It is crucial to diagnose cancers at an early stage to provide prompt intervention to patients.[1,2] Pleural tap or thoracocentesis is a less invasive and less expensive procedure than pleural biopsy. Notably, a pleural biopsy has to be performed using a needle under ultrasound or computed tomography guidance under local anesthesia or by thoracoscopy or open surgery under general anesthesia. In contrast, cytological examination of pleural fluids is simple, easy, and inexpensive for detecting malignancies.[3,4] In addition, an effusion represents cells exfoliated from the entire serosal surface, unlike a focal biopsy from a small area of an extensive serosal surface. Cell blocks prepared from pleural fluid samples preserve tissue architecture, allowing for histopathological diagnoses of malignancies and additional immunohistochemical or molecular studies to subtype different malignancies.[5] The subtyping guides the clinician in selecting a suitable treatment option for patients. For example, unlike non-small cell lung cancers, small cell lung cancer requires chemotherapy regardless of the stage of the disease.[6,7] Moreover, targeted therapy is valuable for treating many oncological diseases (e.g., Epidermal Growth Factor Receptor (EGFR) - or Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS)-positive lung cancers, Estrogen receptor (ER)/Progesterone receptor (PR)-positive breast cancers, human epidermal growth factor receptor 2 (HER2)-positive breast cancers, etc.).[8-10] Furthermore, malignant pleural effusions occur because of a primary pleural malignancy (such as malignant mesothelioma) or secondary (metastatic) pleural malignancy (such as adenocarcinoma, small cell carcinoma, myeloma, lymphoma, etc.) and it is challenging to distinguish between reactive mesothelial, adenocarcinoma, and malignant mesothelial cells owing to their overlapping cytological features.[11,12] Therefore, immunohistochemistry (IHC) is a valuable tool in this regard. Accordingly, this study aimed to determine and characterize various cancers most likely to cause malignant cells in pleural fluids by cell block IHC and determine the mutual diagnostic agreement between conventional cytology and cell block methods.
MATERIAL AND METHODSThis prospective, observational study was conducted at the Department of Pathology (Cytopathology division), Government Medical College Kottayam, India, over 18 months (May 7, 2021–November 6, 2022). The study included pleural fluid samples obtained from clinically suspicious cases of malignancy, whereas duplicate and inadequate samples were excluded. We considered clinically suspicious cases of malignancy as cases with any or combination of the following criteria - the presence of cachexia or significant loss of weight or appetite, rapidly growing swelling or lump or lesion, hard swelling or lump, fixed/non-mobile swelling or lump, non-resolving local site symptoms (such as persistent dyspnea or cough, amenorrhea, bleeding, etc.) after ruling out nonneoplastic causes, elevated serum tumor markers, radiological suggestion or evidence of malignancy, biopsy-proven evidence of cancer at a site other than pleura (such as lung, breast, ovary, endometrium, lymph node, bone marrow, skin, etc.). The clinico-radiological and biopsy details were gathered from patients’ medical records. The Scientific Review Committee and Institutional Review Board approved the study. Patient consent was not required for this study, as advised by the Ethics committee, as we had to use only the patients’ samples sent to the Department of Pathology by various clinicians who had already got informed consent from patients before performing thoracentesis. We obtained permission from the department to use the patients’ samples for this study.
Pleural fluid samples collected from the clinically suspected cases of malignancy were centrifuged at 2000 rpm for 10 min, the supernatants were discarded, and each sediment was split into three, two of which were transferred onto separate glass slides and one was treated with an acid-alcoholic formalin solution (Formalin: Isopropyl alcohol: Glacial acetic acid= 2: 17: 1). One of the glass slides was stained with Papanicolaou stain, whereas the other stained with Giemsa stain and both were then mounted under coverslips to be later examined under the microscope as conventional cytology slides. Next, centrifugation of the sediment that was mixed with acid-alcoholic formalin solution was performed at 2000 rpm for 10 min, followed by keeping the test tubes aside undisturbed for 4–6 h, after which the supernatants were discarded. Subsequently, the sediments were wrapped in filter papers, placed in separate tissue cassettes, and then placed in a formalin solution overnight. The samples were then processed and embedded in paraffin wax to form tissue-embedded wax blocks (known as “cell blocks”), which were sectioned, stained (using Hematoxylin and Eosin), mounted under coverslips and examined under the microscope.
Conventional cytology or cell block samples were considered positive for malignancy when the microscopic examination revealed atypical cells with a high nuclear-to-cytoplasmic ratio and nuclei containing atypical features such as an irregular nuclear contour, hyperchromasia, intranuclear cytoplasmic inclusions, prominent macronucleoli, or atypical mitosis, either isolated or in clusters (especially if large clusters with >12 cells with molding of the cells or smooth outer borders) or in sheets, which may be present in lacunae (pertaining to cell blocks) and which shows nuclear debris or evidence of necrosis.[13] The large conglomerate clusters, known as “proliferation spheres” or “cannon balls,” may be hollow or solid (without or with stromal cores [papillary]) or a combination (including tubulo-papillary).[13,14] Hollow proliferation spheres have well-defined internal spaces (glandular-/tubular-/ductal-like or acinar-like) or ill-defined internal spaces (vague glandular-like).[14,15] Non-cohesive, singly scattered atypical cells or loose clusters would point towards lymphomas or melanomas. If the atypical cells distinctively comprised a second population of cells besides the reactive mesothelial cell population, it was considered metastasis.[13] The absence of a thin rim of cytoplasm between the nucleus and the cell membrane was considered a diagnostic feature of adenocarcinoma rather than malignant mesothelioma or reactive mesothelial cell proliferation.[16] Meanwhile, conventional cytology or cell block samples were considered suspicious of malignancy if there were questionable features such as irregular nuclear contour, hyperchromasia, prominent macronucleoli or atypical mitoses or necrosis. Finally, they were considered negative for malignancy if there were no features such as irregular nuclear contour, hyperchromasia, prominent macronucleoli or atypical mitoses or necrosis.
Moreover, we examined microscopically the histological pattern of arrangement of atypical cells in the cell blocks, which was classified into six groups: cluster (includes simple cluster and solid proliferation sphere), glandular-like hollow proliferation sphere, vague glandular-like hollow proliferation sphere, papillary, sheet, and single-cell patterns. Subsequently, with appropriate controls, we performed IHC on the pleural fluid cell blocks that were positive for malignancy using antibody markers CK7, CK20, TTF1, and calretinin (BioGenex Laboratories, Fremont, CA, USA) and additional IHC markers (BioGenex Laboratories, Fremont, CA, USA) in appropriate cases for subtyping.[17] The results of IHC marker staining were reported positive if ≥5% of the cells (cytoplasm and/or nuclei) were stained, except for ER, which was reported positive if ≥1% of the tumor nuclei were stained.[13,18] We identified various histological subtypes of malignancies based on positive staining by different immunohistochemical markers. In addition, findings from conventional cytology and cell block methods were compared to determine their mutual agreement. Statistical analysis was performed using the SPSS software version 27 (SPSS, Chicago, IL, USA) and kappa statistics.[19,20] The minimum study sample size (258) to be attained was calculated by using the formula: n = Zpq/d2, where Z = 1.96, p = variable with the least proportion, q = 100-p, d = margin of error (taken as 5), and substituting the value of p from the Datta et al.[21] study (metastatic adenocarcinomas from other sites [excluding lung]-21.4%).
RESULTSAmong the pleural fluid samples, most samples were from male patients, and the male-to-female ratio was 1.9:1. Regarding the cell block samples that were positive for malignancy, the male-to-female ratio was 1.3:1. The age of the participants ranged from 45 to 90 years, with a mean age of 55 years. The cases of malignancies diagnosed on cell blocks included patients with a mean age of 60 years.
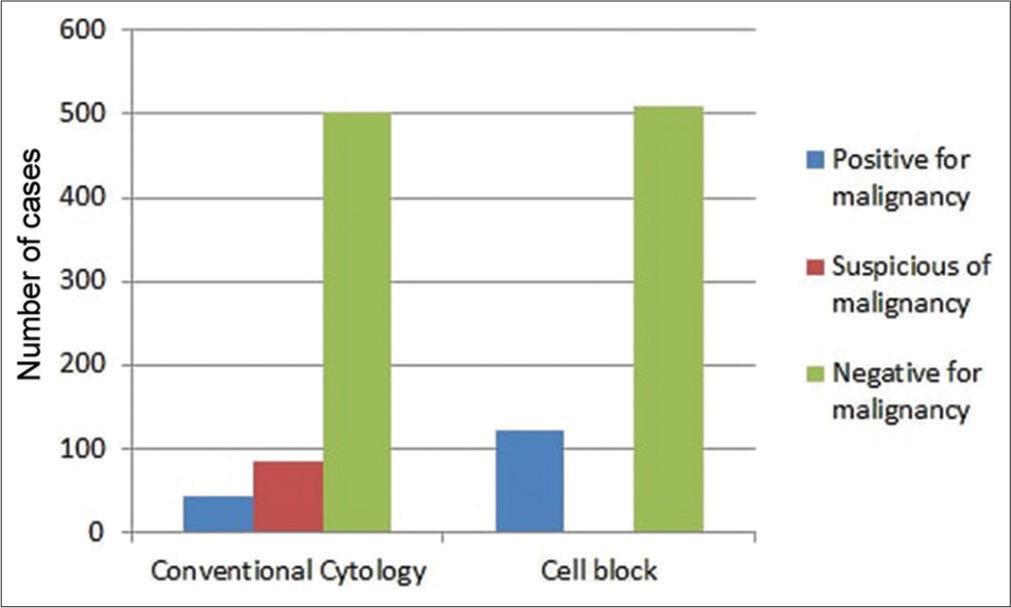
Overall, 503 (80%) negative, 84 (13%) suspicious, and 43 (7%) positive cases of malignancy were diagnosed on conventional cytology, whereas 509 (81%) negative, 0 (0%) suspicious, and 121 (19%) positive cases of malignancy were diagnosed on cell blocks [Figure 1 and Table 1].
Export to PPT
Table 1: Comparison between conventional cytology and cell blocks.
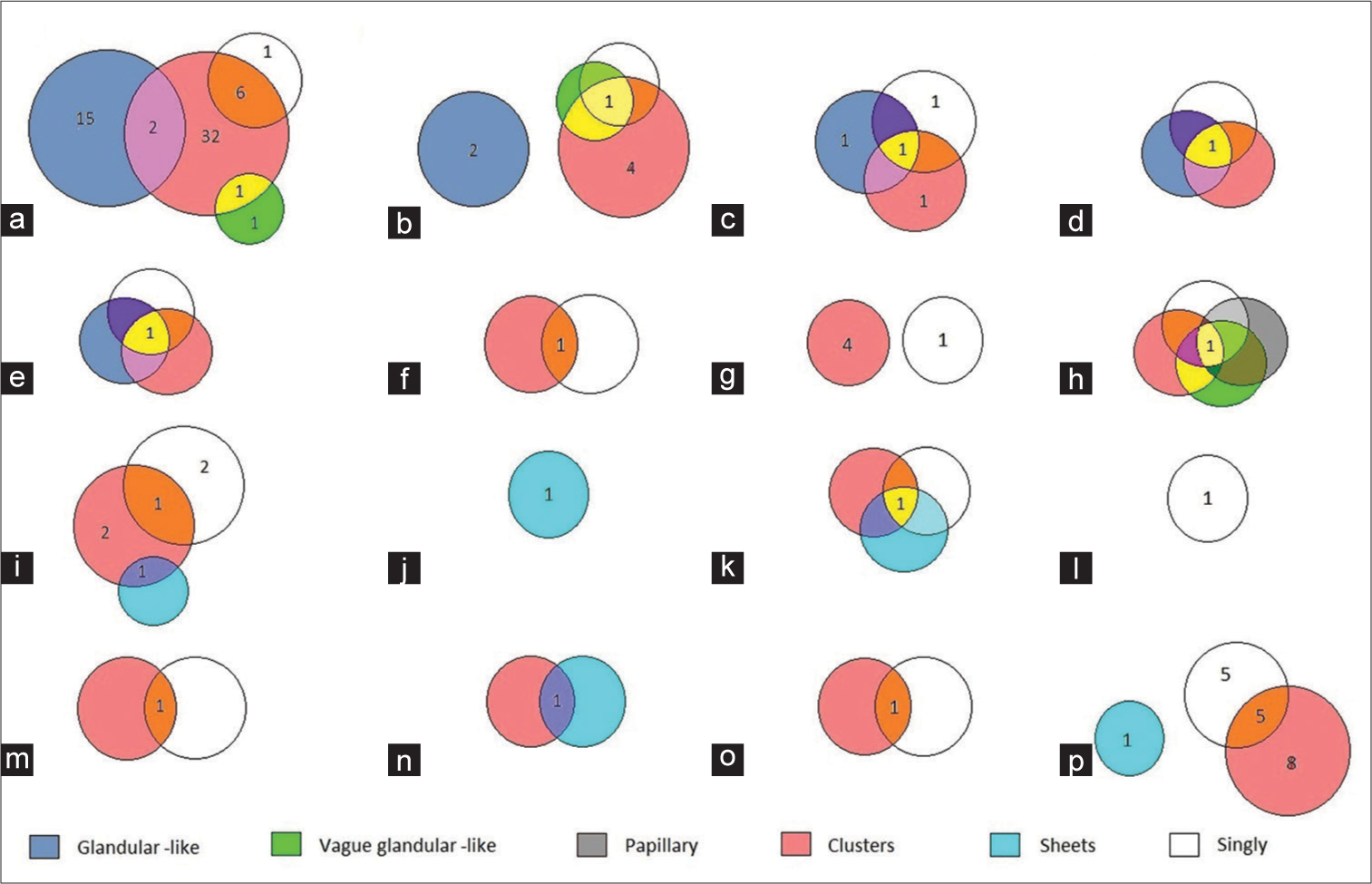
Conventional cytology Cell Block Negative for malignancy Suspicious of malignancy Positive for malignancy Total Negative for malignancy 488 21 0 509 Suspicious of malignancy 0 0 0 0 Positive for malignancy 15 63 43 121 Total 503 84 43 630Based on the microscopic examination of cell block samples, the cells of adenocarcinomas exhibited cluster, glandular-like proliferation sphere, and vague glandular-like proliferation sphere patterns. These cells were compared to those of malignant mesothelioma, which demonstrated cluster, vague glandular-like proliferation sphere, papillary, and single-cell patterns. Moreover, isolated patterns or various combinations of clusters, sheets, and single cells were observed in other malignancies [Figure 2].
Export to PPT
In the present study, immunohistochemical analysis of cell block samples with the initial antibody panel revealed that metastatic adenocarcinomas from the lung [Figure 3] were the most common malignancies (68 cases, 56%), followed by metastatic adenocarcinomas from other sites (21 cases, 17%). In addition, there was 1 case of malignant mesothelioma (1%) and 31 (26%) cases of other malignancies.

Export to PPT
Among the metastatic adenocarcinomas from the lung and metastatic adenocarcinomas from other sites, biopsy-proven cases comprised 6 and 5 cases, respectively. None of the other cases were biopsy-proven before the reporting of cell blocks.
Using additional IHC markers, many metastatic adenocarcinomas from other sites were categorized according to their site of origin; moreover, several other malignancies were also diagnosed [Figure 4 and Table 2]. Among metastatic adenocarcinomas from other sites (21 cases, 17%), metastases from the breast were the most common (7 cases, 33%), followed by metastases from the ovary (4 cases, 19%), endometrium (2 cases, 10%), gastrointestinal tract (2 cases, 10%), and urothelium (1 case, 5%). In addition, there were 5 (23%) cases of metastatic adenocarcinomas from unknown sites. Among other malignancies, there were 6 (19%) squamous cell carcinomas, 1 (3.5%) lymphoma, 1 (3.5%) small-cell carcinoma, 1 (3.5%) myeloma, 1 (3.5%) melanoma, 1 (3.5%) thymoma, and 1 (3.5%) embryonal rhabdomyosarcoma.

Export to PPT
Table 2: Cell block immunohistochemical marker profile of various malignancies.
Metastatic adenocarcinoma from the lung CK7+, CK20−, TTF1+, Calretinin−, Napsin A+ Metastatic adenocarcinoma from the breast CK7+, CK20−, TTF1−, Calretinin−, ER+, GATA3+, Vimentin−, Mammaglobin+ Metastatic adenocarcinoma from the ovary CK7+, CK20−, TTF1−, Calretinin−, ER+, GATA3−, PAX8+, WT1+ Metastatic adenocarcinoma from the endometrium CK7+, CK20−, TTF1−, Calretinin−, ER+, GATA3+, Vimentin+, CEA− Metastatic adenocarcinoma from the esophagusa CK7+, CK20+, TTF1−, Calretinin−, CDX2+, SATB2− Metastatic adenocarcinoma from the colon CK7−, CK20+, TTF1−, Calretinin−, CDX2+, SATB2+, Beta catenin-nuclear+ Metastatic adenocarcinoma from the urothelium CK7+, CK20+, TTF1−, Calretinin+, CDX2+, SATB2−, BetaThe diagnostic agreement between conventional cytology and cell block methods was calculated using kappa statistics. Cohen’s kappa was found to be 0.54, which indicates a moderate agreement according to the Landis and Koch scale.[20]
DISCUSSIONCytological samples are collected by a minimally invasive procedure, unlike biopsy samples. However, in effusion cytology, owing to their broad spectrum of variable morphology, reactive mesothelial cells share many overlapping features with malignant cells of adenocarcinoma or mesothelioma, which poses a significant diagnostic challenge for the cytopathologist. Although there are a few subtle points of differentiation, such as the presence of at least a thin rim of cytoplasm between the cell nucleus and the cell membrane in a mesothelial cell, unlike an adenocarcinoma cell, where the nuclear membrane forms part of the cell outline, not always this is possible for identification.[16] In this context, immunostaining and/or molecular studies are essential in solving the issue. Cytology smears are not recommended for immunocytochemical staining because of issues related to possible interference with immunoreactivity (due to substitution of formalin or alteration of the processing steps), increased background staining (due to the background protein-rich material), and lack of opportunity to evaluate coordinate immunoexpression in the same cells (by Subtractive Coordinate Immunoreactivity Pattern [SCIP] approach). Although immunostaining has been performed successfully on cytology smears (direct smears, cytospin smears, or liquid-based cytology preparations [SurePathTM and ThinPrepTM]) following specific protocols, the results are not always readily reproducible by others. Further, collecting cytology samples in weak alcohol fixatives such as Saccomanno Collection fluid, CytolytTM, PreservCytTM, or CytoRichTM Red would interfere with IHC.[22-27] This is due to the decreased availability of exposed epitopes that can bind to the antibody. It is here where the utility of cell blocks becomes relevant. This technique has a diagnostic accuracy very close to the gold standard, the pleural biopsy. However, it has been found in some cases that the biopsy specimens have been contaminated with non-neoplastic cells, which can hinder the diagnosis. This type of contamination is less likely in cytology.[28] Furthermore, unlike biopsy specimens, cell blocks are prepared from cytological fluids obtained by a procedure that causes much less discomfort to the patient. Cell blocks can be prepared by a variety of methods from body fluids. All require prior centrifugation of the fluid sample unless the sample is a clot or from a fat pad, in which case it is processed directly as a cell block. If there is blood contamination, after performing centrifugation, the sediment is treated with BloodLyzTM solution or FicollHypaque medium before cell block preparation. On the other hand, if there is no blood contamination and the yield of sediment tissue is cellular (>1 mL with Tissuecrit [or Cytocrit] >50%), the fixed sediment method may be sufficient for cell block preparation. Techniques such as the plasma thrombin method, agar method, HistoGelTM, Collodion bag, Shandon CytoblockTM, Micro NextGen CelBlokingTM (Micro-NCGB), Nano NextGen CelBlokingTM (Nano-NCGB), etc. may be used instead for better concentration. Meanwhile, if it is hypocellular (<1 mL with Tissuecrit <50%), Nano-NCGBTM kit should be used for obtaining proper concentration.[22-24,29] Cell block slides are finally viewed under the microscope to arrive at a histopathological diagnosis. Furthermore, IHC or molecular studies can be performed on cell blocks to reveal the true nature of the lesion as well as identify the subset of patients who will likely benefit from targeted therapy.[8-10] Cell block IHC performed by the SCIP approach is rewarding as it gives information about the spatial relationship of various cells in the serial sections of the effusion sample, which is absolutely necessary to track the cells when they are stained with an IHC marker as well as decide what next IHC marker has to be performed. In the SCIP approach, all the diagnostic materials are aligned along the potential cutting surface before gelling or embedding the medium for the best yield, which can be achieved by pre-staining with eosin/hematoxylin or by Shidham’s protocol using a dark-colored beacon-like AV marker (named after the manufacturer AV BioInnovation LLC, Grosse Ile, MI, USA). The AV marker provides precise monitoring during the section-cutting process, thus facilitating the orientation component of the SCIP approach. NCGB kits already have a built-in AV marker.[22-24] Recently, dual color SCIP facilitated easy identification of the foreign populations of malignant cells in effusion fluids.[30] Additionally, special stains may be done on cell-block sections, demonstrating certain organisms, extracellular material or specific histological patterns valuable for diagnosing a particular lesion or neoplasm. Even the frozen section technique has been performed successfully on cell blocks, which allowed rapid diagnosis within a few hours.[31]
The present study used a large sample size of 630 pleural fluid samples and IHC was performed on 121 cell blocks that showed evidence of malignant cells. In contrast, regarding similar studies[4,21,32-37] in the relevant literature, most of them used a much lower number of pleural fluid samples and cell block samples for analysis, except for a study by Porcel et al.,[38] which had a sample size (632) comparable to our study, even though they did not perform cell block IHC.
In our study, compared to female patients, the total number of male patients was higher (1.9:1), and this finding agrees with similar studies.[21,33,36] Further, we demonstrated that as far as cell block samples that were positive for malignancy are concerned, the male-to-female ratio was 1.3:1, which is identical to that observed by Datta et al.,[21] whereas in the studies by Shivakumarswamy et al.[33] and Ranieri et al.,[36] the number of positive cell blocks were higher in female patients. Therefore, the majority of the studies suggest that malignant pleural effusion affects males more than females.
Regarding patients from whom pleural fluid samples were collected in our study, the mean age was 55 years, comparable to that reported by Datta et al.[21] and Shivakumarswamy et al.[33] Moreover, regarding the patients whose cell block samples were positive for malignancy, their mean age (60) was similar to that reported by Datta et al.,[21] and 56% of them were of age ≥60 years. Therefore, studies suggest a higher risk of malignant pleural effusion in individuals of higher age.
Based on conventional cytology, the number of suspicious cases was higher (13%) in the present study than that detected by Shivakumarswamy et al.[33] (8%) and Ranieri et al.[36] (3%). Subsequently, among those 13% suspicious cases, cell block analysis revealed 12% to be positive and 1% to be negative for malignancy. Therefore, the cell block technique eliminated all the suspicious cases that were reported by the conventional cytology method, thereby resolving any issue of diagnostic confusion. Moreover, the cell block method detected more malignancies (19%) than the conventional cytology method (7%), a finding consistent with those of the previous similar studies. The number of malignancies detected on cell blocks by our study (121) lies between those reported by similar studies.[21,33,36,37]
According to work by Datta et al.,[21] the number of cases of metastatic adenocarcinomas from the lung, metastatic adenocarcinomas from other sites, malignant mesotheliomas, and other malignancies were 15 (53.57%), 6 (21.43%), 0 (0%), and 7 (25%), respectively, and this finding is comparable to that in our study. In contrast, Shivakumarswamy et al.[33] and Ranieri et al.[36] reported that the number of metastatic adenocarcinomas from other sites was more than that from the lung.
In the present study, IHC analysis subtyped 85% of all malignancies. However, owing to the unavailability of some specific IHC markers and/or insufficient residual tissue material, 15% of the cases could not be subtyped and thus remained unclassified malignancies.
This study also found a moderate diagnostic agreement between conventional cytology and cell block methods. However, Shivakumarswamy et al.[33] observed a lower agreement.
Thus, the cell block technique has many distinct advantages of samples being obtained through a less invasive procedure than a pleural biopsy while still providing a histopathological diagnosis, thereby having the provision to do ancillary techniques such as IHC for subtyping malignancies and the capability to detect malignancies that would otherwise be missed on a conventional cytology smear.
SUMMARYCell blocks are prepared from pleural fluid samples collected via thoracentesis, a less invasive procedure than a pleural biopsy. In our study, cell block IHC revealed that adenocarcinomas, particularly from the lung, are the major source of malignant cells migrating to the pleural fluid compared to other cancer types. Moreover, cell blocks not only helped detect more malignancies than conventional cytology but also proved to demonstrate better architectural patterns and be a better platform for performing IHC that altogether aid in arriving at a correct diagnosis.
AVAILABILITY OF DATA AND MATERIALSThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
留言 (0)