions, including 0.6% carcinomas.[3] In the United States, the prevalence of high-grade cervical cancer precursors in women with ASC-US ranges from 5.1% to 9%.[8] The prevalence of high-grade cervical lesions in patients with ASC-US and positive hrHPV is 0.8% in the United Arab Emirates.[5] Closer histopathologic follow-up of ASC-US cases, especially when the hrHPV test is positive, is crucial to predicting the malignant transformation of the cervical lesions.
In the present study, the number of cytologic follow-ups within 1 year of ASC-US diagnosis was conducted in only 32% (84/259) of the cases. Most of them regressed into NILM (73.8%) or had persistent ASC-US (17.9%), and these figures are comparable to previously published reports where the majority of ASC-US regressed spontaneously within 6 months of the first diagnosis.[15,16] Most of the cases that regressed into normal cervical cytology were not infected with hrHPV. We found that 7/84 cases progressed into either LSIL or HSIL, and all these cases were positive for hrHPV. Those precursor lesions were more likely to progress into LSIL or HSIL if hrHPV was positive, as published before.[15] This demonstrates that the ASC-US could be the precursor of more advanced cytologic cervical abnormalities and testing cervical lesions for hrHPV may identify those that are likely to progress.
We showed that ASC-US can progress into more advanced cervical cytologic diagnoses or contribute to the malignant transformation of cervical tissue, especially in the presence of hrHPV infection. While hrHPV infection was detected in 30% of ASC-US cases overall, it was detected in all ASC-US cases that went on to develop a more advanced cytological or histological diagnosis. This highlights the importance of hrHPV infection in disease pathogenesis and progression and speculates that using cotesting (a combination of hrHPV genotyping and Pap smear test) could be an effective strategy to identify patients at high risk of premalignant disease. This could also have an additional advantage in that it saves the patient from the inconvenience of a second visit for hrHPV testing.
The limitations of this study are the overall small number of ASC-US cases, and around 40% of them did not undergo hrHPV testing. In addition, the relatively small number of ASC-US cases who had a cervical biopsy within 6 months of diagnosis made it difficult to test for statistically significant associations. In addition, the 1-year cytologic follow-up was not done for more than half of the patients diagnosed with ASC-US; this might be explained by patients not showing up for their follow-up appointments or going to private hospitals for follow-up tests.
SUMMARYThis is the largest and first investigation of hrHPV positivity rates, cytologic progression, and histopathologic follow-up results in women with ASC-US cytology Pap smear results in Bahrain. The findings of this study aided in providing a better understanding of the status of this cervical cytology finding in our population. The reporting rates of ASC-US in Bahrain fell within the worldwide reported range. Although most of the ASC-US cases regressed into NILM, ASC-US can progress into more advanced cervical cytologic diagnoses or contribute to the malignant transformation of cervical tissue, especially in the presence of hrHPV infection, which was positive in 30% of the ASC-US cases, and most of them were infected with the other hrHPV genotype. This emphasizes the role of cotesting (a combination of hrHPV genotyping and Pap smear test) and the importance of colposcopic evaluation of those women and the cytologic follow-up test at 1 year of diagnosis. Implementing these recommendations in the cervical cancer screening practice in Bahrain will help in having a better management plan for ASC-US women and in decreasing avoidable invasive diagnostic and therapeutic procedures.
AVAILABILITY OF DATA AND MATERIALSAll data points generated or analyzed during this study are included in this article and there are no further underlying data necessary to reproduce the results.
ABBREVIATIONSASC-US - Atypical squamous cells of undetermined significance
CIN - Cervical intraepithelial neoplasia
GCC - Gulf Cooperation Council
HPV - Human papillomavirus
HrHPV - High-risk human papillomavirus
HSIL - High-grade squamous intraepithelial lesion
LSIL - Low-grade squamous intraepithelial lesion
NILM - Negative for intraepithelial lesion or malignancy
PCR - Polymerase chain reaction.
AUTHOR CONTRIBUTIONSSA: Concepts, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing and review. AM: Literature search, data acquisition, data analysis, manuscript editing and review, clinical studies. RI: Literature search, clinical studies, data acquisition, data analysis, manuscript editing and review. Each author has participated sufficiently in the work and takes responsibility for appropriate portions of the content of this article. AI: Concepts, design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing and review. All authors read and approved the final manuscript.
留言 (0)