The three approved IL-17 inhibitors consist of IL-17A inhibitors (secukinumab and ixekizumab) and an IL-17 receptor (IL-17R) inhibitor (brodalumab). They exhibit satisfactory therapeutic outcomes and acceptable safety profiles in treating psoriasis and psoriatic arthritis. However, occasional reports highlight less common side effects during anti-IL-17 treatment, such as paradoxical reactions. These reactions are notable for their unmistakable symptoms and the subsequent therapeutic challenge in follow-up care. Paradoxical reactions are generally defined as developing or worsening a preexisting immune-mediated disorder that would typically respond to the same therapeutic agent causing it.1 In this context, we present a case series of patients who developed paradoxical psoriasis during anti-IL-17 treatment at our institution. We also review relevant articles, aiming to propose the most likely mechanisms of action and a relatively optimised therapeutic approach for physicians to consider as a reference.
Materials and MethodsWe conducted a literature review using the PubMed and MEDLINE databases (updated through January 1, 2023). The search employed index terms like “anti-interleukin 17,” “ixekizumab,” “secukinumab,” and “brodalumab,” combined,” coupled with terms such as “paradoxical,” “psoriasis,” “arthritis,” “skin,” and “eruption.” Additionally, we reviewed citations within the articles to ensure comprehensive coverage. Patients with other established trigger factors were excluded from this review. Moreover, we present a case series of patients with preexisting psoriasis who developed paradoxical psoriasis eruptions during anti-IL-17 treatment in our institution from June 2020 to August 2022. All patients provided written informed consent, and the ethics committee approved the study.
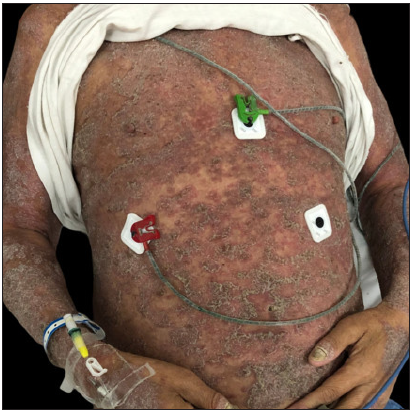
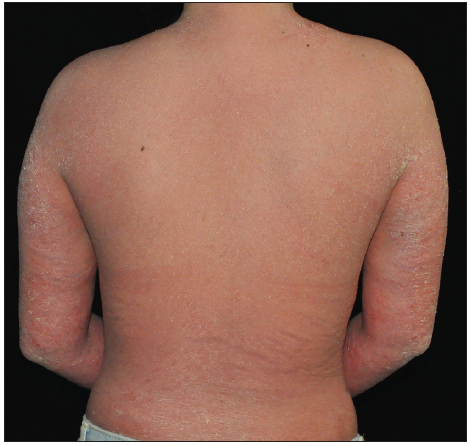
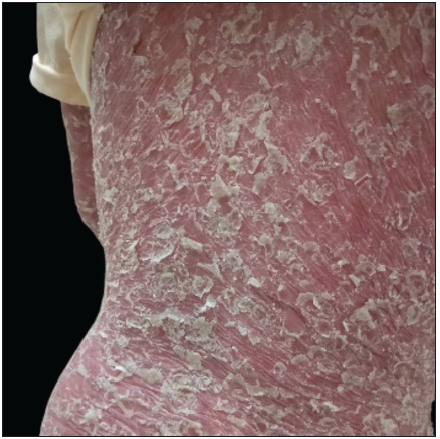
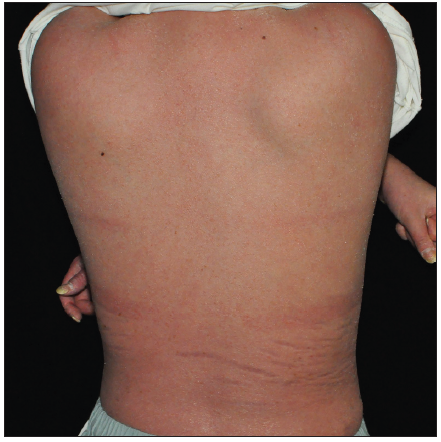
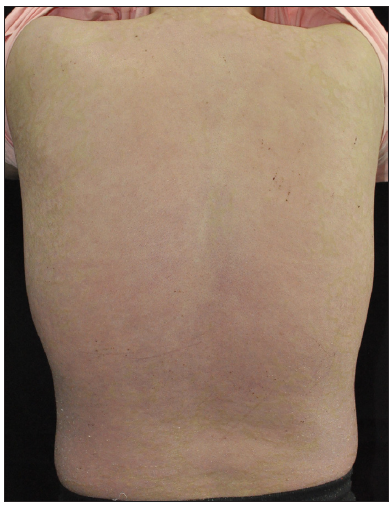
Case SeriesCase 1: A 59-year-old man had plaque psoriasis for over 30 years, along with type 2 diabetes and coronary artery disease for 10 years. And he was referred to our clinic, due to a sudden bout of erythroderma (Body surface area, BSA = 92%; Psoriasis area severity index, PASI = 65) [Figure 1a]. His medical history revealed regular use of Chinese herbal medicines for psoriasis, and he had been on hypoglycemic drugs and antihypertensives for a long time to manage his other health issues. Consequently, ixekizumab treatment was initiated at the prescribed dosages. Ten days later, PASI decreased to 29.2 [Figure 1b]. Unfortunately, the positive response halted in the 3rd week, and erythroderma returned in the first month of ixekizumab treatment (PASI = 64.8) [Figure 1c]. No specific factors were identified as the cause for this deterioration. Due to the worsening of the disease, ixekizumab was stopped, and acitretin combined with cyclosporine was started to improve the condition. After 6 days of this treatment, erythema and edema showed significant improvement, and simultaneously, PASI decreased to 45 [Figure 1d].
Export to PPT

Export to PPT
Export to PPT
Export to PPT
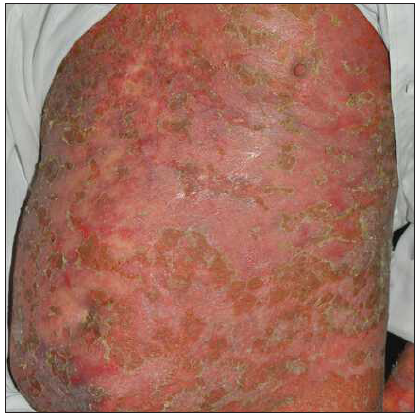
Case 2: An 18-year-old woman, having psoriasis vulgaris for 8 years, was referred to our clinic due to erythroderma and psoriatic arthritis [Figure 2a]. A physical examination showed widespread redness and rough scaly plaques covering nearly all skin (BSA = 98%; PASI = 56.4). After a thorough assessment, ixekizumab was initiated. PASI decreased to 25.6 entirely following the second dose, and troublesome joint symptoms significantly improved [Figure 2b]. However, this positive response lasted for only one more week. During the 6-week follow-up, erythroderma reappeared without apparent cause (PASI = 62.4) [Figure 2c]. Consequently, ixekizumab was discontinued, and adalimumab was started. In another 3-month follow-up, the rash was nearly cleared (PASI = 2) [Figure 2d].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
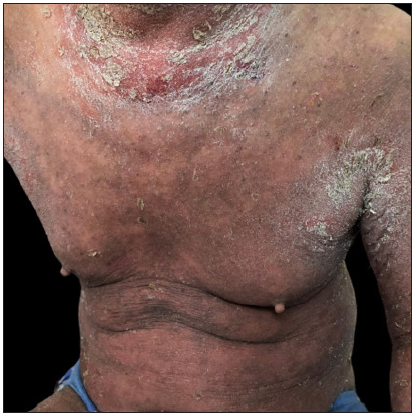
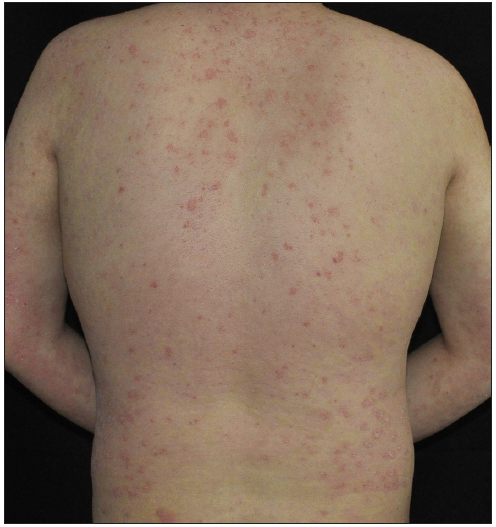
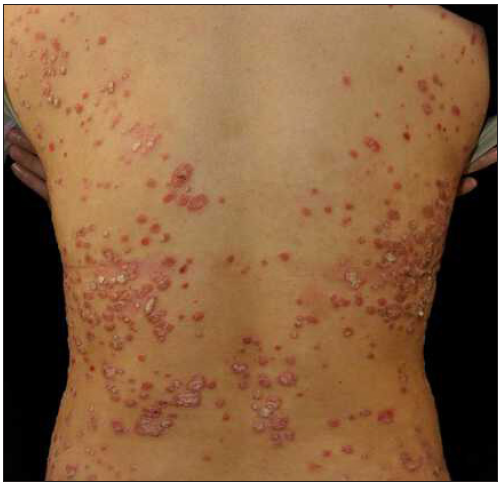
Case 3: A 19-year-old overweight man, diagnosed with plaque psoriasis for 8 years and recently found to have metabolic syndrome, was referred to our clinic due to the significant impact of the disease on his quality of life [Figure 3a]. Previous medical records indicated a refusal of traditional systemic agents due to potential adverse effects. A physical examination revealed widespread redness and thickened scaly plaques covering the skin (BSA = 70%, PASI = 40.8). Consequently, ixekizumab was administered at the recommended dosages. By the 2nd week, PASI decreased to 19.6 [Figure 3b]. However, by the 5th week, guttate psoriasiform eruptions appeared on the ventral and dorsal trunk, and the bilateral lower limbs were covered with coarse plaques without any identifiable triggers (PASI = 29.7) [Figure 3c]. To address this, topical corticosteroids were added for rescue, but the outcomes were unsatisfactory. Subsequently, ixekizumab was stopped, and adalimumab was initiated. Unfortunately, at the 3-month follow-up, the eruption had only partially improved (PASI = 20.7) [Figure 3d].

Export to PPT
Export to PPT
Export to PPT

Export to PPT
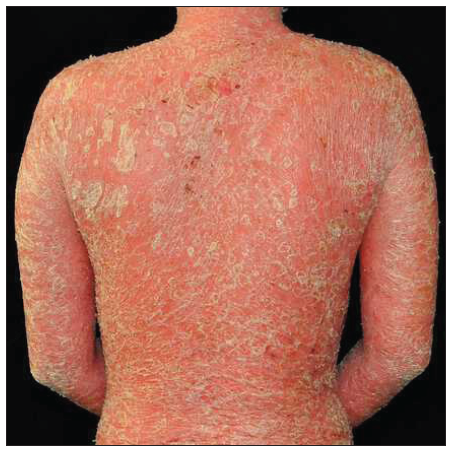
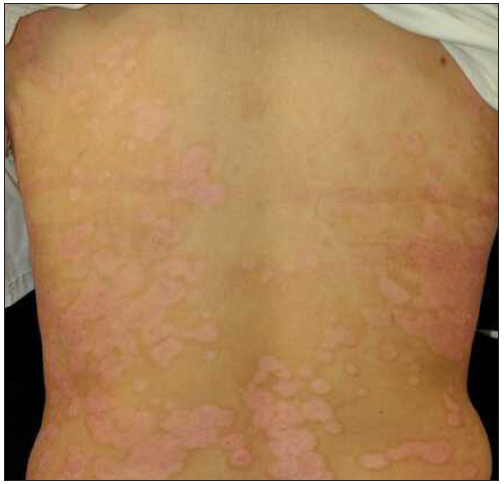
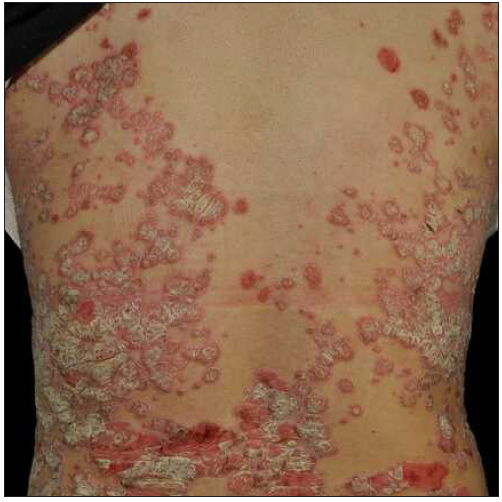
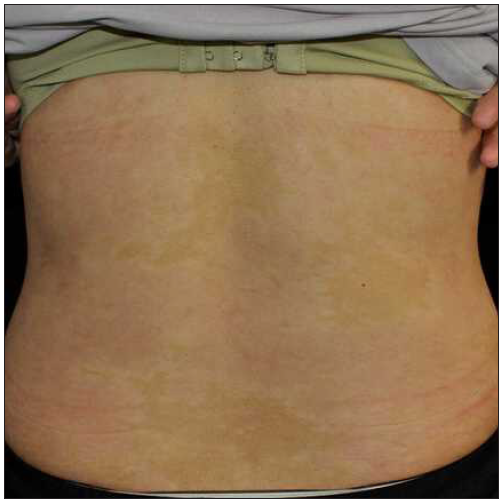
Case 4: A 24-year-old woman, diagnosed with plaque psoriasis for 8 years, was referred to our clinic [Figure 4a]. No prior medical history was available. A physical examination showed generalised thick scaly plaques (BSA = 30%, PASI = 24.3). Ixekizumab was then prescribed. Within 2 weeks, the PASI decreased to 11.1 [Figure 4b]. Unfortunately, by the 3rd week, annular lesions developed and spread on the trunk and gradually onto the limbs despite the application of potent topical corticosteroids (PASI = 29.7) [Figure 4c]. No specific triggers were identified during this time. Consequently, we switched from ixekizumab to adalimumab in the 5th week. At the 3-month follow-up, the lesions were almost resolved (PASI = 0.6) [Figure 4d].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
ResultsA total of 22 articles, including 30 patients, were included in the final analysis. Gender, age, comorbidities, diagnosis, drug administration, the progression of skin eruption, and management details are presented in Tables 1 and 2. As indicated in Table 1, the onset time of paradoxical psoriasis varied, ranging from 1 week to 2 years. Preexisting psoriasis deteriorated in most cases (except for three patients with different major diagnoses). Conversion of lesion morphology during anti-IL-17 therapy was observed in 59.3% (16/27) of patients, with 3 cases developing de novo psoriasis. Among these, the conversion of plaque to pustular was the most common, accounting for approximately 68.8% (11/16).
Table 1: Summary of patients who developed paradoxical psoriasis during IL-17 antagonist treatment in literature
Total (n = 30) PSO (n = 27) AS (n = 2) HS (n = 1) Male 14 (46.6) 14 (51.9) N N Female 16 (53.3) 13 (48.1) 2 (100) 1 (100) Age, mean ± SD years 49.7 ± 12.7 49.8 ± 13.0 55.5 ± 2.1 36.0 Culprit drug Ixekizumab 9 (30.0) 9 (33.3) N N Secukinumab 14 (46.6) 11 (40.7) 1 (100) 1 (100) Brodalumab 7 (23.3) 7 (25.9) N N Types of new onset or exacerbation psoriasis Guttate 1 (3.3) 1 (3.7) N N Plaque 10 (33.3) 10 (37.0) 1 (50) N Pustular 11 (36.6) 9 (33.3) 1 (50) 1 (100) Arthritis 3 (10.0) 3 (11.1) N N Erythroderma 1 (3.3) 1 (3.7) N N Mixed 4 (13.3) 4 (14.8) N N Latent period, mean ± SD weeks 23.0 ± 21.4 23.2 ± 21.9 24.0 ± 28.2 16.0 Management Continuation of anti-IL-17 without adjuvant therapy N N N N Continuation of anti-IL-17 with topical therapy 6 (20.0) 6 (22.2) N N Continuation of anti-IL-17 with systemic therapy 2 (6.6) 2 (9.1) N N Withdrawn anti-IL-17 without adjuvant therapy N N N N Withdrawn anti-IL-17 with topical therapy 6 (20.0) 3 (11.1) 2 (100) 1 (100) Withdrawn anti-IL-17 with systemic therapy 16 (53.3) 16 (59.3) N N Outcome Partial or complete resolution 28 (93.3) 25 (92.6) 2 1 No resolution 2 (6.6) 2 (7.4) N NTable 2: Clinical features and management of patients who developed paradoxical psoriasis during IL-17 antagonist treatment in literature
Number Reference Sex/age (years) Diagnose Comorbidity Anti-IL-17 agent Time to onset or exacerbation of psoriasis (weeks) Conversion of lesion morphology during anti- IL-17 therapy Management Outcome 1 Hoshina et al.4 F/43 PSO PSA Secukinumab 4 Plaque to plaque Secukinumab discontinuation+systemic CsA Gradually improved 2 Pirro et al.5 M/48 PSO N Ixekizumab 16 Plaque to pustular Ixekizumab maintenance+topical steroids Completely resolved 3 Vidal et al6 M/46 PSO N Ixekizumab 2 Plaque to arthritis Ixekizumab discontinuation+ Secukinumab + short-term systemic steroids Completely resolved 4 Martínez-Doménech et al.7 M/43 PSO N Ixekizumab 48 Pustular to Pustular Ixekizumab maintenance + topical steroids No resolution 5 Oiwa et al.8 M/76 PSO N Ixekizumab 8 Plaque to erythroderma Ixekizumab discontinuation + systemic CsA Mostly resolved 6 Cicogna et al.9 F/52 PSO PSA Secukinumab 36 Plaque to pustular Secukinumab discontinuation + systemic acitretin Visibly regressed 7 Caldarola et al.10 M/34 PSO N Ixekizumab 8 Plaque to plaque Ixekizumab maintenance + topical steroids Improved 8 Caldarola et al.10 M/53 PSO N Ixekizumab 20 Plaque to plaque Ixekizumab discontinuation + topical steroids Improved 9 Caldarola et al.10 M/52 PSO N Secukinumab 16 Plaque to plaque Secukinumab discontinuation + topical steroids Improved 10 Caldarola et al.10 M/48 PSO N Ixekizumab 12 Plaque to plaque Ixekizumab maintenance + topical steroids Improved 11 Caldarola et al.10 F/42 PSO N Ixekizumab 26 Plaque to plaque Ixekizumab maintenance + topical steroids Improved 12 Tan et al.11 F/62 PSO Hypertension, diabetes, and essential tremor Ixekizumab 24 Plaque to plaque Ixekizumab discontinuation + short-term systemic steroids Mild improved 13 Takahashi et al.12 F/69 PSO N Brodalumb 16 Plaque to pustular with PSA Brodalumb discontinuation + systemic CsA Rapidly improved 14 Takahashi et al.12 M/63 PSO N Brodalumb 12 Plaque to arthritis Brodalumb discontinuation + adalimumab Rapidly improved 15 Sadik et al.13 F/42 PSO N Brodalumb 24 Plaque to pustular with PSA Brodalumb discontinuation + ustekinumab Gradually resolved 16 Kurtipek et al.14 M/33 PSO N Secukinumab 20 Plaque to guttate Secukinumab maintenance + short-term MTX No regression 17 Honma et al.15 F/64 PSO N Secukinumab 96 Pustular to Pustular Secukinumab maintenance+ topical steroids No worsening 18 Abbruzzese et al.16 F/63 PSO PSA Secukinumab 36 Plaque to pustular Secukinumab maintenance + systemic CsA Completely resolved 19 Currado et al.17 F/54 AS N Secukinumab 44 New onset plaque Secukinumab discontinuation + topical agents Completely resolved 20 Sladden et al.18 F/61 PSO N Secukinumab 12 Plaque to pustular Secukinumab discontinuation+ ustekinumab Significantly improved 21 Noell et al.19 M/53 PSO N Secukinumab 5 Plaque to plaque Secukinumab discontinuation + infliximab Promising improved 22 Dogra et al.20 M/22 PSO N Secukinumab 36 Plaque to pustular Secukinumab discontinuation + Infliximab Completely resolved 23 Mössner et al.21 F/26 PSO N Brodalumb 8 Plaque to pustular Brodalumb discontinuation + ustekinumab Completely resolved 24 Mössner et al.21 F/44 PSO PSA and obesity Secukinumab 64 Plaque to pustular Secukinumab discontinuation + topical steroids Completely resolved 25 Mössner et al.21 F/45 PSO PSA and obesity Secukinumab 56 Plaque to pustular with plaque Secukinumab discontinuation + ustekinumab considerably improved 26 Penalba-Torres et al.22 F/36 HS N Secukinumab 16 New onset pustular Secukinumab discontinuation + topical agents Completely resolved 27 Penalba-Torres et al.22 F/57 AS N Secukinumab 4 New onset pustular Secukinumab discontinuation + topical agents Rapidly improved 28 Hosokawa et al.23 M/67 PSO N Brodalumb 4 Plaque to plaque Brodalumb discontinuation + Guselkumab Visibly improved 29 Kashlan et al.24 M/47 PSO PSA and obesity Brodalumb 1 Plaque to recurred arthritis Brodalumb discontinuation + to facitinib + short-term systemic steroids Improved 30 Caravello et al.25 M/46 PSO N Brodalumb 16 Plaque to pustular with plaque Brodalumb discontinuation + Secukinumab Partial improvedThe management of paradoxical psoriasis varied widely due to patient heterogeneity. Among the 30 patients, 53.3% switched to another systemic agent, 20% suspended the culprit drug and added topical agents, 20% added topical agents without discontinuing the drug, and 6.6% continued the drug with another systemic agent as a complement. In the follow-up, 93.3% of cases showed partial or complete resolution, regardless of drug continuation, while 6.6% exhibited no resolution, primarily in patients maintaining the culprit drug. Interestingly, two cases reintroduced a different class of IL-17 inhibitors for treating paradoxical psoriasiform eruptions, with no observed deterioration and partial improvement of the disease. Additionally, 71.4% (5/7) of patients with comorbidities discontinued the original drug and switched to another systemic agent. For patients without comorbidities, 47.8% (11/23) made the same decision for management. Subgroup analysis generally mirrored the overall group analysis.
DiscussionIL-17A/IL-17R inhibitors are effective and safe in large-scale randomised controlled trials for treating psoriasis and psoriatic arthritis. However, occasional reports of less common side effects, such as paradoxical psoriasis, have emerged. This paper presents representative cases of paradoxical psoriasis induced by IL-17A inhibitors in our institution. Our findings suggest that our patients may be more susceptible to severe attacks with a shorter latency compared to previous studies. Although three out of four patients had comorbidities [Table 3], and one had experienced multiple agents, we attribute the sudden deterioration to paradoxical psoriasis rather than the above mentioned factors. A multicenter retrospective study revealed that ixekizumab response was unaffected by clinical variables like body mass index, disease duration, or psoriatic arthritis, further supporting our opinion.2
Table 3: Details of patients with paradoxical psoriasis induced by ixekizumab in our clinic
Patient Sex/age (years) Diagnose Comorbidity PASI (start) PASI (remission) PASI (exacerbation) PASI (at the last follow-up) Time to exacerbation of psoriasis (weeks) Conversion of lesion morphology Management 1 M/59 EP Type 2 diabetes; cardiovascular diseases 65 29.2 64.8 45 3 No change Acitretin combined with CsA + ixekizumab discontinuation. 2 F/18 EP PSA 56.4 25.6 62.4 2 3 No change Adalimumab + ixekizumab discontinuation 3 M/19 PP Metabolic syndrome 40.8 19.6 29.7 20.7 5 Plaque to guttate Adalimumab + ixekizumab discontinuation 4 F/24 PP N 24.3 11.1 29.7 0.6 3 No change Adalimumab + ixekizumab discontinuationReviewing the literature on IL-17 inhibitor-induced paradoxical psoriasis; we observed that compared with TNF-α inhibitors, the latency was shorter, and the lesion morphology tended to transform plaque into pustular rather than plaque to guttate. Mechanistically, IL-17 inhibitors lead to a decrease in TNF-α concentrations, directly or indirectly causing an increase in IFN-α. This, in turn, leads to the expression of chemokines such as CXCR3 on T cells, promoting T-cell homing to the skin and sustaining the inflammatory mechanisms of psoriasis lesions.3 Inhibition of Th17 may cause repolarization toward Th1, possibly explaining why TNF-α inhibitors can manage paradoxical psoriasis caused by IL-17 inhibitors.
There is no consensus on managing paradoxical psoriasis induced by IL-17 inhibitors due to limited reported data.4–25 In our study, we found that more patients preferred discontinuing the implicated drug and sought additional adjuvant therapy to control paradoxical psoriasis, in contrast to that induced by TNF-α inhibitors (73.3% vs. 33.2%). Patients attempting to continue IL-17 inhibitors without strong intervention faced more significant challenges instead of gradual improvement. This evidence suggests that more aggressive measures should be taken to treat paradoxical psoriasis induced by IL-17 inhibitors than those caused by TNF-α inhibitors. Reintroducing another IL-17 inhibitor is not recommended, as patients rarely improved according to this analysis.
LimitationData are limited to case reports and case series.
ConclusionA substantial body of evidence suggests that patients in similar situations may benefit from more aggressive interventions. Additionally, the re-administration of another IL-17 inhibitor is not recommended.
留言 (0)